A Systematic Literature Review of Industry 4.0 Technologies within Medical Device Manufacturing
Abstract
:1. Introduction
- RQ1. In which ways do smart technologies have the potential to revolutionize supply chain for medical device manufacturing?
- RQ1.1. What are the features and obstacles of digital medical manufacturing?
- RQ1.2. What are some of the most used digital technologies based on the academic literature?
- RQ1.3. What are the ways in which end-to-end manufacturing processes are analyzed and optimized through digital technologies?
2. Materials and Methods
2.1. Search Strategy
(Smart manufacturing OR smart industry OR supply chain OR process mining OR production OR product line OR lean manufacturing) AND (healthcare OR medical) AND (artificial intelligence OR virtual reality OR augmented reality OR digital twin)
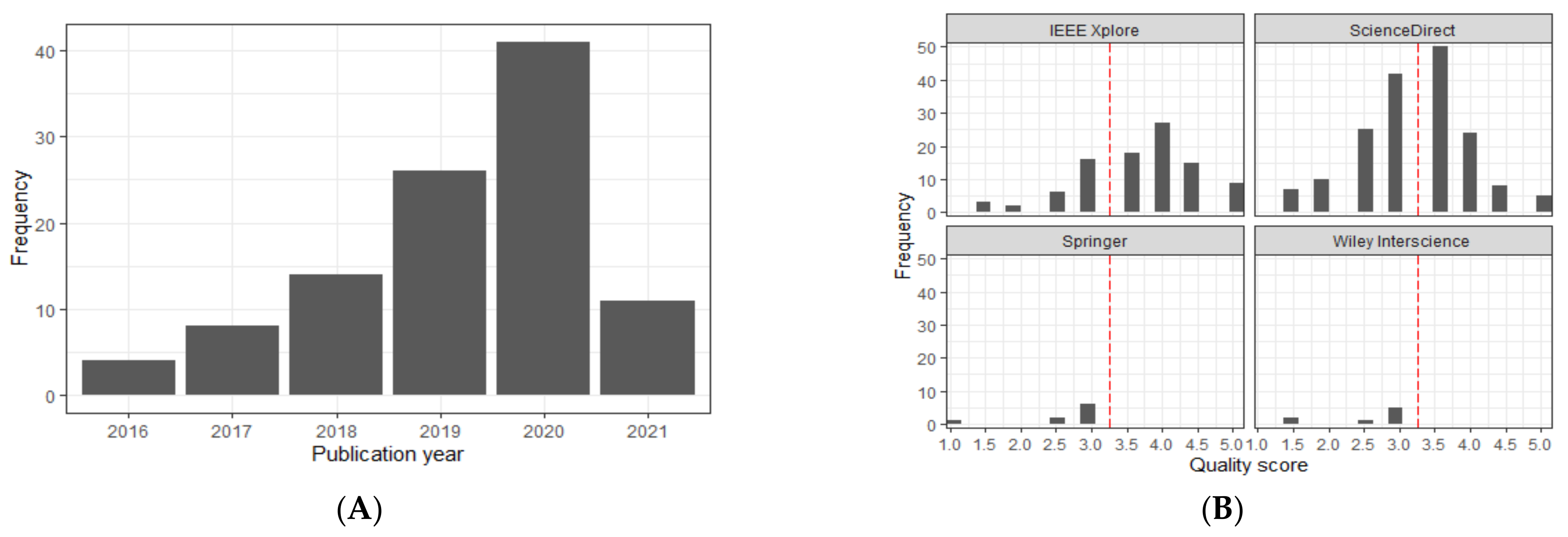
2.2. Quality Assessment
2.3. Data Synthesis
3. Results
3.1. Features
3.2. Smart Manufacturing Principles
3.3. Cyber-Physical Systems
3.4. Internet of Things
3.5. Data-Driven Decision Making
3.6. Digital Twins
3.7. Human-Robot Collaboration
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| (Berger et al., 2016) | (Fang et al., 2016) | (Reuter et al., 2016) | (Shafiq et al., 2016) |
| (Baotong Chen et al., 2017) | (Brian Chen & Chang, 2017) | (B. Li et al., 2017) | (Ren et al., 2017) |
| (Rojas et al., 2017) | (Song et al., 2017) | (Tang et al., 2017) | (F. Tao & Zhang, 2017) |
| (X. Xu & Hua, 2017) | (J. Yan et al., 2017) | (Zhong et al., 2017) | (K. Ding & Jiang, 2018) |
| (Garrido-Hidalgo et al., 2018) | (Hu et al., 2018) | (Kang & Lee, 2018) | (Küfner et al., 2018) |
| (C. Liu et al., 2018) | (Mehta et al., 2018) | (Mengoni et al., 2018) | (Müller et al., 2018) |
| (Subramaniyan et al., 2018) | (W. Tao et al., 2018) | (Bazaz et al., 2019) | (Buhl et al., 2019) |
| (C. C. Lin et al., 2019) | (Damgrave & Lutters, 2019) | (Genge et al., 2019) | (H. K. Lin et al., 2019) |
| (Hinchy et al., 2019) | (Hoppenstedt et al., 2019) | (Ikeda et al., 2019) | (J. Liu et al., 2019) |
| (Kim et al., 2019) | (Kuru & Yetgin, 2019) | (W. J. Lee et al., 2019) | (A. A. Malik & Bilberg, 2019) |
| (Mohamed et al., 2019) | (Mughal et al., 2019) | (O’Brien & Humphries, 2019) | (Pal et al., 2019) |
| (Qi & Tao, 2019) | (Ruiz Garcia et al., 2019) | (Silva et al., 2019) | (Simeone et al., 2019) |
| (X. Liu et al., 2019) | (Y. Xu et al., 2019) | (Zhang et al., 2019) | (Zhu et al., 2019) |
| (Alkhader et al., 2020) | (Borutzky, 2020) | (Brito et al., 2020) | (C. Yang et al., 2020) |
| (Baotong Chen et al., 2020) | (Costa et al., 2020) | (Q. Ding et al., 2020) | (Essien & Giannetti, 2020) |
| (Frustaci et al., 2020) | (Gotzinger et al., 2020) | (Hasan, Salah, Jayaraman, Ahmad, et al., 2020) | (Hasan, Salah, Jayaraman, Omar, et al., 2020) |
| (Hwang et al., 2020) | (Joung et al., 2020) | (Kaynak et al., 2020) | (Khayyam et al., 2020) |
| (Kiangala & Wang, 2020) | (Latif & Starly, 2020) | (C. K. M. Lee et al., 2020) | (Lenz et al., 2020) |
| (M. Li et al., 2020) | (Lou et al., 2020) | (S. Malik & Kim, 2020) | (Matsuda et al., 2020) |
| (Mondal et al., 2020) | (Moyne et al., 2020) | (Nagorny et al., 2020) | (O’Sullivan et al., 2020) |
| (Ou et al., 2020) | (Papananias et al., 2020) | (Parto et al., 2020) | (Sarivan et al., 2020) |
| (W. Tao et al., 2020) | (Q. Wang & Yang, 2020) | (X. V. Wang et al., 2020) | (Y. Wang et al., 2020) |
| (Y. Yang et al., 2020) | (H. Yan et al., 2020) | (Zawadzki et al., 2020) | (L. Zhou et al., 2020) |
| (Zhu & Xu, 2020) | (Fathy et al., 2021) | (T. Zhou et al., 2021) | (J. Wang et al., 2021) |
| (Harrison et al., 2021) | (Aljanabi & Chalechale, 2021) | (Ji et al., 2021) | (Goldman et al., 2021) |
| (Assad, Konstantinov, Rushforth, et al., 2021) | (Zellinger et al., 2021) | (Assad, Konstantinov, Nureldin, et al., 2021) | (Friedl et al., 2021) |
| Term | Description |
|---|---|
| CS | Computer Science |
| IoT | Internet of Things |
| AI | Artificial Intelligence |
| CPS | Cyber-Physical Systems |
| SLR | Systematic Literature Review |
| HRC | Human-Robot Collaboration |
| AR | Augmented Reality |
| VR | Virtual Reality |
| XR | Mixed Reality |
| HMI | Human-Machine Interference |
| HRC | Human-Robot Collaboration |
| RFID | Radio-Frequency Identification |
| DNN | Deep Neural Network |
| MES | Manufacturing Execution System |
| IT | Information Technology |
| OT | Operational Technology |
| OPC UA | OPC Unified Architecture |
| VLC | Visible Light Communication |
| PCA | Principal Component Analysis |
| ML | Machine Learning |
| HMM | Hidden Markov Model |
| ANN | Artificial Neural Network |
| SSD | Single Shot Multibox |
| PLC | Programmable Logic Controller |
| DL | Deep Learning |
| CNC | Computer Numerical Control |
| R&D | Research & Design |
References
- FDA. Overview of Device Regulation. 2021. Available online: https://www.fda.gov/medical-devices/device-advice-comprehensive-regulatory-assistance/overview-device-regulation (accessed on 23 August 2021).
- FDA. Medical Device Overview. 2021. Available online: https://www.fda.gov/industry/regulated-products/medical-device-overview#What%20is%20a%20medical%20device (accessed on 23 August 2021).
- EMA. Medical Devices. 2021. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/medical-devices (accessed on 23 August 2021).
- PMDA. Regulatory Information. Pharmaceuticals and Medical Devices Agency. 2021. Available online: https://www.pmda.go.jp/english/review-services/regulatory-info/0002.html (accessed on 23 August 2021).
- Peter, L.; Hajek, L.; Maresova, P.; Augustynek, M.; Penhaker, M. Medical devices: Regulation, risk classification, and open innovation. J. Open Innov. Technol. Mark. Complex. 2020, 6, 42. [Google Scholar] [CrossRef]
- Citron, P. Ethics considerations for medical device R&D. Prog. Cardiovasc. Dis. 2012, 55, 307–315. [Google Scholar] [CrossRef]
- Iizuka, M.; Ikeda, Y. Regulation and innovation under the 4th industrial revolution: The case of a healthcare robot, HAL by Cyberdyne. Technovation 2021, 108, 102335. [Google Scholar] [CrossRef]
- Gotzinger, M.; Juhasz, D.; Taherinejad, N.; Willegger, E.; Tutzer, B.; Liljeberg, P.; Jantsch, A.; Rahmani, A.M. RoSA: A Framework for modeling self-awareness in cyber-physical systems. IEEE Access 2020, 8, 141373–141394. [Google Scholar] [CrossRef]
- Bongomin, O.; Yemane, A.; Kembabazi, B.; Malanda, C.; Mwape, M.C.; Mpofu, N.S.; Tigalana, D. Industry 4.0 disruption and its neologisms in major industrial sectors: A state of the art. J. Eng. 2020, 2020, 8090521. [Google Scholar] [CrossRef]
- IBM. What is Industry 4.0? Available online: https://www.ibm.com/nl-en/topics/industry-4-0 (accessed on 28 September 2021).
- Gartner. Getting Ready For Industrie 4 0. Available online: https://www.gartner.com/smarterwithgartner/getting-ready-for-industrie-4-0 (accessed on 28 September 2021).
- Wang, Y.; Ma, H.-S.; Yang, J.-H.; Wang, K.-S. Industry 4.0: A way from mass customization to mass personalization production. Adv. Manuf. 2017, 5, 311–320. [Google Scholar] [CrossRef]
- Statista. Global IoT and non-IoT Connections 2010-2025. Available online: https://www.statista.com/statistics/1101442/iot-number-of-connected-devices-worldwide/ (accessed on 28 September 2021).
- Majumdar, A.K. Free-space optical communications. In Optical Wireless Communications for Broadband Global Internet Connectivity; Elsevier: Amsterdam, The Netherlands, 2019; Volume 10, pp. 245–258. [Google Scholar] [CrossRef]
- Parto, M.; Saldana, C.; Kurfess, T. A novel three-layer iot architecture for shared, private, scalable, and real-time machine learning from ubiquitous cyber-physical systems. Procedia Manuf. 2020, 48, 959–967. [Google Scholar] [CrossRef]
- Qi, Q.; Tao, F. A smart manufacturing service system based on edge computing, fog computing, and cloud computing. IEEE Access 2019, 7, 86769–86777. [Google Scholar] [CrossRef]
- Yang, C.; Lan, S.; Wang, L.; Shen, W.; Huang, G.G.Q. Big data driven edge-cloud collaboration architecture for cloud manufacturing: A software defined perspective. IEEE Access 2020, 8, 45938–45950. [Google Scholar] [CrossRef]
- Tao, W.; Amin, A.-; Chen, H.; Leu, M.C.; Yin, Z.; Qin, R. Real-time assembly operation recognition with fog computing and transfer learning for human-centered intelligent manufacturing. Procedia Manuf. 2020, 48, 926–931. [Google Scholar] [CrossRef]
- Sedigh, S.; Hurson, A. Introduction and preface. Adv. Comput. 2012, 87, 1–6. [Google Scholar] [CrossRef]
- Chen, B.; Chang, J.-Y.J. Dynamic analysis of intelligent coil leveling machine for cyber-physical systems implementation. Procedia CIRP 2017, 63, 390–395. [Google Scholar] [CrossRef]
- Bazaz, S.M.; Lohtander, M.; Varis, J. 5-dimensional definition for a manufacturing digital twin. Procedia Manuf. 2019, 38, 1705–1712. [Google Scholar] [CrossRef]
- Demir, K.A.; Cicibas, H. Industry 5.0 and a critique of Industry 4.0. In Proceedings of the 4th International Management Information Systems Conference, Istanbul, Turkey, 17–20 October 2017. [Google Scholar]
- Haleem, A.; Javaid, M. Industry 5.0 and its expected applications in medical field. Curr. Med. Res. Pr. 2019, 9, 167–169. [Google Scholar] [CrossRef]
- Kamble, S.S.; Gunasekaran, A.; Gawankar, S.A. Sustainable industry 4.0 framework: A systematic literature review identifying the current trends and future perspectives. Process. Saf. Environ. Prot. 2018, 117, 408–425. [Google Scholar] [CrossRef]
- Osterrieder, P.; Budde, L.; Friedli, T. The smart factory as a key construct of industry 4.0: A systematic literature review. Int. J. Prod. Econ. 2020, 221, 107476. [Google Scholar] [CrossRef]
- Da Silva, F.S.T.; da Costa, C.A.; Crovato, C.D.P.; Righi, R.D.R. Looking at energy through the lens of Industry 4.0: A systematic literature review of concerns and challenges. Comput. Ind. Eng. 2020, 143, 106426. [Google Scholar] [CrossRef]
- Zonta, T.; da Costa, C.A.; Righi, R.D.R.; de Lima, M.J.; da Trindade, E.S.; Li, G.P. Predictive maintenance in the Industry 4.0: A systematic literature review. Comput. Ind. Eng. 2020, 150, 106889. [Google Scholar] [CrossRef]
- Bueno, A.F.; Filho, M.G.; Frank, A.G. Smart production planning and control in the Industry 4.0 context: A systematic literature review. Comput. Ind. Eng. 2020, 149, 106774. [Google Scholar] [CrossRef]
- Rosa, P.; Sassanelli, C.; Urbinati, A.; Chiaroni, D.; Terzi, S. Assessing relations between Circular Economy and Industry 4.0: A systematic literature review. Int. J. Prod. Res. 2019, 58, 1662–1687. [Google Scholar] [CrossRef] [Green Version]
- Birkel, H.; Müller, J.M. Potentials of industry 4.0 for supply chain management within the triple bottom line of sustainability—A systematic literature review. J. Clean. Prod. 2021, 289, 125612. [Google Scholar] [CrossRef]
- Piccarozzi, M.; Aquilani, B.; Gatti, C. Industry 4.0 in management studies: A systematic literature review. Sustainability 2018, 10, 3821. [Google Scholar] [CrossRef] [Green Version]
- Silvestri, L.; Forcina, A.; Introna, V.; Santolamazza, A.; Cesarotti, V. Maintenance transformation through Industry 4.0 technologies: A systematic literature review. Comput. Ind. 2020, 123, 103335. [Google Scholar] [CrossRef]
- Bodkhe, U.; Tanwar, S.; Parekh, K.; Khanpara, P.; Tyagi, S.; Kumar, N.; Alazab, M. Blockchain for Industry 4.0: A comprehensive review. IEEE Access 2020, 8, 79764–79800. [Google Scholar] [CrossRef]
- Ding, B. Pharma Industry 4.0: Literature review and research opportunities in sustainable pharmaceutical supply chains. Process. Saf. Environ. Prot. 2018, 119, 115–130. [Google Scholar] [CrossRef]
- R: The R Project for Statistical Computing; The R Foundation: Vienna, Austria, 2021.
- Peres, R.S.; Jia, X.; Lee, J.; Sun, K.; Colombo, A.W.; Barata, J. Industrial artificial intelligence in industry 4.0—Systematic review, challenges and outlook. IEEE Access 2020, 8, 220121–220139. [Google Scholar] [CrossRef]
- Kitchenham, B. Procedures for Performing Systematic Reviews; Keele University Technical Report; Keele University: Newcastle, UK, 2004; pp. 1–26. [Google Scholar]
- WUR. WUR Journal Browser. 2021. Available online: https://library.wur.nl/WebQuery/wurbrowser?q=* (accessed on 25 August 2021).
- Mondal, S.; Ruan, L.; Maier, M.; Larrabeiti, D.; Das, G.; Wong, E. Enabling remote human-to-machine applications with AI-enhanced servers over access networks. IEEE Open J. Commun. Soc. 2020, 1, 889–899. [Google Scholar] [CrossRef]
- Zhu, Z.; Xu, X. User-centered information provision of cyber-physical machine tools. Procedia CIRP 2020, 93, 1546–1551. [Google Scholar] [CrossRef]
- Malik, A.A.; Bilberg, A. Human centered Lean automation in assembly. Procedia CIRP 2019, 81, 659–664. [Google Scholar] [CrossRef]
- Assad, F.; Konstantinov, S.; Rushforth, E.J.; Vera, D.A.; Harrison, R. Virtual engineering in the support of sustainable assembly systems. Procedia CIRP 2021, 97, 367–372. [Google Scholar] [CrossRef]
- Damgrave, R.; Lutters, E. Smart industry testbed. Procedia CIRP 2019, 84, 387–392. [Google Scholar] [CrossRef]
- Harrison, R.; Vera, D.A.; Ahmad, B. A connective framework to support the lifecycle of cyber-physical production systems. Proc. IEEE 2021, PP, 1–14. [Google Scholar] [CrossRef]
- Joung, B.G.; Lee, W.J.; Huang, A.; Sutherland, J.W. Development and application of a method for real time motor fault detection. Procedia Manuf. 2020, 49, 94–98. [Google Scholar] [CrossRef]
- Borutzky, W. A Hybrid bond graph model-based—Data driven method for failure prognostic. Procedia Manuf. 2020, 42, 188–196. [Google Scholar] [CrossRef]
- Papananias, M.; McLeay, T.E.; Obajemu, O.; Mahfouf, M.; Kadirkamanathan, V. Inspection by exception: A new machine learning-based approach for multistage manufacturing. Appl. Soft Comput. 2020, 97, 106787. [Google Scholar] [CrossRef]
- Kiangala, K.S.; Wang, Z. An effective predictive maintenance framework for conveyor motors using dual time-series imaging and convolutional neural network in an industry 4.0 environment. IEEE Access 2020, 8, 121033–121049. [Google Scholar] [CrossRef]
- Assad, F.; Konstantinov, S.; Nureldin, H.; Waseem, M.; Rushforth, E.; Ahmad, B.; Harrison, R. Maintenance and digital health control in smart manufacturing based on condition monitoring. Procedia CIRP 2021, 97, 142–147. [Google Scholar] [CrossRef]
- Lin, C.-C.; Deng, D.-J.; Kuo, C.-H.; Chen, L. Concept drift detection and adaption in big imbalance industrial iot data using an ensemble learning method of offline classifiers. IEEE Access 2019, 7, 56198–56207. [Google Scholar] [CrossRef]
- Hinchy, E.; O’Dowd, N.; McCarthy, C. Using open-source microcontrollers to enable digital twin communication for smart manufacturing. Procedia Manuf. 2019, 38, 1213–1219. [Google Scholar] [CrossRef]
- Ding, Q.; Gao, S.; Zhu, J.; Yuan, C. Permissioned blockchain-based double-layer framework for product traceability system. IEEE Access 2019, 8, 6209–6225. [Google Scholar] [CrossRef]
- Lee, C.K.M.; Huo, Y.Z.; Zhang, S.Z.; Ng, K.K.H. Design of a smart manufacturing system with the application of multi-access edge computing and blockchain technology. IEEE Access 2020, 8, 28659–28667. [Google Scholar] [CrossRef]
- Zawadzki, P.; Zywicki, K.; Bun, P.; Gorski, F. Employee training in an intelligent factory using virtual reality. IEEE Access 2020, 8, 135110–135117. [Google Scholar] [CrossRef]
- Shafiq, S.I.; Velez, G.; Toro, C.; Sanin, C.; Szczerbicki, E. Designing intelligent factory: Conceptual framework and empirical validation. Procedia Comput. Sci. 2016, 96, 1801–1808. [Google Scholar] [CrossRef] [Green Version]
- Garrido-Hidalgo, C. Hortelano, D.; Roda-Sanchez, L.; Olivares, T.; Ruiz, M.C.; Lopez, V. IoT heterogeneous mesh network deployment for human-in-the-loop challenges towards a social and sustainable Industry 4. IEEE Access 2018, 6, 28417–28437. [Google Scholar] [CrossRef]
- Gil-Vilda, F.; Sune, A.; Fabra, J.A.Y.; Crespo, C.; Serrano, H. Integration of a collaborative robot in a U-shaped production line: A real case study. Procedia Manuf. 2017, 13, 109–115. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Xu, W.; Liu, J.; Liu, Z.; Zhou, Z.; Pham, D.T. A Reconfigurable modeling approach for digital twin-based manufacturing system. Procedia CIRP 2019, 83, 118–125. [Google Scholar] [CrossRef]
- Wuest, T.; Weimer, D.; Irgens, C.; Thoben, K.-D. Machine learning in manufacturing: Advantages, challenges, and applications. Prod. Manuf. Res. 2016, 4, 23–45. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Zhou, H.; Liu, X.; Tian, G.; Wu, M.; Cao, L.; Wang, W. Dynamic evaluation method of machining process planning based on digital twin. IEEE Access 2019, 7, 19312–19323. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Liu, A. Digital twin-driven supply chain planning. Procedia CIRP 2020, 93, 198–203. [Google Scholar] [CrossRef]
- Zhong, R.Y.; Xu, X.; Wang, L. IoT-enabled smart factory visibility and traceability using laser-scanners. Procedia Manuf. 2017, 10, 1–14. [Google Scholar] [CrossRef]
- Hu, L.; Nguyen, N.-T.; Tao, W.; Leu, M.C.; Liu, X.F.; Shahriar, R.; Al Sunny, S.M.N. Modeling of cloud-based digital twins for smart manufacturing with MT connect. Procedia Manuf. 2018, 26, 1193–1203. [Google Scholar] [CrossRef]
- Tang, H.; Li, D.; Wang, S.; Dong, Z. CASOA: An architecture for agent-based manufacturing system in the context of industry 4. IEEE Access 2017, 6, 12746–12754. [Google Scholar] [CrossRef]
- Kuru, K.; Yetgin, H. Transformation to advanced mechatronics systems within new industrial revolution: A novel framework in automation of everything (AoE). IEEE Access 2019, 7, 41395–41415. [Google Scholar] [CrossRef]
- Hasan, H.R.; Salah, K.; Jayaraman, R.; Ahmad, R.W.; Yaqoob, I.; Omar, M. Blockchain-based solution for the traceability of spare parts in manufacturing. IEEE Access 2020, 8, 100308–100322. [Google Scholar] [CrossRef]
- Ding, K.; Jiang, P. RFID-based production data analysis in an IoT-enabled smart job-shop. IEEE/CAA J. Autom. Sin. 2018, 5, 128–138. [Google Scholar] [CrossRef]
- Chen, B.; Wan, J.; Shu, L.; Li, P.; Mukherjee, M.; Yin, B. Smart factory of industry 4.0: Key technologies, application case, and challenges. IEEE Access 2018, 6, 6505–6519. [Google Scholar] [CrossRef]
- Aljanabi, S.; Chalechale, A. Improving IoT services using a hybrid fog-cloud offloading. IEEE Access 2021, 9, 13775–13788. [Google Scholar] [CrossRef]
- Kaynak, B.; Kaynak, S.; Uygun, O. Cloud manufacturing architecture based on public blockchain technology. IEEE Access 2019, 8, 2163–2177. [Google Scholar] [CrossRef]
- Lou, P.; Chen, Y.; Yan, J. Memetic algorithm with local neighborhood search for bottleneck supplier identification in supply networks. IEEE Access 2020, 8, 148827–148840. [Google Scholar] [CrossRef]
- Subramaniyan, M.; Skoogh, A.; Salomonsson, H.; Bangalore, P.; Bokrantz, J. A data-driven algorithm to predict throughput bottlenecks in a production system based on active periods of the machines. Comput. Ind. Eng. 2018, 125, 533–544. [Google Scholar] [CrossRef]
- Lenz, J.; Pelosi, V.; Taisch, M.; MacDonald, E.; Wuest, T. Data-driven context awareness of smart products in discrete smart manufacturing systems. Procedia Manuf. 2020, 52, 38–43. [Google Scholar] [CrossRef]
- Lee, W.J.; Mendis, G.P.; Sutherland, J.W. Development of an intelligent tool condition monitoring system to identify manufacturing tradeoffs and optimal machining conditions. Procedia Manuf. 2019, 33, 256–263. [Google Scholar] [CrossRef]
- Mohamed, N.; Al-Jaroodi, J.; Lazarova-Molnar, S. Leveraging the capabilities of industry 4.0 for improving energy efficiency in smart factories. IEEE Access 2019, 7, 18008–18020. [Google Scholar] [CrossRef]
- Li, M.; Jiang, M.; Lyu, Z.; Chen, Q.; Wu, H.; Huang, G.Q. Spatial-temporal finite element analytics for cyber-physical system-enabled smart factory: Application in hybrid flow shop. Procedia Manuf. 2020, 51, 1229–1236. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, L.; Horn, B.K. Deep reinforcement learning-based dynamic scheduling in smart manufacturing. Procedia CIRP 2020, 93, 383–388. [Google Scholar] [CrossRef]
- Liu, C.; Xu, X.; Peng, Q.; Zhou, Z. MTConnect-based cyber-physical machine tool: A case study. Procedia CIRP 2018, 72, 492–497. [Google Scholar] [CrossRef]
- Müller, R.; Vette-Steinkamp, M.; Hörauf, L.; Speicher, C.; Burkhard, D. Development of an intelligent material shuttle to digitize and connect production areas with the production process planning department. Procedia CIRP 2018, 72, 967–972. [Google Scholar] [CrossRef]
- Rojas, R.A.; Rauch, E.; Vidoni, R.; Matt, D.T. Enabling connectivity of cyber-physical production systems: A conceptual framework. Procedia Manuf. 2017, 11, 822–829. [Google Scholar] [CrossRef]
- Xu, X.; Hua, Q. Industrial big data analysis in smart factory: Current status and research strategies. IEEE Access 2017, 5, 17543–17551. [Google Scholar] [CrossRef]
- Liu, X.; Wei, X.; Guo, L.; Liu, Y.; Song, Q.; Jamalipour, A. Turning the signal interference into benefits: Towards indoor self-powered visible light communication for IoT Devices in industrial radio-hostile environments. IEEE Access 2019, 7, 24978–24989. [Google Scholar] [CrossRef]
- Genge, B.; Haller, P.; Enăchescu, C. Anomaly detection in aging industrial internet of things. IEEE Access 2019, 7, 74217–74230. [Google Scholar] [CrossRef]
- Hwang, J.; Aziz, A.; Sung, N.; Ahmad, A.; Le Gall, F.; Song, J. AUTOCON-IoT: Automated and scalable online conformance testing for IoT applications. IEEE Access 2020, 8, 43111–43121. [Google Scholar] [CrossRef]
- AlKhader, W.; Alkaabi, N.; Salah, K.; Jayaraman, R.; Arshad, J.; Omar, M. Blockchain-based traceability and management for additive manufacturing. IEEE Access 2020, 8, 188363–188377. [Google Scholar] [CrossRef]
- Li, B.; Zhao, Z.; Guan, Y.; Ai, N.; Dong, X.; Wu, B. Task placement across multiple public clouds with deadline constraints for smart factory. IEEE Access 2017, 6, 1560–1564. [Google Scholar] [CrossRef]
- Essien, A.E.; Giannetti, C. A deep learning model for smart manufacturing using convolutional LSTM neural network autoencoders. IEEE Trans. Ind. Informatics 2020, 16, 6069–6078. [Google Scholar] [CrossRef] [Green Version]
- Goldman, C.V.; Baltaxe, M.; Chakraborty, D.; Arinez, J. Explaining learning models in manufacturing processes. Procedia Comput. Sci. 2021, 180, 259–268. [Google Scholar] [CrossRef]
- Yan, H.; Yang, J.; Wan, J. KnowIME: A system to construct a knowledge graph for intelligent manufacturing equipment. IEEE Access 2020, 8, 41805–41813. [Google Scholar] [CrossRef]
- Chen, B.; Liu, Y.; Zhang, C.; Wang, Z. Time series data for equipment reliability analysis with deep learning. IEEE Access 2020, 8, 105484–105493. [Google Scholar] [CrossRef]
- Wang, J.; Wang, D.; Wang, S.; Li, W.; Song, K. Fault diagnosis of bearings based on multi-sensor information fusion and 2D convolutional neural network. IEEE Access 2021, 9, 23717–23725. [Google Scholar] [CrossRef]
- Frustaci, F.; Perri, S.; Cocorullo, G.; Corsonello, P. An embedded machine vision system for an in-line quality check of assembly processes. Procedia Manuf. 2020, 42, 211–218. [Google Scholar] [CrossRef]
- Küfner, T.; Uhlemann, T.H.-J.; Ziegler, B. Lean data in manufacturing systems: Using artificial intelligence for decentralized data reduction and information extraction. Procedia CIRP 2018, 72, 219–224. [Google Scholar] [CrossRef]
- O’Brien, K.; Humphries, J. Object detection using convolutional neural networks for smart manufacturing vision systems in the medical devices sector. Procedia Manuf. 2019, 38, 142–147. [Google Scholar] [CrossRef]
- Sarivan, I.-M.; Greiner, J.N.; Álvarez, D.D.; Euteneuer, F.; Reichenbach, M.; Madsen, O.; Bøgh, S. Enabling real-time quality inspection in smart manufacturing through wearable smart devices and deep learning. Procedia Manuf. 2020, 51, 373–380. [Google Scholar] [CrossRef]
- Pal, D.; Vanijja, V.; Arpnikanondt, C.; Zhang, X.; Papasratorn, B. A quantitative approach for evaluating the quality of experience of smart-wearables from the quality of data and quality of information: An end user perspective. IEEE Access 2019, 7, 64266–64278. [Google Scholar] [CrossRef]
- Hoppenstedt, B.; Probst, T.; Reichert, M.; Schlee, W.; Kammerer, K.; Spiliopoulou, M.; Schobel, J.; Winter, M.; Felnhofer, A.; Kothgassner, O.D.; et al. Applicability of immersive analytics in mixed reality: Usability study. IEEE Access 2019, 7, 71921–71932. [Google Scholar] [CrossRef]
- Tao, W.J.; Lai, Z.H.; Leu, M.C.; Yin, Z.Z. Worker Activity Recognition in Smart Manufacturing Using IMU and sEMG Signals with Convolutional Neural Networks. In Proceedings of the 46th Sme North American Manufacturing Research Conference, Namrc 46, College Station, TX, USA, 18–22 June 2018; Volume 26, pp. 1159–1166. [Google Scholar]
- Zellinger, W.; Wieser, V.; Kumar, M.; Brunner, D.; Shepeleva, N.; Gálvez, R.; Langer, J.; Fischer, L.; Moser, B. Beyond federated learning: On confidentiality-critical machine learning applications in industry. Procedia Comput. Sci. 2021, 180, 734–743. [Google Scholar] [CrossRef]
- Mengoni, M.; Ceccacci, S.; Generosi, A.; Leopardi, A. Spatial augmented reality: An application for human work in smart manufacturing environment. Procedia Manuf. 2018, 17, 476–483. [Google Scholar] [CrossRef]
- Tao, F.; Zhang, M. Digital twin shop-floor: A new shop-floor paradigm towards smart manufacturing. IEEE Access 2017, 5, 20418–20427. [Google Scholar] [CrossRef]
- Latif, H.; Starly, B. A simulation algorithm of a digital twin for manual assembly process. Procedia Manuf. 2020, 48, 932–939. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, Y.; Liu, X.; Zheng, Y. A digital-twin-assisted fault diagnosis using deep transfer learning. IEEE Access 2019, 7, 19990–19999. [Google Scholar] [CrossRef]
- O’Sullivan, J.; O’Sullivan, D.; Bruton, K. A case-study in the introduction of a digital twin in a large-scale smart manufacturing facility. Procedia Manuf. 2020, 51, 1523–1530. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, C.; Xu, X. Visualisation of the digital twin data in manufacturing by using augmented reality. Procedia CIRP 2019, 81, 898–903. [Google Scholar] [CrossRef]
- Hasan, H.R.; Salah, K.; Jayaraman, R.; Omar, M.; Yaqoob, I.; Pesic, S.; Taylor, T.; Boscovic, D. A blockchain-based approach for the creation of digital twins. IEEE Access 2020, 8, 34113–34126. [Google Scholar] [CrossRef]
- Garcia, M.A.R.; Rojas, R.; Gualtieri, L.; Rauch, E.; Matt, D. A human-in-the-loop cyber-physical system for collaborative assembly in smart manufacturing. Procedia CIRP 2019, 81, 600–605. [Google Scholar] [CrossRef]
- Ji, S.; Lee, S.; Yoo, S.; Suh, I.; Kwon, I.; Park, F.C.; Lee, S.; Kim, H. Learning-based automation of robotic assembly for smart manufacturing. Proc. IEEE 2021, 109, 423–440. [Google Scholar] [CrossRef]
- Ikeda, M.; Ganglbauer, M.; Ashok, P.; Maddukuri, S.; Hofmann, M.; Pichler, A. Instrumented tool based robot programming—Parameterization of screwing process macros. Procedia Manuf. 2019, 38, 415–422. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.; Chen, Y.; Li, Y.; Ren, T.; Ren, Y. Design and automatic fabrication of novel bio-inspired soft smart robotic hands. IEEE Access 2020, 8, 155912–155925. [Google Scholar] [CrossRef]
- Brito, T.; Queiroz, J.; Piardi, L.; Fernandes, L.A.; Lima, J.; Leitão, P. A machine learning approach for collaborative robot smart manufacturing inspection for quality control systems. Procedia Manuf. 2020, 51, 11–18. [Google Scholar] [CrossRef]
- Kim, B.S.; Jin, Y.; Nam, S. An integrative user-level customized modeling and simulation environment for smart manufacturing. IEEE Access 2019, 7, 186637–186645. [Google Scholar] [CrossRef]
- Syberfeldt, A.; Danielsson, O.; Gustavsson, P. Augmented reality smart glasses in the smart factory: Product evaluation guidelines and review of available products. IEEE Access 2017, 5, 9118–9130. [Google Scholar] [CrossRef]
- Berger, C.; Hees, A.; Braunreuther, S.; Reinhart, G. Characterization of cyber-physical sensor systems. Procedia CIRP 2016, 41, 638–643. [Google Scholar] [CrossRef]
- Fang, F.Z.; Li, Z.; Arokiam, A.; Gorman, T. Closed loop pmi driven dimensional quality lifecycle management approach for smart manufacturing system. Procedia CIRP 2016, 56, 614–619. [Google Scholar] [CrossRef] [Green Version]
- Reuter, C.; Brambring, F.; Weirich, J.; Kleines, A. Improving data consistency in production control by adaptation of data mining algorithms. Procedia CIRP 2016, 56, 545–550. [Google Scholar] [CrossRef] [Green Version]
- Ren, G.; Hua, Q.; Deng, P.; Yang, C.; Zhang, J. A multi-perspective method for analysis of cooperative behaviors among industrial devices of smart factory. IEEE Access 2017, 5, 10882–10891. [Google Scholar] [CrossRef]
- Song, Z.; Sun, Y.; Yan, H.; Wu, D.; Niu, P.; Wu, X. Robustness of smart manufacturing information systems under conditions of resource failure: A complex network perspective. IEEE Access 2017, 6, 3731–3738. [Google Scholar] [CrossRef]
- Yan, J.; Meng, Y.; Lu, L.; Li, L. Industrial big data in an Industry 4.0 environment: Challenges, schemes, and applications for predictive maintenance. IEEE Access 2017, 5, 23484–23491. [Google Scholar] [CrossRef]
- Kang, H.S.; Lee, J.Y. A real-time cyber modeling approach in MTConnect-based cyber-physical production environment. Procedia CIRP 2018, 72, 462–467. [Google Scholar] [CrossRef]
- Mehta, P.; Butkewitsch-Choze, S.; Seaman, C. Smart manufacturing analytics application for semi-continuous manufacturing process—A use case. Procedia Manuf. 2018, 26, 1041–1052. [Google Scholar] [CrossRef]
- Buhl, J.F.; Grønhøj, R.; Jørgensen, J.K.; Mateus, G.; Pinto, D.; Sørensen, J.K.; Bøgh, S.; Chrysostomou, D. A dual-arm collaborative robot system for the smart factories of the future. Procedia Manuf. 2019, 38, 333–340. [Google Scholar] [CrossRef]
- Lin, H.-K.; Hsieh, C.-H.; Wei, N.-C.; Peng, Y.-C. Association rules mining in R for product performance management in industry 4. Procedia CIRP 2019, 83, 699–704. [Google Scholar] [CrossRef]
- Mughal, M.A.; Shi, P.; Ullah, A.; Mahmood, K.; Abid, M.; Luo, X. Logical tree based secure rekeying management for smart devices groups in IoT enabled WSN. IEEE Access 2019, 7, 76699–76711. [Google Scholar] [CrossRef]
- Silva, J.D.C.; Rodrigues, J.J.P.C.; Saleem, K.; Kozlov, S.A.; Rabelo, R. M4DN.IoT-A networks and devices management platform for internet of things. IEEE Access 2019, 7, 53305–53313. [Google Scholar] [CrossRef]
- Simeone, A.; Caggiano, A.; Boun, L.; Deng, B. Intelligent cloud manufacturing platform for efficient resource sharing in smart manufacturing networks. Procedia CIRP 2019, 79, 233–238. [Google Scholar] [CrossRef]
- Costa, S.; Silva, F.; Campilho, R.; Pereira, T. Guidelines for machine tool sensing and smart manufacturing integration. Procedia Manuf. 2020, 51, 251–257. [Google Scholar] [CrossRef]
- Khayyam, H.; Jamali, A.; Bab-Hadiashar, A.; Esch, T.; Ramakrishna, S.; Jalili, M.; Naebe, M. A novel hybrid machine learning algorithm for limited and big data modeling with application in industry 4. IEEE Access 2020, 8, 111381–111393. [Google Scholar] [CrossRef]
- Malik, S.; Kim, D. A hybrid scheduling mechanism based on agent cooperation mechanism and fair emergency first in smart factory. IEEE Access 2020, 8, 227064–227075. [Google Scholar] [CrossRef]
- Matsuda, M.; Nishi, T.; Hasegawa, M.; Terunuma, T. Construction of a virtual supply chain using enterprise e-catalogues. Procedia CIRP 2020, 93, 688–693. [Google Scholar] [CrossRef]
- Moyne, J.; Qamsane, Y.; Balta, E.C.; Kovalenko, I.; Faris, J.; Barton, K.; Tilbury, D.M. A requirements driven digital twin framework: Specification and opportunities. IEEE Access 2020, 8, 107781–107801. [Google Scholar] [CrossRef]
- Nagorny, K.; Scholze, S.; Colombo, A.W.; Oliveira, J.B. A DIN Spec 91345 RAMI 4.0 compliant data pipelining model: An approach to support data understanding and data acquisition in smart manufacturing environments. IEEE Access 2020, 8, 223114–223129. [Google Scholar] [CrossRef]
- Ou, X.; Huang, J.; Chang, Q.; Hucker, S.; Lovasz, J.G. First time quality diagnostics and improvement through data analysis: A study of a crankshaft line. Procedia Manuf. 2020, 49, 2–8. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, H. Sensor-based recurrence analysis of energy efficiency in machining processes. IEEE Access 2020, 8, 18326–18336. [Google Scholar] [CrossRef]
- Wang, X.V.; Zhang, X.; Yang, Y.; Wang, L. A human-robot collaboration system towards high accuracy. Procedia CIRP 2020, 93, 1085–1090. [Google Scholar] [CrossRef]
- Fathy, Y.; Jaber, M.; Brintrup, A. Learning with imbalanced data in smart manufacturing: A comparative analysis. IEEE Access 2021, 9, 2734–2757. [Google Scholar] [CrossRef]
- Zhou, T.; Tang, D.; Zhu, H.; Wang, L. Reinforcement learning with composite rewards for production scheduling in a smart factory. IEEE Access 2021, 9, 752–766. [Google Scholar] [CrossRef]
- Friedl, J.; Zimmer, B.; Perkhofer, L.; Zenisek, J.; Hofer, P.; Jetter, H.-C. An empirical study of task-specific limitations of the overview+detail technique for interactive time series analysis. Procedia Comput. Sci. 2021, 180, 628–638. [Google Scholar] [CrossRef]






| Nr | Selection Criteria |
|---|---|
| SQ1 | Paper is open access. |
| SQ2 | Paper is written in English. |
| SQ3 | Paper is not a duplicate. |
| SQ4 | Paper relates to manufacturing. |
| SQ5 | Paper validates current study. |
| Nr | Quality Assessment |
|---|---|
| QA1 | Are the aims of the article clearly stated? |
| QA2 | Are the scope, context, and experimental design of the study clearly defined? |
| QA3 | Is the research process documented adequately? |
| QA4 | Is the journal in which the article is published considered highly ranked in the respective field? |
| QA5 | Is the research coupled with a real-life application? |
| QA6 | Is there a direct link to the research focus of this study? |
| Fault Detection | Predictive Maintenance | Communication | Virtualization | Human-Machine Interference |
|---|---|---|---|---|
| Anomaly detection | Big data | Blockchain | Augmented reality | Adaptability, flexibility |
| High accuracy | Condition-based maintenance | Cloud computing | Cost minimization | Agility |
| Improved performance | Equipment reliability | Cloud-assistance | Mixed reality | Cobot programming by demonstration |
| Quality improvement | Labor activity monitoring | Computational self-awareness | Task placement | Digital twin |
| Real-time stress prediction | Production control | Decentralized | Virtual training | High scalability |
| Reduction of breakdown risk | Reinforcement learning | Edge computing | Virtual reality | Human capability enhancement |
| Root cause diagnosis | Scrap reduction | Energy efficiency | Immersive analytics | |
| Uncertainty reduction | Fog computing | Improved ergonomic conditions | ||
| Usage prediction | IoT | Process planning | ||
| What-if analysis | Less downtime | Remote control |
| Data Governance | Predictive Analytics | Quality | Other |
|---|---|---|---|
| Data integration | Imbalance causes issues | Solutions only for simple manufacturing systems | Cost |
| Security | Parameter configuration | Architecture | Latency |
| Data acquisition | Data preparation | Lack of standards | Communication |
| Data validity | Automation | Adherence to standards | Ethics |
| Data aggregation across systems | Optical noise | Not generalizable | |
| Privacy | User position tracking indoors | Safety | |
| Data ownership | Dealing with unexpected scenarios | AR cannot be used for long hours | |
| Real time application | High computing power required |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lepasepp, T.K.; Hurst, W. A Systematic Literature Review of Industry 4.0 Technologies within Medical Device Manufacturing. Future Internet 2021, 13, 264. https://doi.org/10.3390/fi13100264
Lepasepp TK, Hurst W. A Systematic Literature Review of Industry 4.0 Technologies within Medical Device Manufacturing. Future Internet. 2021; 13(10):264. https://doi.org/10.3390/fi13100264
Chicago/Turabian StyleLepasepp, Tuuli Katarina, and William Hurst. 2021. "A Systematic Literature Review of Industry 4.0 Technologies within Medical Device Manufacturing" Future Internet 13, no. 10: 264. https://doi.org/10.3390/fi13100264
APA StyleLepasepp, T. K., & Hurst, W. (2021). A Systematic Literature Review of Industry 4.0 Technologies within Medical Device Manufacturing. Future Internet, 13(10), 264. https://doi.org/10.3390/fi13100264







