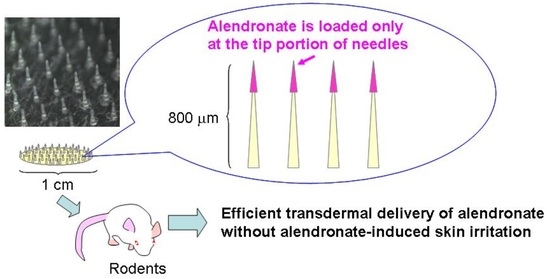
Efficient Transdermal Delivery of Alendronate, a Nitrogen-Containing Bisphosphonate, Using Tip-Loaded Self-Dissolving Microneedle Arrays for the Treatment of Osteoporosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
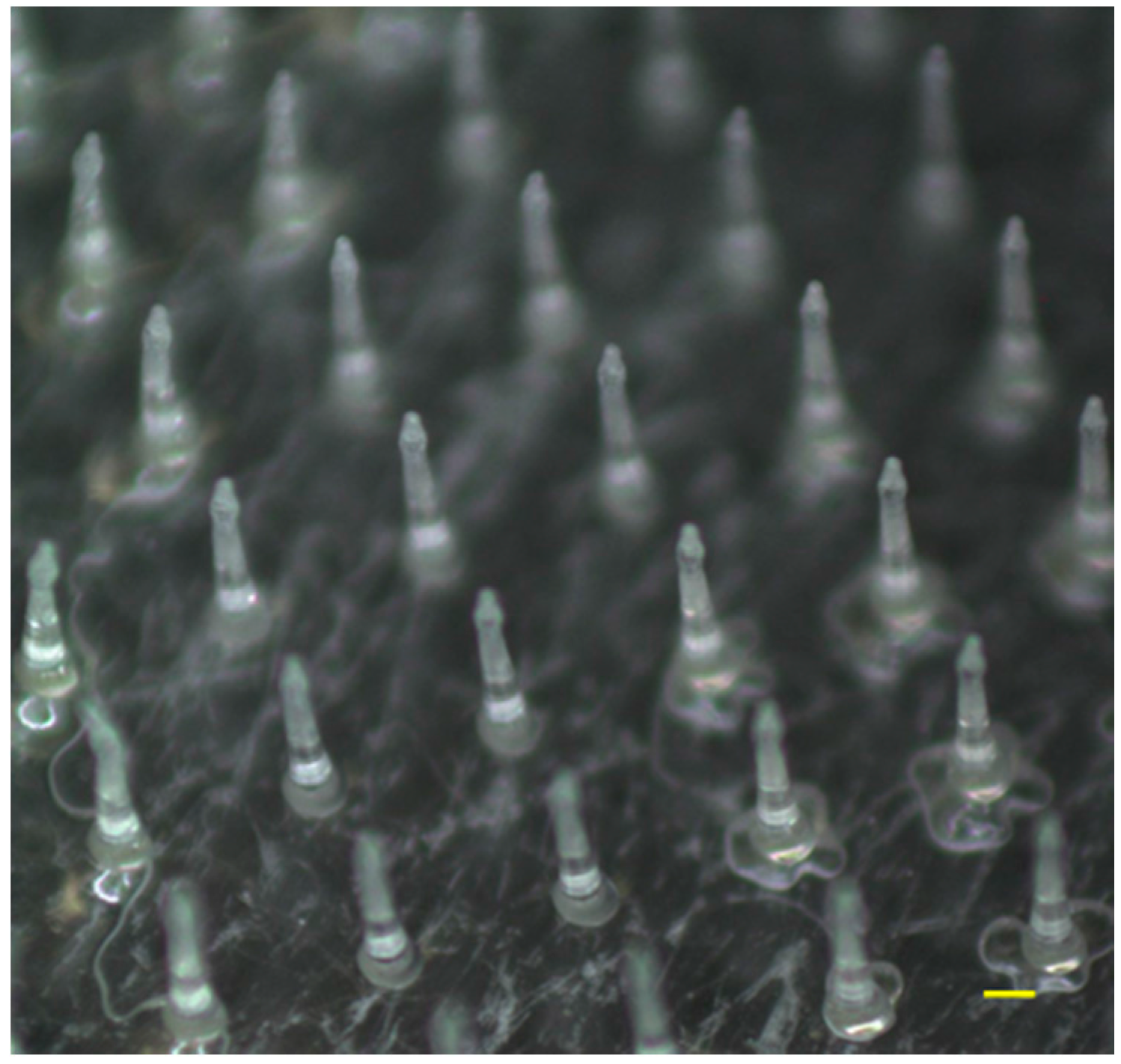
2.3. Fabrication of ALN(TIP)-MN
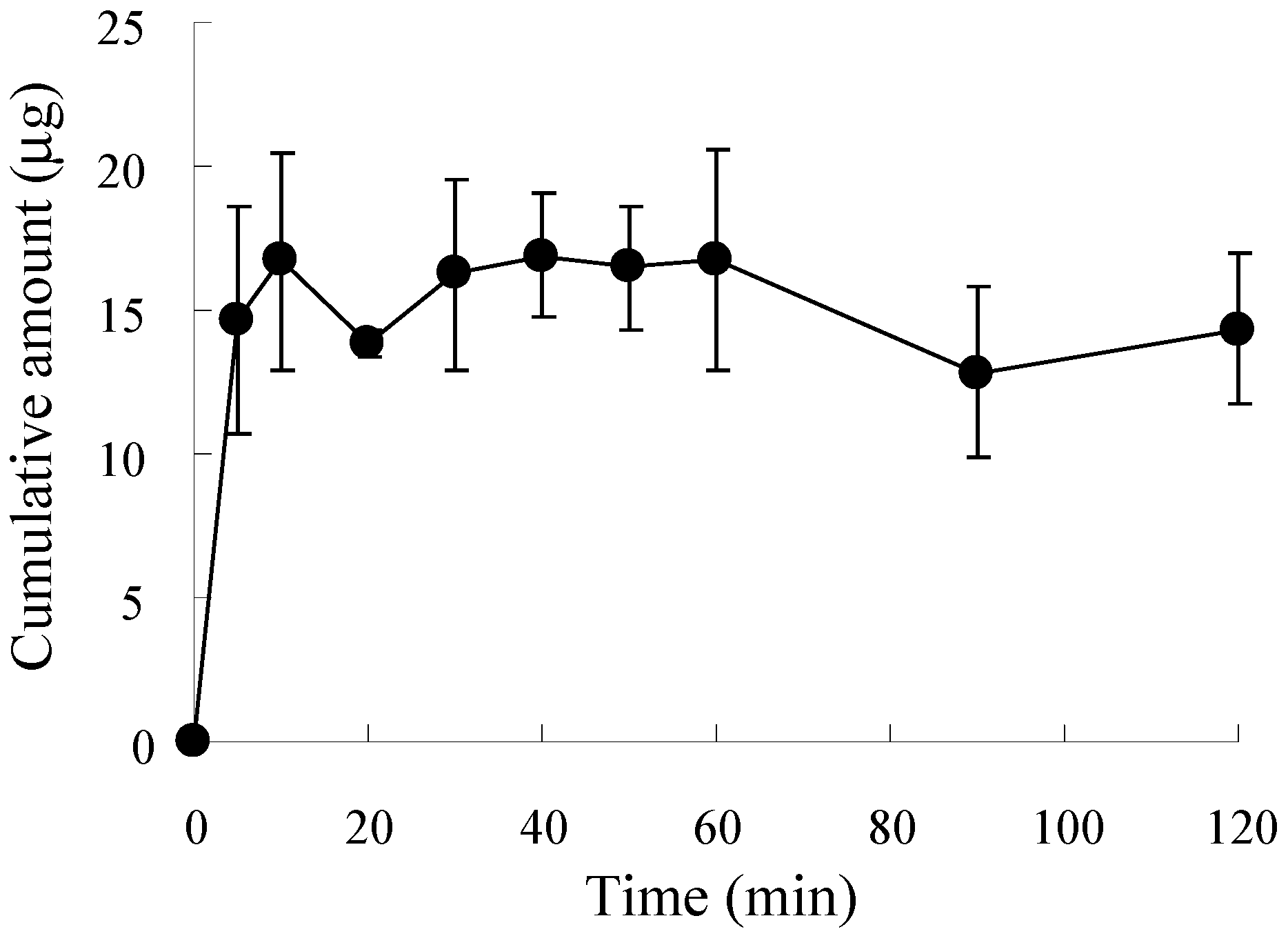
2.4. In Vitro Release of ALN from ALN(TIP)–MN
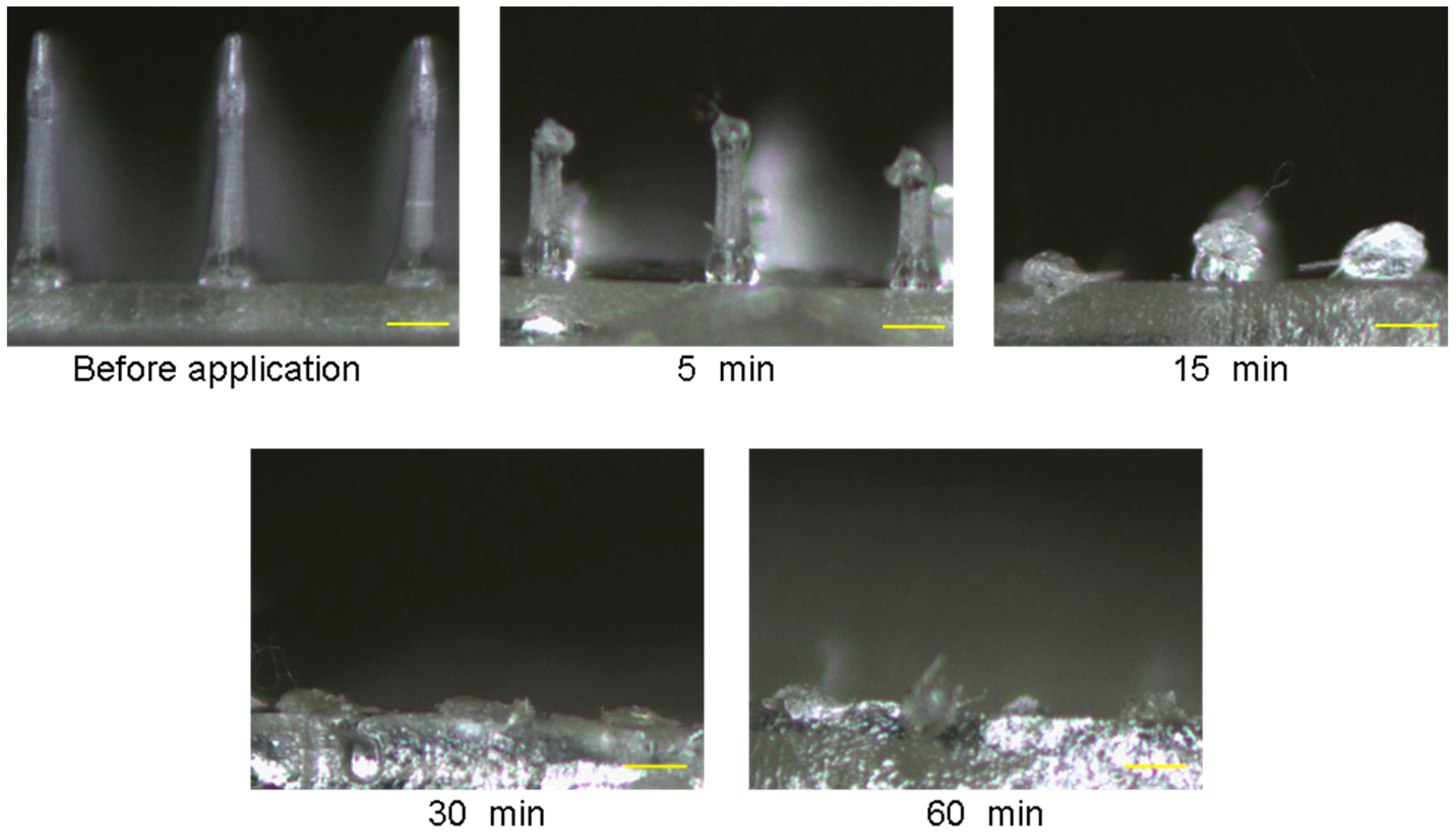
2.5. Dissolution of ALN(TIP)–MN after Application to the Rat Skin
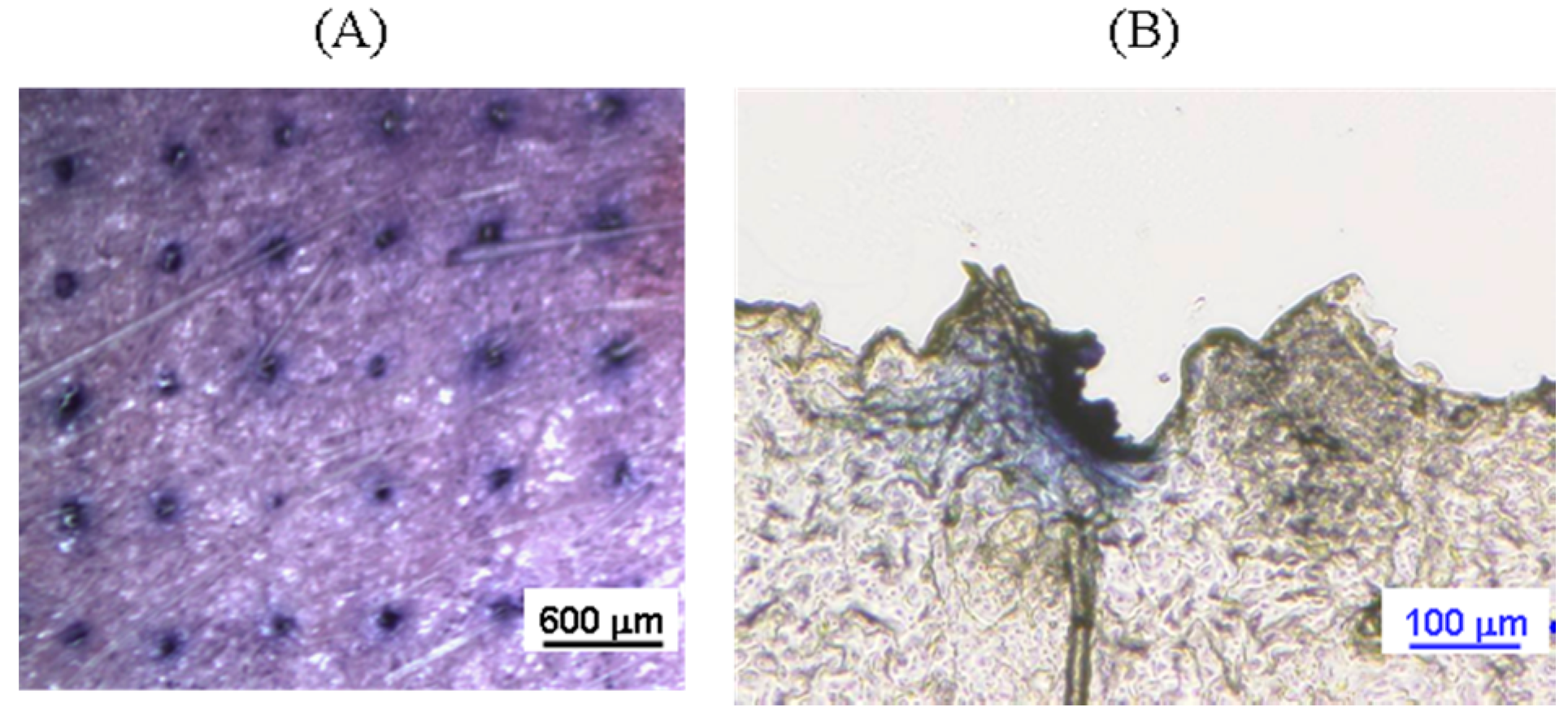
2.6. Characterization of Rat Skin Pierced by ALN(TIP)–MN
2.7. Transdermal Absorption Study
2.8. Osteoporosis Experiment
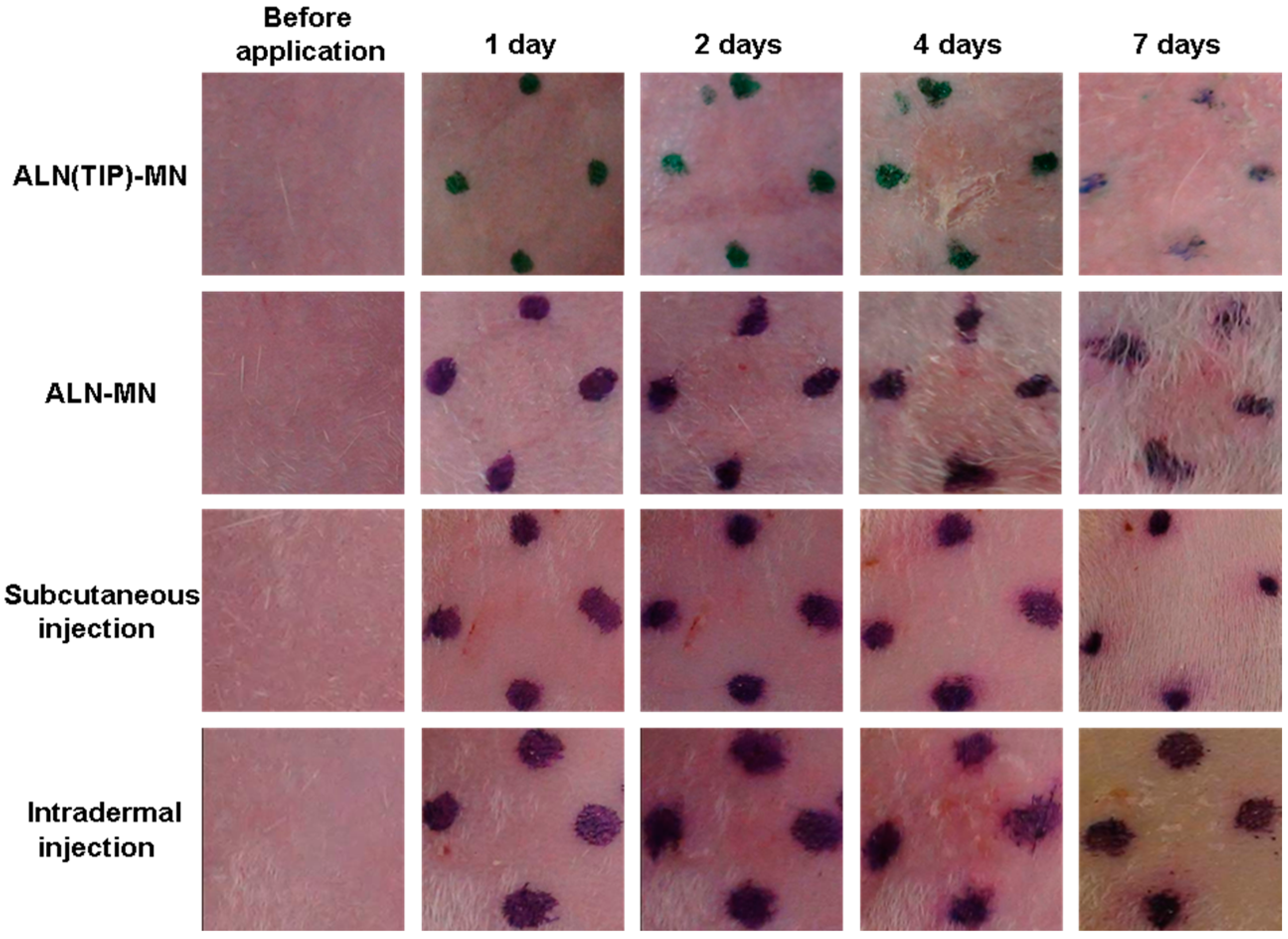
2.9. Skin Irritation in Response to ALN(TIP)–MN
3. Results
3.1. Characteristics of ALN(TIP)–MN
3.2. In Vitro Release Profile of ALN from ALN(TIP)–MN
3.3. Dissolution Process of ALN(TIP)–MN after Application to Rat Skin
3.4. Piercing Ability of ALN(TIP)–MN across Rat Skin
3.5. Pharmacokinetics of ALN after Various Administration Methods
3.6. Preventive and Therapeutic Effect of ALN(TIP)–MN on Osteoporosis
3.7. Skin Irritation Caused by ALN after Various Administration Methods
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Russell, R.G. Bisphosphonates: Mode of action and pharmacology. Pediatrics 2007, 119, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Lowe, C.E.; Depew, W.T.; Vanner, S.J.; Paterson, W.G.; Meddings, J.B. Upper gastrointestinal toxicity of alendronate. Am. J. Gastroenterol. 2000, 95, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Gertz, B.J.; Holland, S.D.; Kline, W.F.; Matuszewski, B.K.; Freeman, A.; Quan, H.; Lasseter, K.C.; Mucklow, J.C.; Porras, A.G. Studies of the oral bioavailability of alendronate. Clin. Pharmacol. Ther. 1995, 58, 288–298. [Google Scholar] [CrossRef]
- Guy, R.H. Transdermal drug delivery. Handb. Exp. Pharmacol. 2010, 197, 399–410. [Google Scholar]
- Wiechers, J.W. The barrier function of the skin in relation to percutaneous absorption of drugs. Pharm Weekbl. Sci. 1989, 11, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Aungst, B.J. Absorption enhancers: Applications and advances. AAPS J. 2012, 14, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Dixit, N.; Bali, V.; Baboota, S.; Ahuja, A.; Ali, J. Iontophoresis—An approach for controlled drug delivery: A review. Curr. Drug Deliv. 2007, 4, 1–10. [Google Scholar] [PubMed]
- Rao, R.; Nanda, S. Sonophoresis: Recent advancements and future trends. J. Pharm Pharmacol. 2009, 61, 689–705. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Suzuki, T.; Todo, H.; Kamimura, M.; Sugibayashi, K. Iontophoresis-facilitated delivery of prednisolone through throat skin to the trachea after topical application of its succinate salt. Pharm Res. 2011, 4, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.W.; Ko, S.F.; Hui, S.W. Enhancing transdermal drug delivery with electroporation. Recent Pat. Drug Deliv. Formul. 2008, 2, 51–57. [Google Scholar] [PubMed]
- Van der Maaden, K.; Jiskoot, W.; Bouwstra, J. Microneedle technologies for (trans)dermal drug and vaccine delivery. J. Control. Release 2012, 161, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, M.; Mousoulis, C.; Ziaie, B. Polymeric microdevices for transdermal and subcutaneous drug delivery. Adv. Drug Deliv. Rev. 2012, 64, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.R.; Lee, H.S.; Choi, I.J.; Park, J.H. Considerations in the use of microneedles: pain, convenience, anxiety and safety. J. Drug Target. 2017, 1, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R. Microneedles for transdermal drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.P.; Koutsonanos, D.G.; Del Pilar Martin, M.; Lee, J.W.; Zarnitsyn, V.; Choi, S.O.; Murthy, N.; Compans, R.W.; Skountzou, I.; Prausnitz, M.R. Dissolving polymer microneedle patches for influenza vaccination. Nat. Med. 2010, 16, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Chen, J.; Qiu, Y.; Zhang, S.; Xu, B.; Gao, Y. Enhanced transcutaneous immunization via dissolving microneedle array loaded with liposome encapsulated antigen and adjuvant. Int. J. Pharm. 2013, 447, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Choi, S.O.; Felner, E.I.; Prausnitz, M.R. Dissolving microneedle patch for transdermal delivery of human growth hormone. Small 2011, 7, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Ita, K.; Simon, L. Modelling of dissolving microneedles for transdermal drug delivery: Theoretical and experimental aspects. Eur. J. Pharm Sci. 2015, 68, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Katsumi, H.; Liu, S.; Tanaka, Y.; Hitomi, K.; Hayashi, R.; Hirai, Y.; Kusamori, K.; Quan, Y.S.; Kamiyama, F.; Sakane, T.; Yamamoto, A. Development of a novel self-dissolving microneedle array of alendronate, a nitrogen-containing bisphosphonate: Evaluation of transdermal absorption, safety, and pharmacological effects after application in rats. J. Pharm Sci. 2012, 101, 3230–3238. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wu, D.; Quan, Y.S.; Kamiyama, F.; Kusamori, K.; Katsumi, H.; Sakane, T.; Yamamoto, A. Improvement of transdermal delivery of exendin-4 using novel tip-loaded microneedle arrays fabricated from hyaluronic acid. Mol. Pharm 2016, 13, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Haj-Ahmad, R.; Khan, H.; Arshad, M.S.; Rasekh, M.; Hussain, A.; Walsh, S.; Li, X.; Chang, M.W.; Ahmad, Z. Microneedle Coating Techniques for Transdermal Drug Delivery. Pharmaceutics 2015, 7, 486–502. [Google Scholar] [CrossRef] [PubMed]
- Amodwala, S.; Kumar, P.; Thakkar, H.P. Statistically optimized fast dissolving microneedle transdermal patch of meloxicam: A patient friendly approach to manage arthritis. Eur. J. Pharm Sci. 2017, 104, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Mehta, P.; Msallam, H.; Armitage, D.; Ahmad, Z. Smart microneedle coatings for controlled delivery and biomedical analysis. J. Drug Target. 2014, 22, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.A.; Renton, K.W.; Crocker, J.F.; O’Regan, P.A.; Acott, P.D. Determination of pamidronate in human whole blood and urine by reversed-phase HPLC with fluorescence detection. Biomed. Chromatogr. 2004, 18, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, K.; Tanigawara, Y.; Nakagawa, T.; Uno, T. A pharmacokinetic analysis program (MULTI) for microcomputer. J. Pharm. -Dyn. 1981, 4, 879–885. [Google Scholar] [CrossRef]
- Yamaoka, K.; Nakagawa, T.; Uno, T. Statistical moments in pharmacokinetics. J. Pharmacokinet. Biopharm. 1978, 6, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Xiang, A.; Kanematsu, M.; Mitamura, M.; Kikkawa, H.; Asano, S.; Kinoshita, M. Analysis of change patterns of microcomputed tomography 3-dimensional bone parameters as a high-throughput tool to evaluate antiosteoporotic effects of agents at an early stage of ovariectomy-induced osteoporosis in mice. Invest. Radiol. 2006, 41, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Kogan, G.; Soltés, L.; Stern, R.; Gemeiner, P. Hyaluronic acid: A natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol. Lett. 2007, 29, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jin, M.N.; Quan, Y.S.; Kamiyama, F.; Katsumi, H.; Sakane, T.; Yamamoto, A. The development and characteristics of novel microneedle arrays fabricated from hyaluronic acid, and their application in the transdermal delivery of insulin. J. Control. Release 2012, 161, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jin, M.N.; Quan, Y.S.; Kamiyama, F.; Kusamori, K.; Katsumi, H.; Sakane, T.; Yamamoto, A. Transdermal delivery of relatively high molecular weight drugs using novel self-dissolving microneedle arrays fabricated from hyaluronic acid and their characteristics and safety after application to the skin. Eur. J. Pharm Biopharm. 2014, 86, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Sandby-Møller, J.; Poulsen, T.; Wulf, H.C. Epidermal thickness at different body sites: Relationship to age, gender, pigmentation, blood content, skin type and smoking habits. Acta. Derm. Venereol. 2003, 83, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.P.; do Espírito Santo, R.F.; Line, S.R.; Pinto, M.; Santos Pde, M.; Toralles, M.B.; do Espírito Santo, A.R. Bisphosphonates: Pharmacokinetics, bioavailability, mechanisms of action, clinical applications in children, and effects on tooth development. Environ. Toxicol. Pharmacol. 2016, 42, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Suri, S.; Mönkkönen, J.; Taskinen, M.; Pesonen, J.; Blank, M.A.; Phipps, R.J.; Rogers, M.J. Nitrogen-containing bisphosphonates induce apoptosis of Caco-2 cells in vitro by inhibiting the mevalonate pathway: A model of bisphosphonate-induced gastrointestinal toxicity. Bone 2001, 29, 336–343. [Google Scholar] [CrossRef]
- Lichtenberger, L.M.; Romero, J.J.; Gibson, G.W. Effect of bisphosphonates on surface hydrophobicity and phosphatidylcholine concentration of rodent gastric mucosa. Blank MA. Dig. Dis. Sci. 2000, 45, 1792–1801. [Google Scholar] [CrossRef] [PubMed]
- Reszka, A.A.; Halasy-Nagy, J.; Rodan, G.A. Nitrogen-bisphosphonates block retinoblastoma phosphorylation and cell growth by inhibiting the cholesterol biosynthetic pathway in a keratinocyte model for esophageal irritation. Mol. Pharmacol. 2001, 59, 193–202. [Google Scholar] [PubMed]
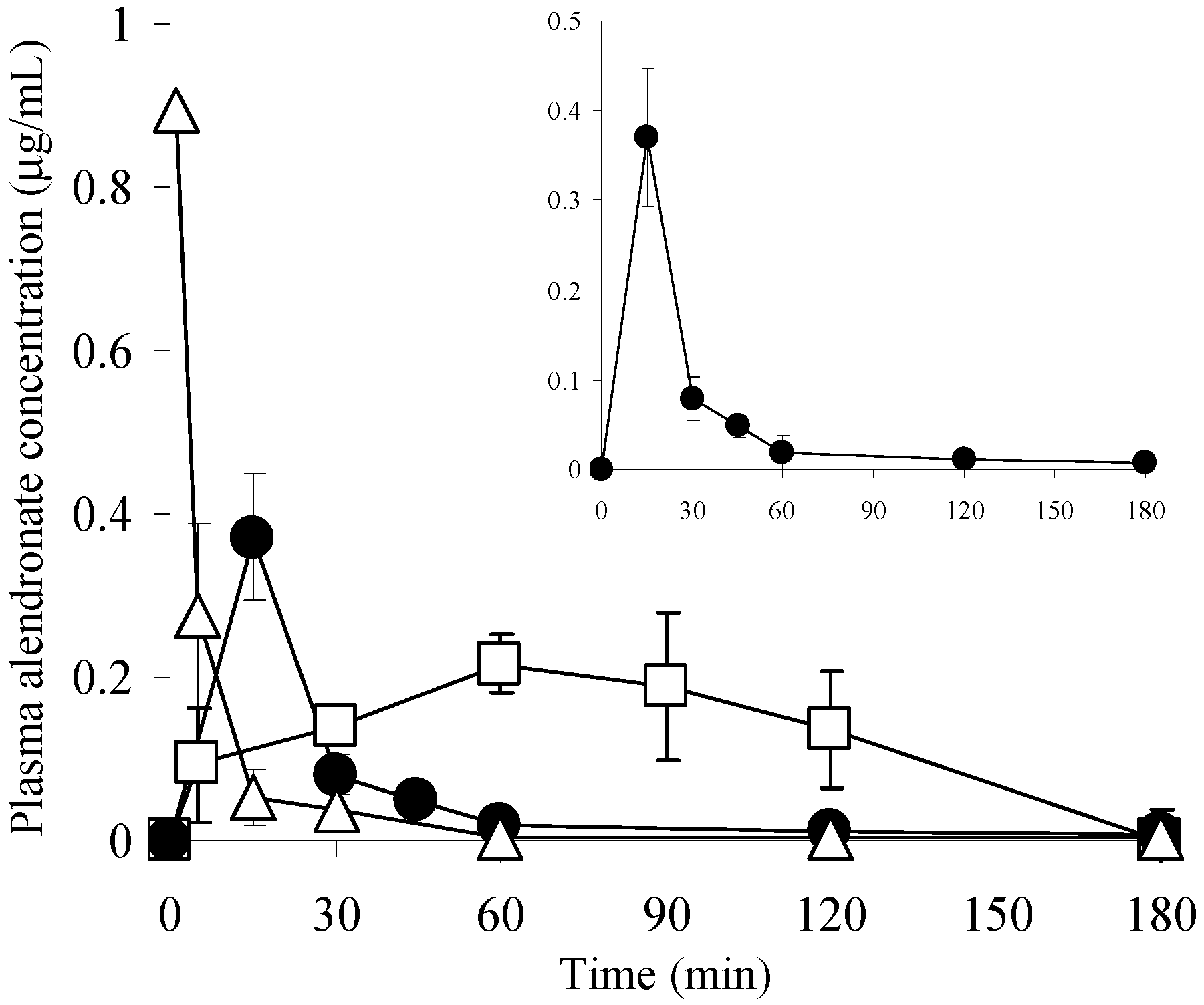

| Dose (mg/kg) | Cmax (μg/mL) | Tmax (min) | AUC (μg·min/mL) | BA (%) | |
|---|---|---|---|---|---|
| i.v. | 1 | - | - | 5.64 | - |
| p.o. | 50 | 0.22 ± 0.04 | 60 | 23.5 | 8.3 |
| ALN(TIP)–MN | 1.5 | 0.37 ± 0.08 | 15 | 8.16 | 96 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katsumi, H.; Tanaka, Y.; Hitomi, K.; Liu, S.; Quan, Y.-s.; Kamiyama, F.; Sakane, T.; Yamamoto, A. Efficient Transdermal Delivery of Alendronate, a Nitrogen-Containing Bisphosphonate, Using Tip-Loaded Self-Dissolving Microneedle Arrays for the Treatment of Osteoporosis. Pharmaceutics 2017, 9, 29. https://doi.org/10.3390/pharmaceutics9030029
Katsumi H, Tanaka Y, Hitomi K, Liu S, Quan Y-s, Kamiyama F, Sakane T, Yamamoto A. Efficient Transdermal Delivery of Alendronate, a Nitrogen-Containing Bisphosphonate, Using Tip-Loaded Self-Dissolving Microneedle Arrays for the Treatment of Osteoporosis. Pharmaceutics. 2017; 9(3):29. https://doi.org/10.3390/pharmaceutics9030029
Chicago/Turabian StyleKatsumi, Hidemasa, Yutaro Tanaka, Kaori Hitomi, Shu Liu, Ying-shu Quan, Fumio Kamiyama, Toshiyasu Sakane, and Akira Yamamoto. 2017. "Efficient Transdermal Delivery of Alendronate, a Nitrogen-Containing Bisphosphonate, Using Tip-Loaded Self-Dissolving Microneedle Arrays for the Treatment of Osteoporosis" Pharmaceutics 9, no. 3: 29. https://doi.org/10.3390/pharmaceutics9030029
APA StyleKatsumi, H., Tanaka, Y., Hitomi, K., Liu, S., Quan, Y.-s., Kamiyama, F., Sakane, T., & Yamamoto, A. (2017). Efficient Transdermal Delivery of Alendronate, a Nitrogen-Containing Bisphosphonate, Using Tip-Loaded Self-Dissolving Microneedle Arrays for the Treatment of Osteoporosis. Pharmaceutics, 9(3), 29. https://doi.org/10.3390/pharmaceutics9030029