Synthesis of Octacalcium Phosphate Containing Glutarate Ions with a High Incorporation Fraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Characterisation
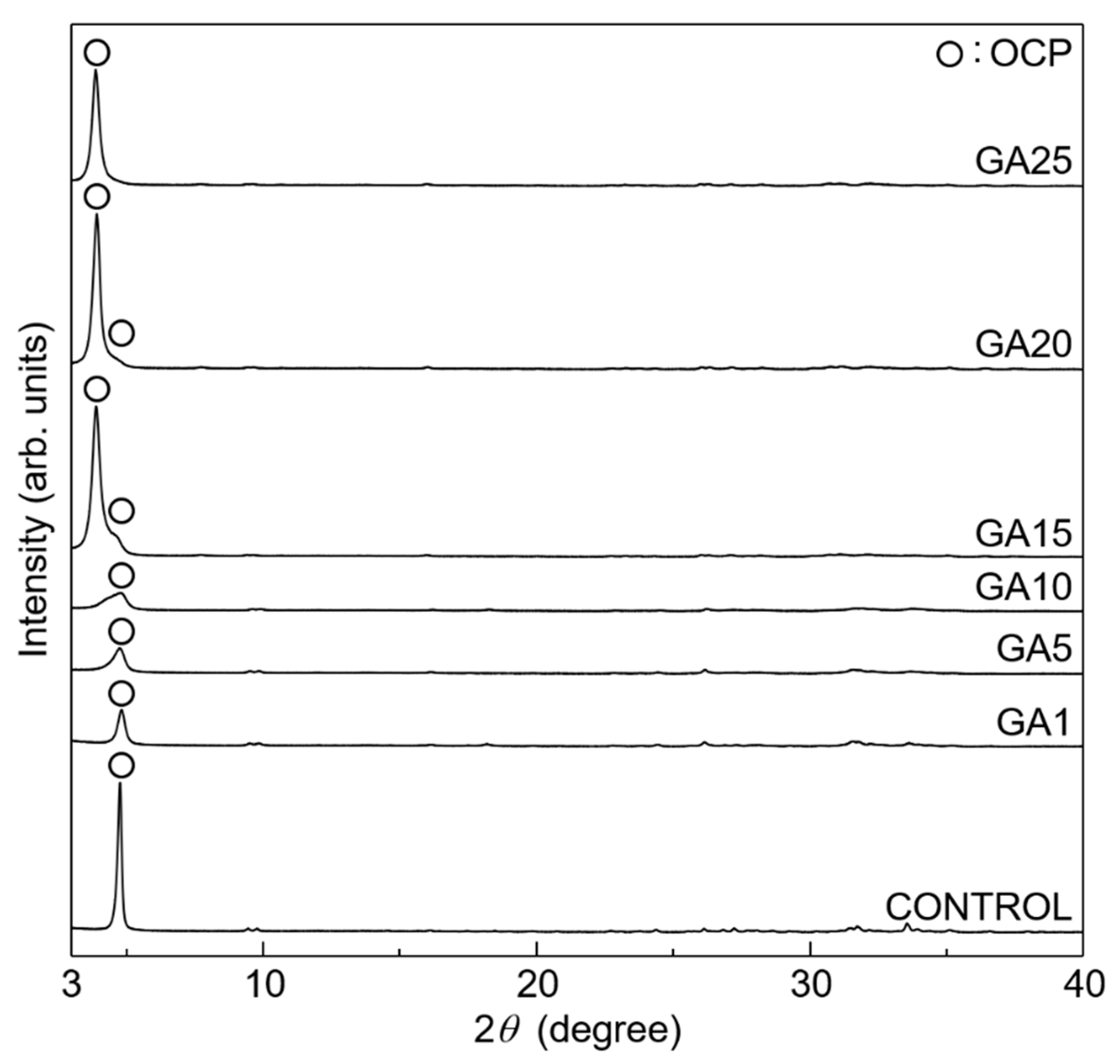
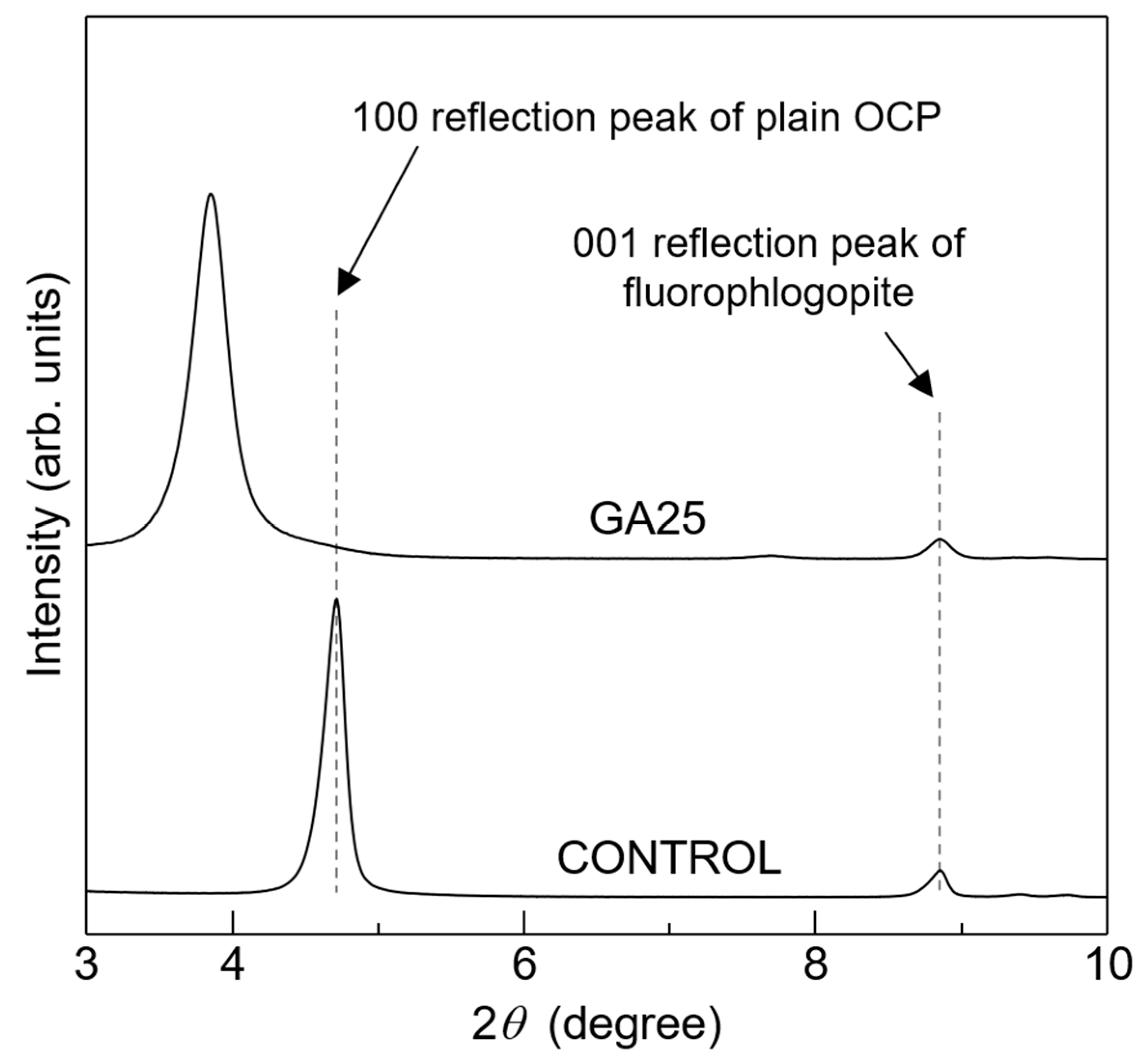
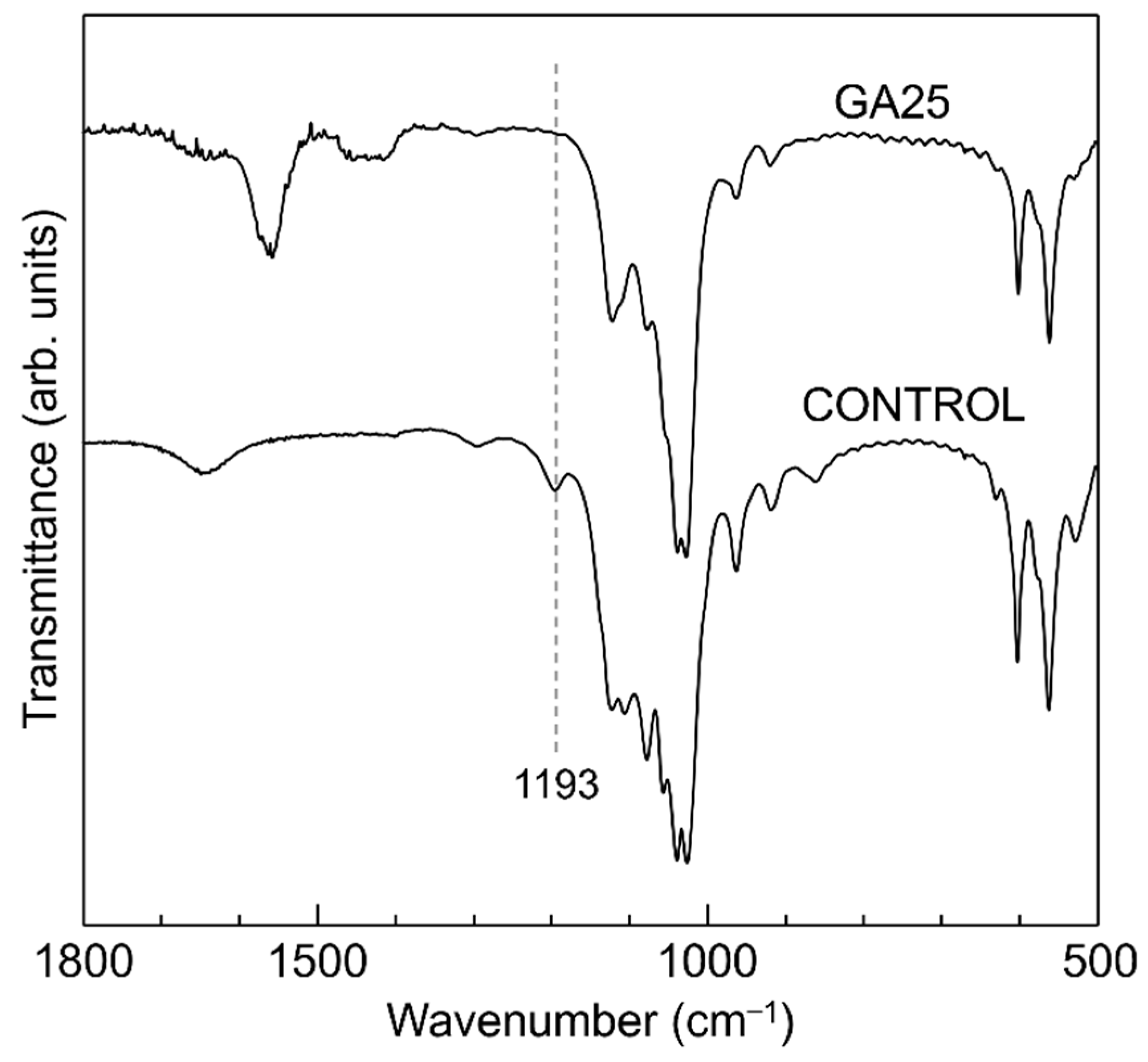
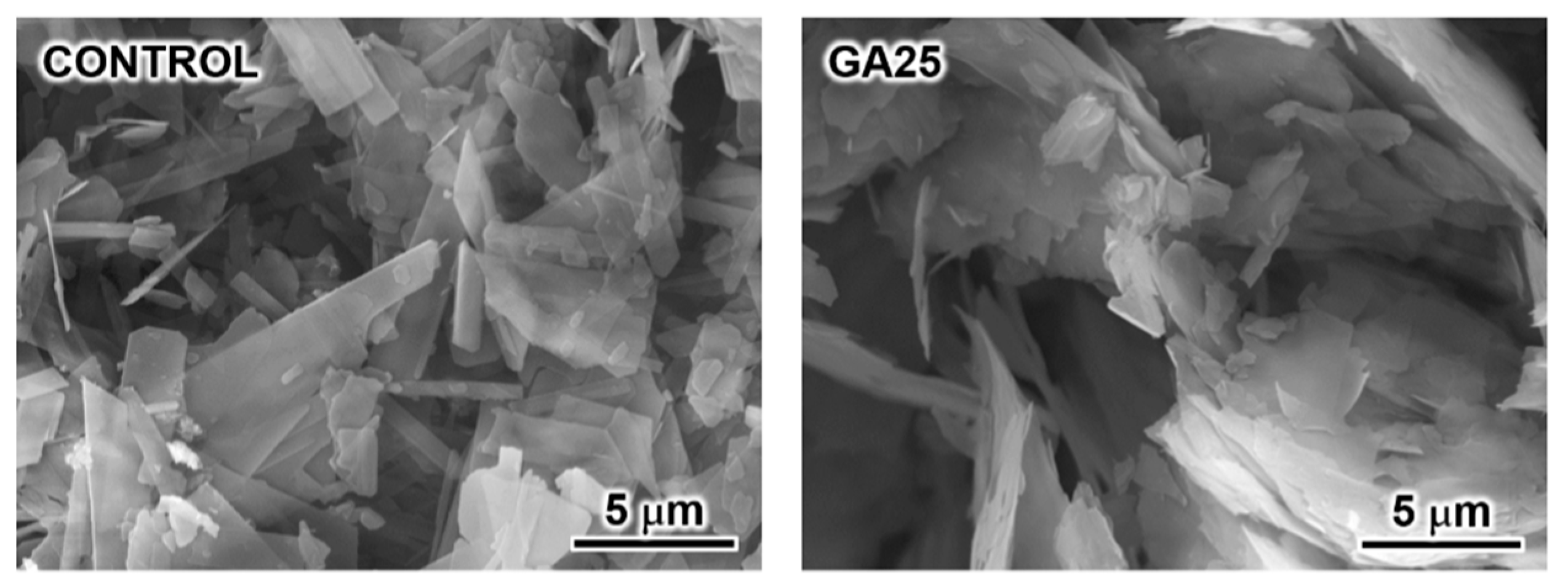
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suzuki, O.; Insley, G. Octacalcium Phosphate Biomaterials: Understanding of Bioactive Properties and Application; Woodhead Publishing: Duxford, UK, 2019; ISBN 978-0-08-102511-6. [Google Scholar]
- Kim, J.-S.; Jang, T.-S.; Kim, S.-Y.; Lee, W.-P. Octacalcium phosphate bone substitute (Bontree®): From basic research to clinical case study. Appl. Sci. 2021, 11, 7921. [Google Scholar] [CrossRef]
- Shiwaku, Y.; Tsuchiya, K.; Xiao, L.; Suzuki, O. Effect of calcium phosphate phases affecting the crosstalk between osteoblasts and osteoclasts in vitro. J. Biomed. Mater. Res. Part A 2019, 107, 1001–1013. [Google Scholar] [CrossRef]
- Anada, T.; Pan, C.-C.; Stahl, A.M.; Mori, S.; Fukuda, J.; Suzuki, O.; Yang, Y. Vascularized bone-mimetic hydrogel constructs by 3D bioprinting to promote osteogenesis and angiogenesis. Int. J. Mol. Sci. 2019, 20, 1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sai, Y.; Shiwaku, Y.; Anada, T.; Tsuchiya, K.; Takahashi, T.; Suzuki, O. Capacity of octacalcium phosphate to promote osteoblastic differentiation toward osteocytes in vitro. Acta Biomater. 2018, 69, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Sakai, S.; Anada, T.; Tsuchiya, K.; Yamazaki, H.; Margolis, H.C.; Suzuki, O. Comparative study on the resorbability and dissolution behavior of octacalcium phosphate, beta-tricalcium phosphate, and hydroxyapatite under physiological conditions. Dent. Mater. J. 2016, 35, 216–224. [Google Scholar] [CrossRef] [Green Version]
- Yokoi, T.; Kim, I.Y.; Ohtsuki, C. Mineralization of calcium phosphate on octacalcium phosphate in a solution mimicking in vivo conditions. Phosphorus Res. Bull. 2012, 26, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Okuyama, K.; Shiwaku, Y.; Hamai, R.; Mizoguchi, T.; Tsuchiya, K.; Takahashi, T.; Suzuki, O. Differentiation of committed osteoblast progenitors by octacalcium phosphate compared to calcium-deficient hydroxyapatite in Lepr-cre /Tomato mouse tibia. Acta Biomater. 2022, 142, 332–344. [Google Scholar] [CrossRef]
- Koyama, S.; Hamai, R.; Shiwaku, Y.; Kurobane, T.; Tsuchiya, K.; Takahashi, T.; Suzuki, O. Angio-osteogenic capacity of octacalcium phosphate co-precipitated with copper gluconate in rat calvaria critical-sized defect. Sci. Technol. Adv. Mater. 2022, 23, 120–139. [Google Scholar] [CrossRef]
- Hamai, R.; Sakai, S.; Shiwaku, Y.; Anada, T.; Tsuchiya, K.; Ishimoto, T.; Nakano, T.; Suzuki, O. Octacalcium phosphate crystals including a higher density dislocation improve its materials osteogenecity. Appl. Mater. Today 2022, 26, 101279. [Google Scholar] [CrossRef]
- Hamada, S.; Mori, Y.; Shiwaku, Y.; Hamai, R.; Tsuchiya, K.; Baba, K.; Oizumi, I.; Kanabuchi, R.; Miyatake, N.; Aizawa, T.; et al. Octacalcium phosphate/gelatin composite (OCP/Gel) enhances bone repair in a critical-sized transcortical femoral defect rat model. Clin. Orthop. Rel. Res. 2022, 480, 2043–2055. [Google Scholar] [CrossRef]
- Shiwaku, Y.; Hamai, R.; Sato, S.; Sakai, S.; Tsuchiya, K.; Baba, K.; Takahashi, T.; Suzuki, O. Bone tissue response to different grown crystal batches of octacalcium phosphate in rat long bone intramedullary canal area. Int. J. Mol. Sci. 2021, 22, 9770. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Hamai, R.; Shiwaku, Y.; Hasegawa, T.; Sakai, S.; Tsuchiya, K.; Sai, Y.; Iwama, R.; Amizuka, N.; Takahashi, T.; et al. Involvement of distant octacalcium phosphate scaffolds in enhancing early differentiation of osteocytes during bone regeneration. Acta Biomater. 2021, 129, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, H.; Hamai, R.; Shiwaku, Y.; Sakai, S.; Tsuchiya, K.; Suzuki, O. Mutual chemical effect of autograft and octacalcium phosphate implantation on enhancing intramembranous bone regeneration. Sci. Technol. Adv. Mater. 2021, 22, 345–362. [Google Scholar] [CrossRef] [PubMed]
- Oizumi, I.; Hamai, R.; Shiwaku, Y.; Mori, Y.; Anada, T.; Baba, K.; Miyatake, N.; Hamada, S.; Tsuchiya, K.; Nishimura, S.-N.; et al. Impact of simultaneous hydrolysis of OCP and PLGA on Bone Induction of a PLGA-OCP composite scaffold in a rat femoral defect. Acta Biomater. 2021, 124, 358–373. [Google Scholar] [CrossRef]
- Chiba, S.; Anada, T.; Suzuki, K.; Saito, K.; Shiwaku, Y.; Miyatake, N.; Baba, K.; Imaizumi, H.; Hosaka, M.; Itoi, E.; et al. Effect of resorption rate and osteoconductivity of biodegradable calcium phosphate materials on the acquisition of natural bone strength in the repaired bone. J. Biomed. Mater. Res. Part A 2016, 104, 2833–2842. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Anada, T.; Handa, T.; Kanda, N.; Yoshinari, M.; Takahashi, T.; Suzuki, O. Osteoconductive property of a mechanical mixture of octacalcium phosphate and amorphous calcium phosphate. ACS Appl. Mater. Interfaces 2014, 6, 22602–22611. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.; Song, I. Biomimetic octacalcium phosphate bone has superior bone regeneration ability compared to xenogeneic or synthetic bone. Materials 2021, 14, 5300. [Google Scholar] [CrossRef]
- Kibe, T.; Maeda-Iino, A.; Takahashi, T.; Kamakura, S.; Suzuki, O.; Nakamura, N. A follow-up study on the clinical outcomes of alveolar reconstruction using octacalcium phosphate granules and atelocollagen complex. J. Oral Maxillofac. Surg. 2021, 79, 2462–2471. [Google Scholar] [CrossRef]
- Kawai, T.; Kamakura, S.; Matsui, K.; Fukuda, M.; Takano, H.; Iino, M.; Ishikawa, S.; Kawana, H.; Soma, T.; Imamura, E.; et al. Clinical study of octacalcium phosphate and collagen composite in oral and maxillofacial surgery. J. Tissue Eng. 2020, 11, 2041731419896449. [Google Scholar] [CrossRef]
- Kawai, T.; Suzuki, O.; Matsui, K.; Tanuma, Y.; Takahashi, T.; Kamakura, S. Octacalcium phosphate collagen composite facilitates bone regeneration of large mandibular bone defect in humans. J. Tissue Eng. Regen. Med. 2017, 11, 1641–1647. [Google Scholar] [CrossRef]
- Kawai, T.; Echigo, S.; Matsui, K.; Tanuma, Y.; Takahashi, T.; Suzuki, O.; Kamakura, S. First clinical application of octacalcium phosphate collagen composite in human bone defect. Tissue Eng. Part A 2014, 20, 1336–1341. [Google Scholar] [CrossRef] [PubMed]
- Teterina, A.Y.; Smirnov, I.V.; Fadeeva, I.S.; Fadeev, R.S.; Smirnova, P.V.; Minaychev, V.V.; Kobyakova, M.I.; Fedotov, A.Y.; Barinov, S.M.; Komlev, V.S. Octacalcium phosphate for bone tissue engineering: Synthesis, modification, and in vitro biocompatibility assessment. Int. J. Mol. Sci. 2021, 22, 12747. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, T.; Goto, T.; Hara, M.; Sekino, T.; Seki, T.; Kamitakahara, M.; Ohtsuki, C.; Kitaoka, S.; Takahashi, S.; Kawashita, M. Incorporation of tetracarboxylate ions into octacalcium phosphate for the development of next-generation biofriendly materials. Comm. Chem. 2021, 4, 4. [Google Scholar] [CrossRef]
- Mathew, M.; Brown, W.E.; Schroeder, L.W.; Dickens, B. Crystal structure of octacalcium bis(hydrogenphosphate) tetrakis(phosphate)pentahydrate, Ca8(HPO4)2(PO4)4·5H2O. J. Crystallogr. Spectrosc. Res. 1988, 18, 235–250. [Google Scholar] [CrossRef]
- Brown, W.E.; Smith, J.P.; Lehr, J.R.; Frazier, A.W. Crystallographic and chemical relations between octacalcium phosphate and hydroxyapatite. Nature 1962, 196, 1050–1055. [Google Scholar] [CrossRef]
- Brown, W.E.; Lehr, J.R.; Smith, J.P.; Frazier, A.W. Crystallography of octacalcium phosphate. J. Am. Chem. Soc. 1957, 79, 5318–5319. [Google Scholar] [CrossRef]
- Yokoi, T.; Shimabukuro, M.; Kawashita, M. Octacalcium phosphate with incorporated carboxylate ions: A review. Sci. Technol. Adv. Mater. 2022, 23, 434–445. [Google Scholar] [CrossRef]
- Monma, H.; Goto, M. Succinate-complexed octacalcium phosphate. Bull. Chem. Soc. Jpn. 1983, 56, 3843–3844. [Google Scholar] [CrossRef] [Green Version]
- Nakahira, A.; Aoki, S.; Sakamoto, K.; Yamaguchi, S. Synthesis and evaluation of various layered octacalcium phosphates by wet-chemical processing. J. Mater. Sci.: Mater. Med. 2001, 12, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Aoki, S.; Sakamoto, K.; Yamaguchi, S.; Nakahira, A. Syntheses of octacalcium phosphate containing dicarboxylic acids and effects of the side groups on the crystal growth of octacalcium phosphate. J. Ceram. Soc. Jpn. 2000, 108, 909–914. [Google Scholar] [CrossRef]
- Marković, M.; Fowler, B.; Brown, W. Octacalcium phosphate carboxylates. 1. Preparation and identification. Chem. Mater. 1993, 5, 1401–1405. [Google Scholar] [CrossRef]
- Monma, H. Apatitic intercalation compounds containing dicarboxylates. Gypsum Lime 1992, 237, 108–114. [Google Scholar]
- Monma, H. The incorporation of dicarboxylates into octacalcium bis(hydrogenphosphate) tetrakis(phosphate) pentahydrate. Bull. Chem. Soc. Jpn. 1984, 57, 599–600. [Google Scholar] [CrossRef] [Green Version]
- Yokoi, T.; Goto, T.; Sekino, T.; Kawashita, M. Fluorescent properties of octacalcium phosphate with incorporated isophthalate ions. J. Ceram. Soc. Jpn. 2022, 130, 337–340. [Google Scholar] [CrossRef]
- Yokoi, T.; Machida, S.; Sugahara, Y.; Hashimoto, M.; Kitaoka, S. Enantioselective Incorporation of Dicarboxylate Guests by Octacalcium Phosphate. Chem. Commun. 2017, 53, 6524–6527. [Google Scholar] [CrossRef]
- Yokoi, T.; Kamitakahara, M.; Ohtsuki, C. Continuous Expansion of the Interplanar Spacing of Octacalcium Phosphate by Incorporation of Dicarboxylate Ions with a Side Chain. Dalton Trans. 2015, 44, 7943–7950. [Google Scholar] [CrossRef]
- Yokoi, T.; Kamitakahara, M.; Kawashita, M.; Ohtsuki, C. Formation of Organically Modified Octacalcium Phosphate in Solutions Containing Various Amounts of Benzenedicarboxylic Acids. J. Ceram. Soc. Jpn. 2013, 121, 219–225. [Google Scholar] [CrossRef] [Green Version]
- Yokoi, T.; Kato, H.; Kim, I.Y.; Kikuta, K.; Kamitakahara, M.; Kawashita, M.; Ohtsuki, C. Formation of Octacalcium Phosphates with Co-Incorporated Succinate and Suberate Ions. Dalton Trans. 2012, 41, 2732–2737. [Google Scholar] [CrossRef]
- Yokoi, T.; Kato, H.; Kim, I.Y.; Kikuta, K.; Kawashita, M.; Ohtsuki, C. Synthesis of octacalcium phosphate with incorporated succinate and suberate ions. Ceram. Int. 2012, 38, 3815–3820. [Google Scholar] [CrossRef]
- Yokoi, T.; Kawashita, M. Understanding the steric structures of dicarboxylate ions incorporated in octacalcium phosphate crystals. Materials 2021, 14, 2703. [Google Scholar] [CrossRef]
- Marković, M.; Fowler, B.; Brown, W. Octacalcium phosphate carboxylates. 2. Characterization and structural considerations. Chem. Mater. 1993, 5, 1406–1416. [Google Scholar] [CrossRef]
- Boanini, E.; Torricelli, P.; Gazzano, M.; Giardino, R.; Bigi, A. Nanocomposites of hydroxyapatite with aspartic acid and glutamic acid and their interaction with osteoblast-like cells. Biomaterials 2006, 27, 4428–4433. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Hossain, M.I.; Hossain, M.K.; Rubel, M.H.K.; Hossain, K.M.; Mahfuz, A.M.U.B.; Anik, M.I. Recent progress in nanostructured smart drug delivery systems for cancer therapy: A review. ACS Appl. Bio. Mater. 2022, 5, 971–1012. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Hossain, M.K. Classification and properties of nanoparticles. In Nanoparticle-Based Polymer Composites; Mavinkere Rangappa, S., Parameswaranpillai, J., Yashas Gowda, T.G., Siengchin, S., Seydibeyoglu, M.O., Eds.; Woodhead Publishing: Cambridge, MA, USA, 2022; pp. 15–54. ISBN 978-0-12-824272-8. [Google Scholar]
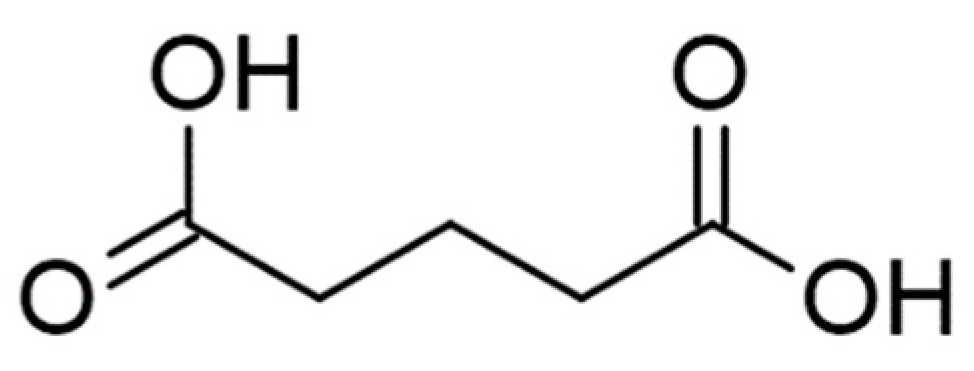
| Incorporated Anion | Ca/P Molar Ratio | Substitution Fraction 1 (%) |
|---|---|---|
| Hydrogen phosphate ion (control) | 1.37 | N/A |
| Glutarate ion (GA25) | 1.57 | 90 2 |
| Malonate ion | 1.47 3 | 42 3 |
| Succinate ion | 1.55 3 | 83 3 |
| Glutarate ion | 1.45 3 | 35 3 |
| Adipate ion | 1.56 3 | 86 3 |
| Pimerate ion | 1.41 3 | 22 3 |
| Suberate ion | 1.55 3 | 92 3 |
| Azelate ion | 1.45 3 | 50 3 |
| Sebacate ion | 1.53 3 | 79 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yokoi, T.; Watanabe, M.; Goto, T.; Meng, S.; Sekino, T.; Shimabukuro, M.; Kawashita, M. Synthesis of Octacalcium Phosphate Containing Glutarate Ions with a High Incorporation Fraction. Materials 2023, 16, 64. https://doi.org/10.3390/ma16010064
Yokoi T, Watanabe M, Goto T, Meng S, Sekino T, Shimabukuro M, Kawashita M. Synthesis of Octacalcium Phosphate Containing Glutarate Ions with a High Incorporation Fraction. Materials. 2023; 16(1):64. https://doi.org/10.3390/ma16010064
Chicago/Turabian StyleYokoi, Taishi, Masahiro Watanabe, Tomoyo Goto, Sikun Meng, Tohru Sekino, Masaya Shimabukuro, and Masakazu Kawashita. 2023. "Synthesis of Octacalcium Phosphate Containing Glutarate Ions with a High Incorporation Fraction" Materials 16, no. 1: 64. https://doi.org/10.3390/ma16010064





