Abstract
Alzheimer’s disease (AD) is a devastating neurodegenerative disorder without a cure. Hence, developing an effective treatment or protective agent is crucial for public health. The present study aims to characterize orange peel extract (OPE) through in vitro and in silico studies. Furthermore, it examines the protective effect of OPE against experimentally-induced Alzheimer’s disease in rats. The total phenolic and flavonoid content of OPE was 255.86 ± 1.77 and 52.06 ± 1.74 (mg/100 g), respectively. Gallic acid, the common polyphenol in OPE detected by HPLC was 3388.60 μg/100 g. OPE antioxidant IC50 was 67.90 ± 1.05, 60.48 ± 0.91, and 63.70 ± 0.30 by DPPH, ABTS and Hydroxyl radical scavenging activity methods, respectively. In vitro anti-acetylcholinesterase (AChE) IC50 was 0.87 ± 0.025 mg/mL for OPE and 2.45 ± 0.001 mg/mL for gallic acid. Molecular docking analysis for human AChE (4EY7) with donepezil, gallic acid, and acetylcholine showed binding energy ΔG values of −9.47, −3.72, and −5.69 Kcal/mol, respectively. Aluminum chloride injection (70 mg/Kg/day for 6 weeks) induced Alzheimer’s-like disease in male rats. OPE (100 and 200 mg/kg/d) and gallic acid (50 mg/kg/d) were administered orally to experimental animals for 6 weeks in addition to aluminum chloride injection (as protective). OPE was found to protect against aluminum chloride-induced neuronal damage by decreasing both gene expression and activity of acetylcholinesterase (AChE) and a decrease in amyloid beta (Aβ42) protein level, thiobarbituric acid-reactive substances (TBARS), and nitric oxide (NO), and increased reduced glutathione (GSH) level and activity of the antioxidant enzymes in the brain tissues. Additionally, gene expressions for amyloid precursor protein (APP) and beta secretase enzyme (BACE1) were downregulated, whereas those for presinilin-2 (PSEN2) and beta cell lymphoma-2 (BCL2) were upregulated. Furthermore, the reverse of mitochondrial alternation and restored brain ultrastructure might underlie neuronal dysfunction in AD. In conclusion, our exploration of the neuroprotective effect of OPE in vivo reveals that OPE may be helpful in ameliorating brain oxidative stress, hence protecting from Alzheimer’s disease progression.
1. Introduction
Dementia caused by Alzheimer’s disease (AD) is a complex illness without a treatment [1]. The prevalence of Alzheimer’s disease (AD) is rising as the number of elderly people increases [2]. Currently, more than 55 million people live with dementia worldwide, and there are nearly 10 million new cases every year. Dementia results from a variety of diseases and injuries that primarily or secondarily affect the brain. Alzheimer’s disease is the most common form of dementia and may contribute to 60–70% of cases [3].
Protein amyloid beta (plaques) and tau (tangles) accumulate outside and inside of neurons, respectively, as characteristic pathologies of Alzheimer’s disease [4]. These alterations are associated with neuronal death and brain tissue damage [5]. Several studies have found that mitochondrial dysfunction is highly prevalent in AD, pointing to a crucial role for mitochondrial homeostasis in the progression of this disease [6]. It is well-reported that several pathological conditions of AD, including Aβ pathology, disturb mitochondrial dynamics via direct or indirect processes [7].
The majority of the metals found in the crust of the Earth are aluminum. It’s in everything we consume: food, water, dust, air, beverages, flavored drinks, energy drinks, and medicine [8]. Due to its extremely high affinity to transferrin receptors, aluminum is able to cross the blood–brain barrier (BBB) and accumulate in the brain [9]. Kawahara states that this metal causes neuronal death both in vivo and in vitro [10]. Through its ability to cross-link hyperphosphorylated proteins, aluminum may actively contribute to the etiology of major neuropathologic lesions in Alzheimer’s disease and similar illnesses [11].
Six medications for the treatment of Alzheimer’s disease have been greenlighted by the Food and Drug Administration (FDA) in the United States. There are now five medications that temporarily treat Alzheimer’s symptoms but do not change the underlying brain changes of Alzheimer’s or alter the course of the disease. These medications include donepezil, rivastigmine, galantamine, memantine, and memantine coupled with donepezil [3].
With the exception of memantine, these medications alleviate symptoms by boosting levels of neurotransmitters. Memantine prevents glutamate, a neurotransmitter that can overstimulate and even destroy neurons, from doing so in the brain. The potential adverse effects of these five medications are generally minor and may include side effects such as headaches and nausea [3]. As of June 2021, aducanumab, the sixth medicine on the list, is the first drug approved by the FDA to treat the underlying biology of Alzheimer’s disease rather than its symptoms. Beta-amyloid plaques in the brain are diminished as a result. However, not everyone with Alzheimer’s should try this treatment, as it is not a cure. Amyloid-related imaging abnormalities (ARIA) are a sign of brain swelling and are more common with aducanumab than with other medications approved to treat Alzheimer’s disease [12]. These synthetic drugs are associated with a number of side effects, possess much economic burden and are not implemented for all cases of AD [13]. Therefore, there is a high demand for discovering new remedies of natural origin obtained from medicinal plants to be directed for protection from, slowing down or halting of AD disease progression in its early stages. The protective effect of polyphenols against Alzheimer’s disease was recently reviewed by Chiara Colizzi [14]. In addition, research has shown that phenolic compounds derived from plants and fruits have a variety of beneficial biological effects, such as antioxidant, antimicrobial, anti-inflammatory, anticancer, and hepato-protective properties [15,16,17].
Aqueous orange peel extract had many food applications such as supplementation in minced beef to suppress lipid oxidation [18] and milk for increasing the antioxidant activity and total polyphenol content, and decreasing the total microbial count [19].
Peels tend to be the predominant byproduct of citrus juice production, contributing to environmental contamination [19]. Over 100 million tons of citrus fruit is harvested every year, making it the world’s most prolific fruit crop. It is estimated that typical food processing processes squander 20% of the entire weight of citrus peels as by-products [20]. Orange peels are sources of various bioactive ingredients, such as phenolics, flavonoids, tannins, and limonoids especially, that are unique to other plants [21]. Antimicrobial, antioxidant, and anti-cancer properties are just a few of the many biological functions exhibited by these bioactive chemicals [22]. Therefore, citrus by-products applications should be studied in order to turn them into value-added products [23]. In this study, in vitro and computer simulations are conducted to learn more about orange peel extract (OPE). Moreover, we assess a number of biochemical, molecular, and brain ultrastructural biomarkers to investigate OPE’s protective impact against a rat model of Alzheimer’s disease induced by aluminum chloride injection (70 mg/Kg/day for 6 weeks).
2. Results
2.1. Phytochemical and HPLC Analysis
Total phenolics (TP) and flavonoids (TF) concentrations were 255.86 ± 1.77 and 52.06 ± 1.74 (mg/100 g), respectively (Table 1). Twelve polyphenolic compounds were detected by HPLC in OPE (Table 2). The most abundant polyphenol was gallic acid with 3388.60 μg/100 g concentration. Moreover, rutin, naringenin, ferulic acid, and quercetin was detected with concentrations 1952.64, 1967.43, 1335.96, and 1266.97 μg/100 g, respectively.

Table 1.
Orange peel extract (OPE) total phenolic, total flavonoids, and its functional properties.

Table 2.
Phenolic compounds identified by high-performance liquid chromatography (HPLC) (µg/g) OPE.
2.2. Biochemical Properties of OPE
2.2.1. Antioxidant Activity
The antioxidant activities of OPE was evaluated using DPPH, ABTS and Hydroxyl radical scavenging activity and the results in IC50 were 67.90 ± 1.05, 60.48 ± 0.91, and 63.70 ± 0.30 μg/mL, respectively (Table 1).
2.2.2. Anti-Acetylcholinesterase Power
The effectiveness of OPE and gallic acid in inhibiting AChE, an enzyme that is hyperactivated in AD, was investigated in vitro. Critical concentrations for inhibition (IC50) of OPE, gallic acid, and donepezil were 0.87 ± 0.025, 2.45± 0.001, and 0.0034 ± 0.0 mg/mL, respectively (Table 1).
2.3. Computational Docking Analysis
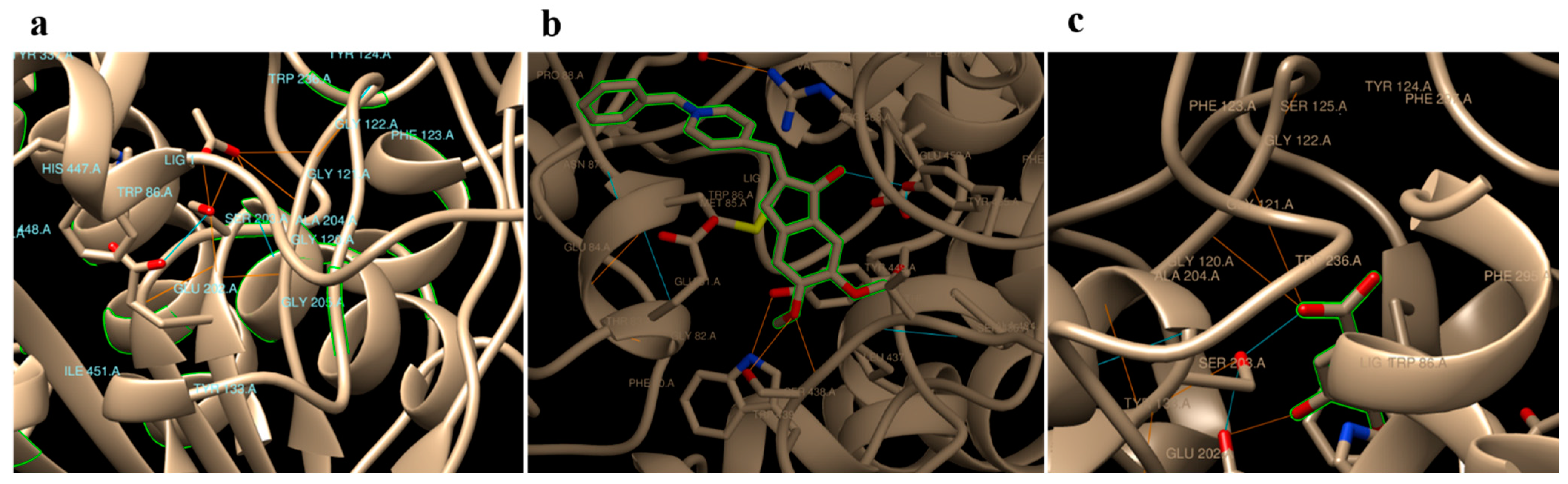
In silico docking analyses of gallic acid, donepezil and native substrate (acetylcholine) separately against Homo sapiens AChE (PDB code: 4EY7) revealed hydrogen binding energy (ΔG) values −3.72, −9.47, and −5.69 Kcal/mol, respectively (Table 3). Donepezil, a commonly used medicine, was found to interact with residues TYR 465, TYR 124, HIS 447, and SER 203 in the AChE binding site. In addition to GLU 202 and SER 203, the GLY 121 and GLY 122 residues on AChE were anticipated to be binding sites for gallic acid. Acetylcholine, on the other hand, was predicted to bind to a number of different residues on AChE, particularly SER 203, GLY 121, GLY 122, ALA 204, ARG 296, and PHE 295 (Table 3 and Figure 1).

Table 3.
Docking substrates with AChE (PDB: 4EY7) and predicted binding information.
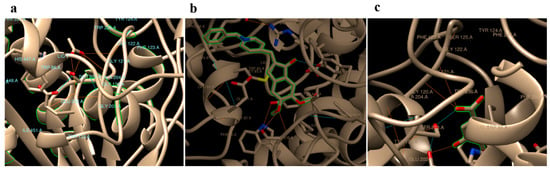
Figure 1.
Predicted binding modes of acetylcholine (a), Aricept (b), and gallic (c) with hAChE (PDB Code: 4EY7).
2.4. In Vivo Anti Alzheimer Potentials
After 6 weeks of treatment, the rats’ body weights were measured, the body weight gain was significantly more decreased in the induction group than the control group. Moreover, the body weight remained significantly less than the control group after treatment with gallic acid and OPE (Table 4).

Table 4.
Effect of Aricept, gallic, OPE 100, and OPE 200 on body weight gain, brain structure, and other oxidative stress parameters in brain tissue of different experimental rat groups treated with aluminum chloride.
2.4.1. Effect of Donepezil, Gallic Acid and OPE Treatment on Brain Aβ42, Total Cholesterol and Phospholipids Levels
Brain Aβ42 levels (pg/g tissue) were measured by sandwich ELISA. Aluminum chloride injection at a dose of 70 mg/kg significantly increased the level of Aβ42 3.60-fold comparing to the control (19.18 ± 1.15 vs. 5.32 ± 0.98). Oral administration of donepezil (2.25 mg/kg), gallic acid (50 mg/kg), OPE (100 mg/kg), and OPE (200 mg/kg) before aluminum chloride injection significantly reduced the level of Aβ42 to 7.97 ± 0.63, 13.86 ± 1.70, 16.73 ± 0.97, and 11.55 ± 1.02 pg/g tissue, respectively at (p < 0.05) (Table 4).
The brain’s total cholesterol (TC) and phospholipid (Ph. Lipid) levels (mg/g protein) and TC/phospholipid ratio analysis showed that brain TC levels were significantly elevated by a factor of two in the aluminum chloride group. However, phospholipid levels decreased from 72.33 ± 1.19 mg/g of protein in the control group to 33.50 ± 0.86 mg/g protein in the AD-induced group. Moreover, aluminum chloride significantly increased the TC/phospholipid ratio 4.5-fold as compared to control (Table 4). In donepezil-, gallic acid-, OPE 100-, and OPE 200-treated groups, the brain TC levels were 70.65 ± 1.72, 51.43 ± 1.46, 58.83 ± 1.04, and 44.45 ± 0.90 (mg/mg protein), respectively. There was no significant different in brain phospholipid level and TC/phospholipid ratio between the OPE 200-treated group and the control group.
2.4.2. Effect of Donepezil, Gallic Acid, and OPE Treatment on Oxidative Stress Parameters in Brain
Particularly in rat brain tissue, lipid peroxidation (LPO) was evaluated to assess the oxidative stress. Thiobarbituric acid-reactive substances (TBARS) (umol/g × 10−3) measurement in brain (Table 4) showed that aluminum chloride injection increased TBARS levels more significantly than the control group at 5.43 ± 0.83 and 1.32 ± 0.15, respectively, at (p < 0.05), while their levels in either donepezil-, gallic acid-, OPE 100-, or OPE 200-treated groups were significantly decreased to 3.67 ± 0.59, 2.41 ± 0.21, 4.25 ± 0.38, and 2.34 ± 0.22, respectively, in the brain than that of the aluminum chloride-treated rats. The NO level (µm/mg protein) in the brain (Table 4) was 12.43 ± 0.96 in control group. After treatment with aluminum chloride, the brain NO levels increased significantly to 32.76 ± 1.47 compared to the control. In donepezil-, gallic acid-, OPE 100-, and OPE 200-treated groups, the NO levels decreased significantly (p< 0.05) to 23.35 ± 1.31, 23.54 ± 0.73, 22.58 ± 0.79, and 21.86 ± 1.68, respectively, compared to the aluminum chloride group. Brain GSH level (mg/mg protein) × 10−2 in brain (Table 4) significantly decreased at (p < 0.05) in the aluminum chloride-injected group (0.24 ± 0.12) as compared to the control group (0.43 ± 0.03). In donepezil-, gallic acid-, OPE 100,- and OPE 200-treated groups the brain GSH levels were increased significantly compared to the aluminum chloride group.
2.4.3. Effect of Donepezil, Gallic Acid, and OPE Treatment on Brain Antioxidant Enzymes
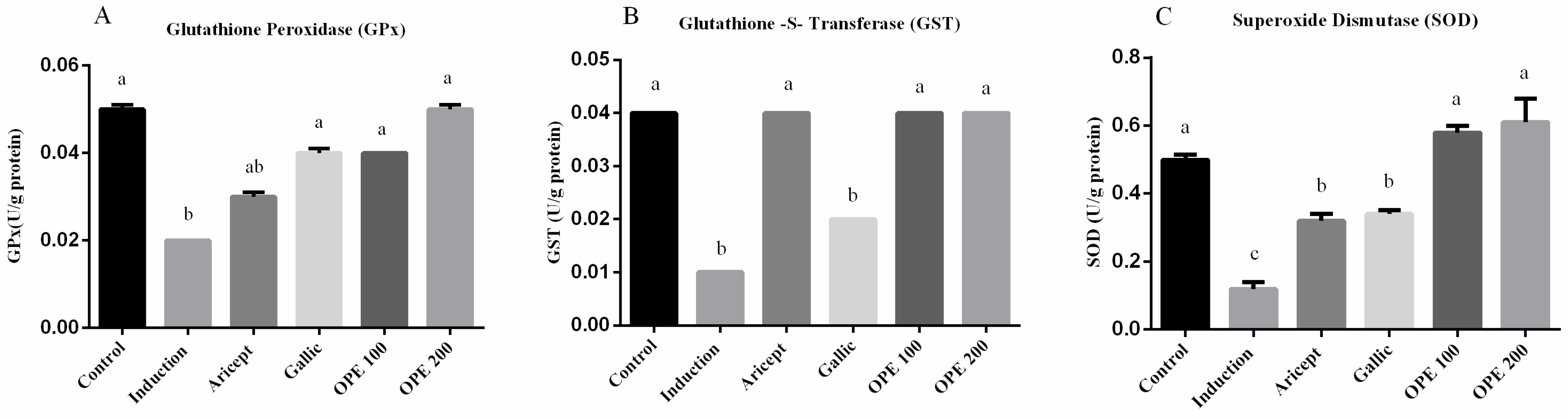
Brain GPx, GST and SOD activities (U/g protein) were measured and shown in (Figure 2A–C, respectively). The administration of aluminum chloride resulted in a significant decrease in GPx, GST, and SOD activities in brain at p < 0.05, comparing to their corresponding values in the control group. Daily administration of either donepezil, gallic acid, OPE 100, and OPE 200 with aluminum chloride injection resulted in a significant increase in the activities of GPx, GST, and SOD in brain tissue (GPx: 1.5-, 2-, 2-, and 2.5-fold, respectively); (GST: 4-, 2-, 4-, and 4-fold, respectively); (SOD: 2.7-, 2.8-, 4.8-, and 5.1-fold, respectively), comparing to the aluminum chloride group (induced group).
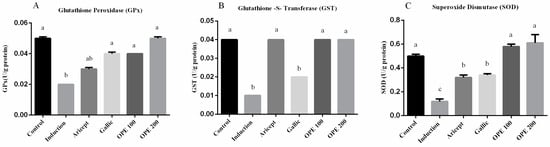
Figure 2.
Effect of Aricept, gallic, OPE 100, and OPE 200 on brain antioxidant enzymes. Glutathione peroxidase (GPx) (A), Glutathione-S-transferase (GST) (B) and superoxide dismutase (SOD) (C) activities. Values represent the mean ± SD of six rats. ANOVA (one-way) followed by Tukey’s test. Values bearing the same letters showed no significant difference (p < 0.05). The results are sorted in descending order: a < b < c.
2.4.4. Effect of Donepezil, Gallic Acid, and OPE Treatment on Brain AChE Activity and Gene Expression Levels (Hypothalamus)
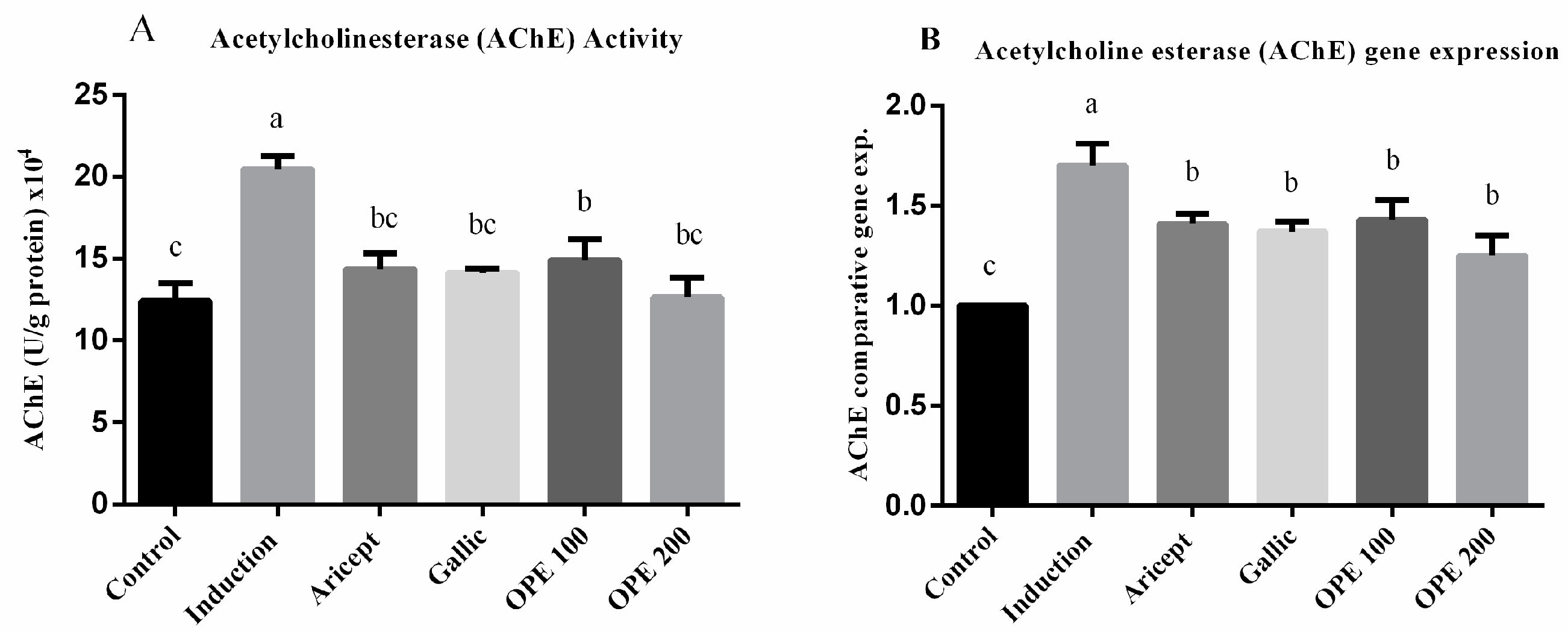
AChE activity (U/g protein) × 104 was significantly rose (1.6-fold) in the aluminum chloride treated group as compared to the control group (20.46 ± 0.81 vs. 12.4 ± 2.10) at p < 0.05 (Figure 3A). AChE activity in the brain tissue of aluminum chloride-treated rats that received either donepezil (14.36 ± 0.96), gallic acid (14.13 ± 0.25), OPE 100 (14.90 ± 1.30), or OPE 200 (12.64 ± 1.20) was significantly less than that of the aluminum chloride-treated rats (p < 0.05). Daily administration of donepezil (2.25 mg/kg) significantly decreased brain AChE activity as compared to the aluminum chloride group. It also showed a non-significant change in brain AChE activity compared to the control. The mRNA levels of AChE were measured using qRT-PCR analysis and the results are shown in (Figure 3B). The results showed that daily intraperitoneal injection of aluminum chloride for 6 weeks significantly upregulated the mRNA expression level of AChE as compared with the control group (1.70 ± 0.11 vs. 1.000 ± 0.000). Animals administered donepezil, gallic acid, OPE 100, and OPE 200 registered a significant decrease in the mRNA expression levels of AChE as compared with the aluminum chloride-treated group. Gallic acid-, OPE 100-, and OPE 200-treated groups showed a non-significance in AChE gene expression level compared to the donepezil-treated group (p < 0.05).
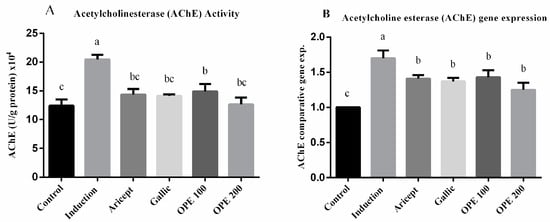
Figure 3.
Effect of Aricept, gallic, OPE treatment on brain AChE activity (A) and gene expression levels (hypothalamus) (B). Values represent the mean ± SD of six rats. ANOVA (one-way) followed by Tukey’s test. Values bearing the same letters showed no significant difference (p < 0.05). The results are sorted in descending order: a < b < c.
2.4.5. Brain (Hippocampus) Gene Expression
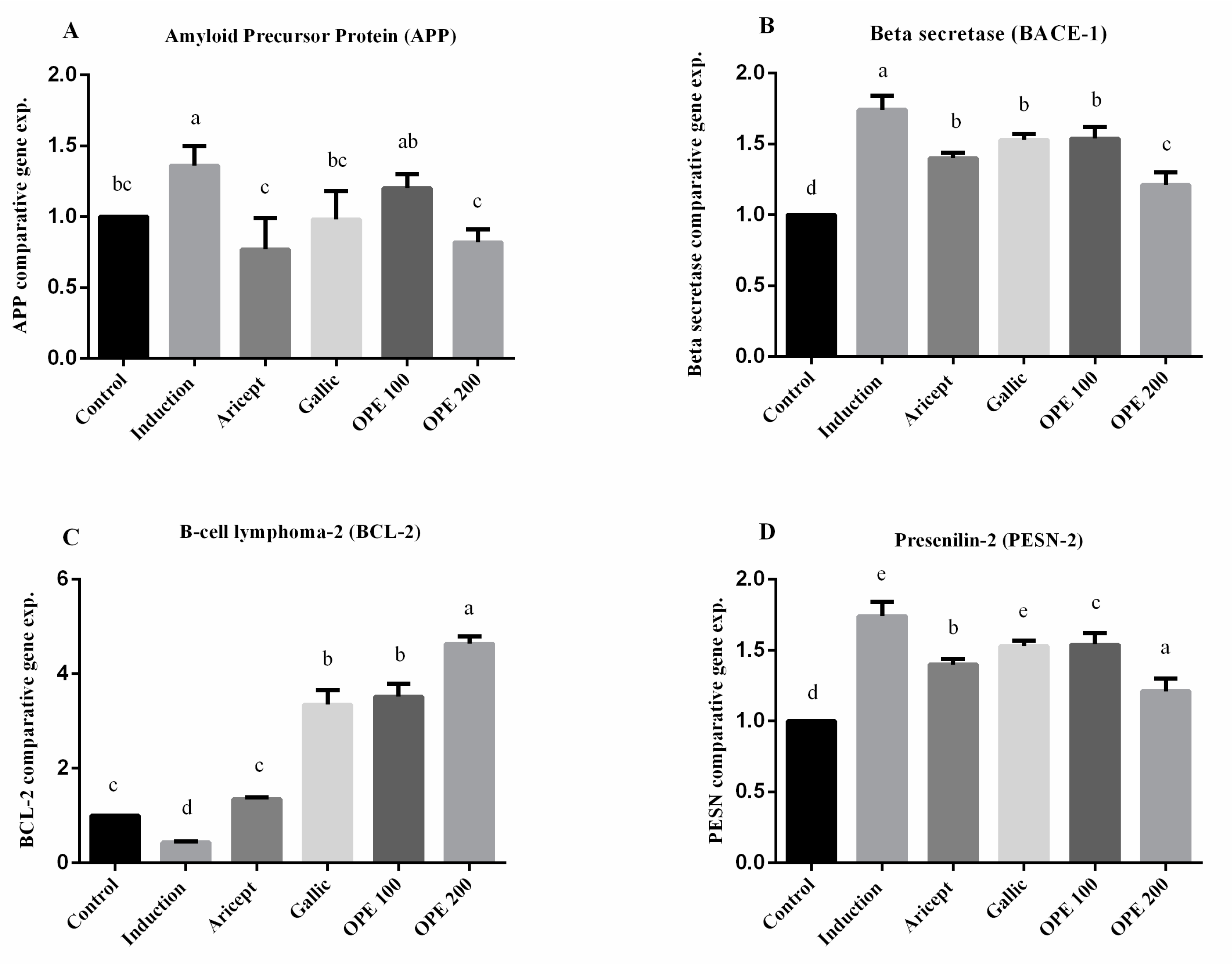
Brain (hippocampus) gene expression showed that intraperitoneal injection (ip) injection of aluminum chloride for 6 weeks significantly upregulated the mRNA expression level of APP as compared with the control group (1.36 ± 0.14 vs. 1.000 ± 0.000). Animals administered donepezil, gallic acid, OPE 100, and OPE 200 exhibited a significant decrease in the mRNA expression levels of APP as compared with the aluminum chloride-treated group. Results reveal that there was no significance in mRNA expression level of APP between the gallic acid-treated group and the control group (0.98 ± 0.20 vs. 1.000 ± 0.000). Additionally, OPE 200 treatment showed significant decrease in APP gene expression compared to the control group (0.82 ± 0.09 vs. 1.000 ± 0.000) (p < 0.05) (Figure 4A). Considering the beta secretase (BACE-1) gene, the results showed that intraperitoneal injection (ip) injection of aluminum chloride significantly upregulated the mRNA expression level of BACE-1 as compared with the control group (1.74 ± 0.10 vs. 1.000 ± 0.000). Animals administered donepezil, gallic acid, OPE 100, and OPE 200 represented a significant decrease in the mRNA expression levels of BACE-1 as compared with the aluminum chloride-treated group. Moreover, there was no significant difference in BACE-1 gene expression in donepezil-, gallic acid-, and OPE 100-treated groups (1.40 ± 0.04, 1.53 ± 0.04, 1.54 ± 0.08), respectively, at (p < 0.05) (Figure 4B). However, in the case of BCL-2 gene expression, results showed that daily ip of aluminum chloride significantly downregulated the mRNA expression level of BCL-2 as compared with the control group (0.43 ± 0.02 vs. 1.000 ± 0.000). Animals administered donepezil, gallic acid, OPE 100, and OPE 200 registered a significant increase in the mRNA expression levels of BCL-2 as compared with the aluminum chloride treated group. Gallic acid-, OPE 100-, and OPE 200-treated groups showed a significant increase in BCL-2 gene expression level compared to the donepezil-treated group (p < 0.05) (Figure 4C). Gene expression analysis of PSEN-2 showed that daily intraperitoneal injection of aluminum chloride significantly downregulated the mRNA expression level of PSEN-2 as compared with the control group (0.55 ± 0.16 vs. 1.000 ± 0.000) (Figure 3). Animals administered donepezil, OPE 100, and OPE 200 registered a significant increase in the mRNA expression levels of PSEN-2 as compared with the aluminum chloride-treated group. The gallic acid-treated group showed a non-significant change in PSEN-2 gene expression level compared to the induced group (p < 0.05). At the same time, OPE 100 and OPE 200 showed a more significant increase in PSEN-2 levels than the control group at (p < 0.05).
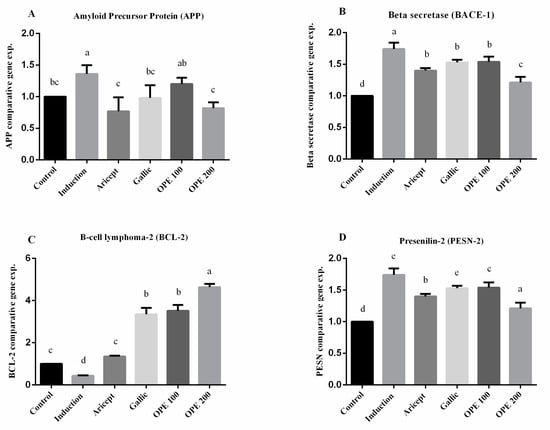
Figure 4.
Effect of Aricept, gallic, OPE 100, and OPE 200 on brain (hippocampus) gene expression of amyloid precursor protein (APP) (A), beta secretase (BACE-1) (B), B-cell lymphoma-2 (BCL-2) (C) and presenilin-2 (PSEN-2) (D). Values represent the mean ± SD of six rats. ANOVA (one-way) followed by Tukey’s test. Values bearing the same letters showed no significant difference (p < 0.05). The results are sorted in descending order: a < b < c < d < e.
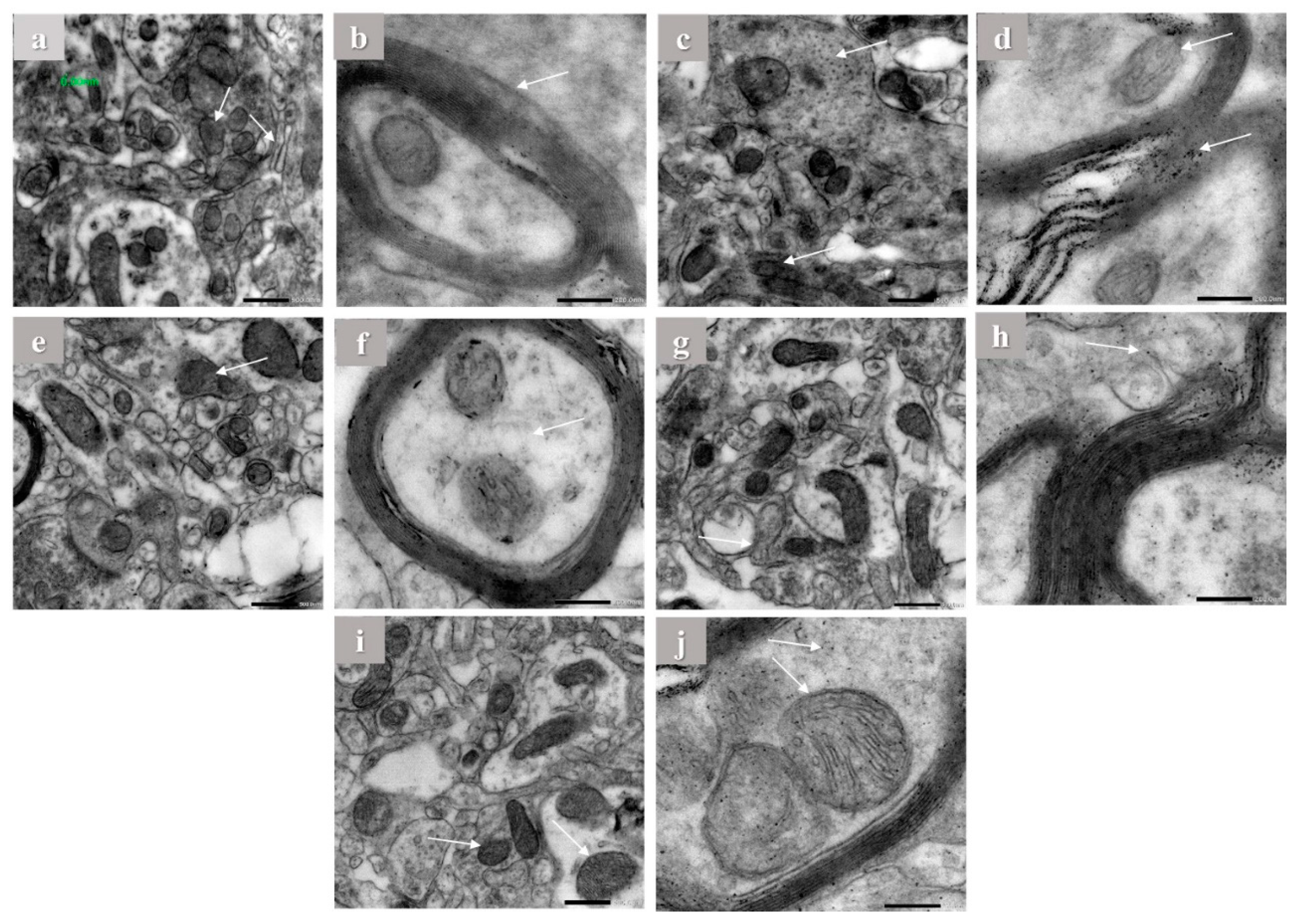
2.5. Electron Microscope Observations
Hippocampus of brain tissues of control, aluminum chloride, and treated groups were examined using electron microscope by the end of the experiment. The hippocampus of control rats showed normal mitochondrial configuration, Golgi apparatus, and asymmetric concave synapses without any abnormal structures as shown in (Figure 5a,b). On the other hand, it is clearly visible that aluminum chloride group showed more abnormal structures and organelles compared to other groups. This is indicated by flat and convex synapses, a concentric configuration of mitochondrial cristae, dilation of Golgi apparatus, and extra amyloid beta deposition compared to the normal group (Figure 5c,d). The donepezil-treated group showed concave synapses curvature and no more amyloid beta deposition appeared (Figure 5e,f). Furthermore, the gallic acid-treated group showed asymmetric synapses with concave curvature and slight amyloid beta deposition (Figure 5g,h). In addition, in the OPE 200-treated group, Golgi apparatus showed less dilation than in the induced group, less amyloid beta deposition and the treated group was mostly near to the control group, which showed some protective effect against aluminum chloride-induced abnormal structures as shown in (Figure 5i,j), which are consistent with the biochemical marker profile. Considering mitochondria length and width, the results showed that daily intraperitoneal injection of aluminum chloride significantly decreased the mitochondrial length from 435.67 ± 1.24 to 186.30 ± 0.65 nm and the mitochondrial width from 261 ± 1.00 to 157 ± 2 in control group comparing to the induced group (Table 5). Animals administered donepezil registered a significant increase in mitochondrial length and width as compared with the aluminum chloride treated group. Moreover, animal groups administered gallic acid and OPE 200 separately registered a significant change in mitochondrial length and width as compared with the donepezil-treated group (p < 0.05); the are results shown in (Table 5).
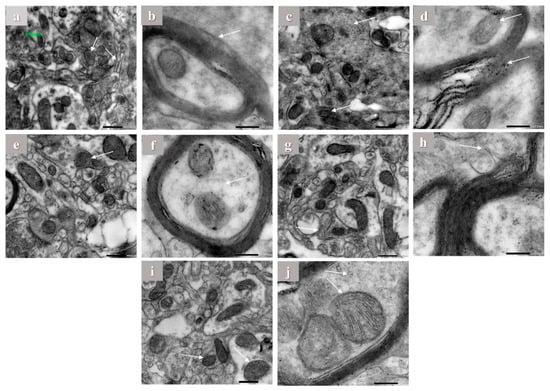
Figure 5.
The electron microscope imaging of the brain hippocampus of Aricept-, gallic-, caffeine-, OPE 100-, and OPE 200-treated groups. Normal control group showed normal Golgi apparatus architecture without dilation (a,b), induced group showed amyloid beta deposition in myelin sheath (c,d), Aricept protective group showed concave synapse curvature and no amyloid beta deposition appeared (e,f), OPE 200 protective group showed asymmetric synapses with concave curvature and no amyloid beta deposition (g,h), and gallic acid protective group showed Golgi apparatus showed less dilation than induced group (i,j).

Table 5.
Effect of OPE on brain (hippocampus) mitochondrial length and width (nm) of different experimental rat groups treated with aluminum chloride.
3. Discussion
3.1. Phytochemicals Content and HPLC Analysis
Polyphenols are bioactive plant secondary metabolites with many important functions. These include long-term protection from vascular illnesses, chemoprotective action, antioxidant, neuroprotective, and anti-inflammatory effects [24]. Numerous studies have been conducted, and more are planned, to determine the feasibility of recovering polyphenolic compounds from agri-food wastes as a high-value biorefining option [24]. Orange peel extract (OPE) contains a moderate concentration of polyphenols and flavonoids (Table 1); fractionation and identification of these polyphenols by HPLC reveals that the extract contains twelve different polyphenolic and flavonoid compounds ranged from 3388.60 to 47.08 (μg/100 g) for gallic acid and cinnamic acid, respectively (Table 2). The most prevalent polyphenol in OPE, gallic acid, was found to have beneficial effects in treating a variety of diseases and conditions, including those related to the gut, the brain, the metabolism, and the heart [15]. In addition, it is a known antioxidant substance with neuroprotective activities in several types of neurodegeneration, neurotoxicity, and oxidative stress [25]. Another polyphenol found in high concentrations in OPE, rutin, protects neurons from the neurodegenerative consequences of prion buildup by boosting neurotropic factor synthesis and blocking apoptotic pathway activation in neural cells [26]. In regard to naringenin, another polyphenol containing OPE, it has demonstrated a wide variety of therapeutic effects, primarily due to its antioxidant activity. They have a direct impact on scavenging free radicals, and they also interact with various signal transduction pathways involved in the redox response, which leads to an increase in the internal antioxidant defense system and promotes neuroprotection [27]. Additionally, ferulic acid has been shown to have neuroprotective benefits in Parkinson’s disease cell models by inducing autophagy [28]. Moreover, quercetin possess neuroprotective action by acting with different mechanisms on the microglial cells of CNS and influences microRNA expression in order to regulate the inflammation, differentiation, proliferation, apoptosis, and immune responses [29]. Many other polyphenols and flavonoids were present in OPE with different biological activity. The interactions between these bioactive compounds may greatly improve their therapeutic effects and reduce their negative effects [30]. It has been found that the pharmacological effects of many commonly used herbs are significantly enhanced when administered together [31]. Thus, using the OPE as a mixture of bioactive compounds was interesting and may display a new properties added for each individual compounds.
3.2. Characterization of OPE
Antioxidant and anti-acetylcholinesterase activities were investigated as a first step to evaluate the biological activities of OPE. The ABTS, DPPH, and hydroxyl radical scavenging assays are significant in vitro analyses for studying OPE’s antioxidant efficacy (Table 1). Both techniques (ABTS and DPPH) use decolorization assays to identify antioxidants that prevent the formation of ABTS radical cations and DPPH radicals [32]. Because both techniques result in the same chemical characteristic of H or electron donation to the antioxidant, ABTS activity was closely associated with DPPH in the majority of experiments to measure antioxidant properties [33]. Hydroxyl radicals are very reactive and transient [34]. Because of their extreme reactivity, hydroxyl radicals cause tremendous harm to cells and their individual components, which then affects organisms as a whole [35]. It has previously been shown that a number of flavonoids can scavenge OH radicals [36]. Since free radicals have a detrimental role in Alzheimer’s disease progression, radical scavenging activities are crucial [37]. The present study indicates the half maximal inhibitory concentration (IC50) values denoted the concentration of OPE required to scavenge 50% of free radicals. OPE’s potential efficacy in combating free radical-induced diseases is likely attributable to the presence of a number of phenolic compounds and flavonoids. According to previous results with donepezil, the standard AD treatment exhibited IC50 133 ± 4.5 μg/mL using DPPH assay [23]. In this study, OPE showed IC50 67.90 ± 1.05 μg/mL. Thus, OPE has antioxidant activity, which is absent in donepezil. The previous antioxidant result of OPE is promising, suggesting that OPE can be used as a natural antioxidant. The effectiveness of OPE and gallic acid in inhibiting AChE, an enzyme that is hyperactivated in AD, was also investigated in vitro to compare with the standard AChE inhibitor, donepezil. The inhibitory concentrations (IC50) of OPE and gallic acid were 0.87 ± 0.025 and 2.45± 0.001 (mg/mL), respectively (Table 1). Results reveal that the inhibitory effect of OPE did not depend on the presence of gallic acid only but the presence of gallic acid in combination with other compounds such as polyphenols or flavonoids. OPE contain ellagic acid (EA) at the concentration of 336.16 (μg/100 g), which may contribute the anti-acetylcholinesterase activity of the extract. According to another study, artichoke waste extract was found to have an IC50 for AChE inhibition of 5.705 ± 0.0157 mg/mL; this may be because of ellagic acid contents [23]. In addition, the presence of gallic acid and ellagic acid in the methanolic extract of Terminalia chebula resulted in considerable cholinesterase inhibition (IC50 = 180 ± 14.6 mg/mL) [38]. In further addition, ellagic acid inhibited AChE in an animal model of Alzheimer’s disease [39]. According to our results, OPE might express a good result in treating Alzheimer disease model by its potential antioxidant and anti-acetylcholinesterase activity.
One way to screen for drugs that will work on a certain biological target is by computational docking, in which test ligands are placed in a docking simulation and their binding mode to the target is predicted [40]. Variable hydrogen binding sites were predicted using in silico docking investigations of donepezil, gallic acid, and acetylcholine against human acetylcholinesterase (PDB code: 4EY7) (Table 3; Figure 1). These findings show that gallic acid, when compared to the native substrate (acetylcholine) and donepezil, exhibited a lower binding energy. More research is needed to confirm that OPE, rather than its individual polyphenols, has anti-AChE activity in vivo.
3.3. The Possible Protective Effect of OPE against AD Rat Model Induced by Aluminum Chloride Injection
Aluminum chloride injection produced severe decline in cognitive capabilities and related symptoms of neurodegeneration in the brain. Furthermore, altered mitochondrial function presented, which might underlie neuronal dysfunction in AD [41]. In the current study, daily i.p. injection of aluminum chloride for 6 weeks produced severe decline in cognitive capabilities and related symptoms of neurodegeneration in the brain, including increased both gene expression and activity of AChE and Aβ42 levels. Aluminum chloride administration also caused oxidative damage (elevation in TBARS, NO, and decrease of reduced GSH level and reduce activity of the antioxidant enzyme). Furthermore, aluminum chloride produced downregulation of the amyloidogenic-related genes PSEN-2, BCL2, and upregulation of APP and BACE-1 gene expressions. Aluminum is a cholinotoxin, according to the literature, because it disrupts the cholinergic system [42]. It has been reported that aluminum chloride cholinotoxin activity in rats induces senile dementia of Alzheimer’s in animal models [43]. Its neurotoxic effects could be explained by many reported mechanisms, such as Aβ deposition in neurons. Additionally, stimulating AChE activity in the neuritic plaques and neurofibrillary tangles around regions [44]. Aluminum chloride injection at dose of 70 mg/kg for 6 weeks exerts significant effect on biochemical and behavioral than the dose of 50 mg/kg [43]. Thus, the present dose of aluminum chloride used was 70 mg/kg daily for 6 weeks. It has been reported that gallic acid (GA) has a protective role in Alzheimer’s disease (AD) and neurodegeneration at dosages equivalent to 20 mg/kg bw [45]. Antioxidant [46] and neuroprotective properties of purified GA were reported in multiple scientific studies [47,48]. The present study is the first to deal with OPE as a source of gallic acid with other compounds mixed that may play a role in AD disease.
3.4. Effect of Donepezil, Gallic Acid, and OPE Treatment on Brain Aβ42 Level, TC, and Phospholipids in the Brain
The amyloid cascade hypothesis proposed that the key clinical hallmarks of Alzheimer’s disease were extracellular deposits of A peptides as senile plaques, intraneuronal neurofibrillary tangles (NFTs), and extensive neuronal death [49,50]. Therefore, Aβ peptides have dominated new therapeutic research over the past twenty years as a possible target for AD [51]. Thus, Aβ42 level in rat’s brain was an important marker to evaluate [52]. Aluminum chloride injection for 6 weeks resulted in a significant 3.6-fold increase in Aβ42 level in rat’s brain compared to control (Table 4). Aβ42 has been reported to bind with the membrane negatively charged phospholipids, leading to conformational change of other nearby lipids leading to membrane disruption and cell death [53]. On the other hand, oral administration of OPE (200 mg/kg) before aluminum chloride injection significantly reduced the level of Aβ42 by 40.04% compared to those of the aluminum chloride-treated group (p < 0.05). In the case of administration of gallic acid at the concentration of 50 mg/kg the Aβ42 deposition significantly decreased compared to the aluminum chloride-treated group by 27.73%. Gallic acid is one of many chemicals found in tea extracts that have been demonstrated to have anti-amyloid characteristics, and it has been linked to the positive health effects [54]. This common polyphenol is thought to not only mediate oxidative stress and inflammation, but also to directly prevent the development of amyloid fibrils, both of which are major contributors to neurodegenerative disorders [55,56]. Many other detected polyphenols such as naringenin may influence the OPE results. Pretreatment with naringenin reduces Aβ-induced impairment of learning and memory by inhibiting lipid peroxidation and apoptosis, as shown by Ghofrani et al. [57]. One of the metabolites of curcuma is ferulic acid, which can be found in OPE. In addition to being able to scavenge reactive oxygen species, it has been shown to have neuroprotective properties by interacting directly with Aβ mature fibrils, which may lead to the disintegration of these aggregations [58,59]. Moreover, quercetin destabilizes preformed mature Aβ fibrils by strengthening the hydrophobic interaction between the phenyl rings and the β-sheet structures of Aβ [60,61].
Aluminum chloride injection increased the brain’s total cholesterol (TC) twofold and reduced the level of brain phospholipids to near half and also increased the TC/phospholipids ratio 4.71-fold as compared to control (Table 4). This adverse effect was reversed by oral intake of donepezil, gallic acid, and OPE. Oral administration of OPE restored the brain TC and phospholipids to near normal control values. The biochemical evidence is overwhelming that hypercholesterolemia is an important causal factor in the pathogenesis of AD. The biochemical evidence is overwhelming that hypercholesterolemia is an important causal factor in the pathogenesis of AD; moreover, cholesterol intracellularly influences Alzheimer’s disease APP synthesis and amyloid-beta formation [62]. A critical reason that Aβ aggregation is modulated by changes in cholesterol levels in the brain, which results in both β- and γ-secretase complexes located in cholesterol-rich lipid rafts of the plasma membrane in the brain; depleting cellular cholesterol as one of the therapeutic mechanism appears to inhibit BACE-1 enzyme [63]. Consistent with prior findings, aluminum chloride injection significantly increased Aβ42 levels through boosting cholesterol and lowering phospholipid levels in brain tissue of this generated model. Measuring of TC and phospholipid levels determine the mechanism of OPE and gallic acid protective mechanism against AD.
3.5. Effect of Donepezil, Gallic Acid, and OPE Treatment on Oxidative Stress Parameters in Brain
Many prooxidant markers were assessed in order to determine the effect of OPE and their polyphenol GA, regarding to TBARS, there were significant decreases in its levels in groups treated with donepezil, gallic acid, and OPE compared to the AD-induced group. There were no significant changes in TBARS levels between donepezil and OPE 100-treated groups. On the other hand, NO level showed significant decrease in groups treated with donepezil, gallic acid, and OPE compared to the AD-induced group (Table 4). The current results indicated that aluminum chloride increased the oxidative stress significantly (NO and TBARS) throughout the brain in agreement with Yuan et al. [64]. Groups treated with donepezil, gallic acid, and OPE exhibited a significant elevation in the antioxidant state in the rat’s brain via increase the level of GSH near to normal levels (Table 4). Aluminum chloride has also been found to disrupt metabolism, especially for antioxidants of low molecular weight such as glutathione, and thus exacerbate the rate of lipid peroxidation in the brain [65].
3.6. Effect of Donepezil, Gallic Acid, and OPE Treatment on Brain Antioxidant Enzymes
Results of brain antioxidant enzymes (GST, GPx, and SOD) in Figure 2 demonstrated that donepezil, gallic acid, and OPE treatment restores the antioxidant enzyme activity in normal levels near to the control values. It has been reviewed that in order to maintain normal neuronal function, reactive nitrogen species (RNS) and reactive oxygen species (ROS) are required [66]. Brain tissue is particularly vulnerable to oxidative stress due to high levels of oxygen (20% of the body’s oxygen), metals, polyunsaturated fatty acids and low antioxidant capacity [67]. So, improving the antioxidant state by administration of natural extract such as OPE could be helping to prevent the disease development or progression of AD.
3.7. Brain AChE Activity and Its Gene Expression Level on Brain (Hypothalamus)
In the present study, acetylcholinesterase activity and gene expression were investigated in brain hypothalamus (Figure 3). Aluminum chloride injection at 70 mg/kg increased AChE activity 1.65-fold over the control group and significantly upregulated the mRNA expression level of AChE as compared to the control group (1.706 ± 0.11 vs. 1.000 ± 0.17). The present data together with the Kaizer report indicated that aluminum chloride produced severe cholinergic neuronal dysfunction deficits as well as increased AChE activity and hypothalamic AChE expression, which could explain the brain neurodegeneration [68]; it may then evaluate the effects of oral administration of donepezil (2.5 mg/kg), gallic acid (50 mg/kg), OPE (100 mg/kg), and OPE (200 mg/kg) before aluminum chloride injection on AChE activity and correlate these activities with their anti-AD effect. A previous study reported that prolonged administration of donepezil (Aricept®, 1.5 mg/kg orally, twice daily for 21 days) inhibited AChE activity and increase the ACh concentration in rats’ brains [69]. The obtained results from the in vivo Alzheimer study, regarding AChE activity and AchE gene expression, explained the obtained results from the in vitro anti-acetylcholinesterase activity and docking studies.
3.8. Brain (Hippocampus) Gene Expression
Numerous human diseases are characterized by alterations in gene expression, which have been successfully exploited to anticipate molecular and cellular pathways linked with pathological processes [70]. In the brain, the single-pass transmembrane protein amyloid precursor protein (APP) is rapidly and digested by a number of successive proteases, including the intramembranous γ-secretase complex [71]. Alternative cleavage of the APP by β-secretase (BACE1) and the γ-secretase complex lead to production of Aβ species, e.g., Aβ38, Aβ40, and Aβ42 [72]. From all the previous facts, APP gene expression level was estimated in this study to follow the possible mechanism of OPE and its polyphenol gallic acid in neural action and brain protection; animal groups administered donepezil, gallic acid, and OPE registered a significant decrease in the mRNA expression levels of APP as compared with the aluminum chloride-treated group. The OPE 200-treated group showed nonsignificant APP gene expression level with the donepezil-treated group. In addition, OPE 200 expressed a significant decrease in APP gene expression level compared to the control group (p < 0.05) (Figure 4). Since β-secretase (BACE-1) cleavage is a bottleneck for Aβ42 production, it is a promising therapeutic target for the development of inhibitors that reduce Aβ42 [73]. The pharmaceutical industry has concentrated on BACE1 inhibitors as a therapeutic strategy for AD to block the first stage of amyloid development [74]. However, in clinical trials, BACE1 inhibitors have failed due to potential side effects or lack of beneficial cognitive outcomes [75]. In this study, results showed that aluminum chloride injection caused a significant increase in BACE1 gene expression level when compared to the control group (Figure 4). There were no significant differences in BACE1 gene expression level between donepezil (2.5 mg/kg), gallic acid (50 mg/kg), as well as OPE 100. However, oral treatment by OPE 200 exhibited a more significant decrease BACE1 gene expression than donepezil (2.5 mg/kg), gallic acid (50 mg/kg), as well as OPE 100 treated groups. From the above results, the treatment with gallic acid and OPE contribute to Aβ42 production through the modulation of amyloid precursor protein (APP) and the attenuation of Aβ42 generator enzyme (BACE1) gene expressions. Amyloid beta peptide induces neuronal apoptosis and contributes to the pathophysiology of AD. Key apoptosis proteins such as Bax, Bcl-2, and cytochrome c have been demonstrated to regulate the triggered apoptotic pathway involving mitochondria [76]. Our results showed that aluminum chloride induced apoptosis via down regulate of BCL-2 gene expression that in agreement with Justin et al. [77] who use aluminum chloride at dose of 100 mg/kg. mRNA expression levels of BCL-2 in donepezil-, gallic acid-, OPE 100-, and OPE 200-treated groups showed significant 1.34-, 3.35-, 3.52-, and 4.64-fold increases, respectively, comparing to the control group (p < 0.05) (Figure 4). The relationship between the increase in the BCL-2 gene expression levels of OPE- and gallic acid-treated groups were much higher compared to the donepezil-treated group within the used doses. These are considered good results in the treatment of Alzheimer’s disease, as evidenced by the APP and BACE1 gene expression results shown above. This early onset of the inherited form of Alzheimer’s disease has been linked to a lack of PSEN-2 function caused by mutation, which leads to incomplete digestion of the amyloid peptide and may contribute to increased vulnerability in the brain [78]. Oral administration of donepezil (2.5 mg/kg), gallic acid (50 mg/kg), OPE 100 (100 mg/kg), and OPE 200 (200 mg/kg) showed significant upregulation in the PSEN-2 gene expression comparing to the aluminum chloride-induced model (Figure 4). The findings in the present study are consistent with previous studies, which reported that quercetin doses (25 and 50 mg/kg) that were orally administered for 28 days inhibited the amyloidogenic pathway via suppression of APP, BACE1, APH1, and PSEN1 gene expression levels in the hippocampus [79]. From our previous results, the positive impact of OPE treatment on reversing aluminum-induced AD was confirmed through protein and gene analysis.
3.9. Electron Microscope
Neurodegenerative diseases have a vicious cycle of cell death caused by oxidative stress and mitochondrial malfunction [80]. Both Aβ42 and APP have been found to localize to mitochondrial membranes, where they are thought to interact with mitochondrial proteins, block the transport of nuclear-encoded mitochondrial proteins to mitochondria, disrupt the electron transport chain, increase production of reactive oxygen species, damage mitochondria, and ultimately impair neuronal function [81]. Recent studies demonstrated that altered mitochondrial function might underlie neuronal dysfunction in AD [82]. Accordingly, in order to investigate the mitochondrial ultrastructures and possible mechanical changes, electron microscopic studies were assessed. Administration of aluminum chloride for 6 weeks resulted in ultrastructure alterations in the hippocampus (Figure 5), such as rupture of the mitochondrial membrane and a concentric configuration of mitochondrial cristae. Synaptic loss is the best correlate of cognitive dysfunction in AD [83]. The present transmission electron microscopy showed morphological abnormalities including flat and convex curvatures of synapses. These findings are in line with the idea that mitochondrial damage in both the pre- and post-synaptic regions contributes to age-related synaptic loss. As the number of asymmetric synapses with concave curvature declined with age, the number of asymmetric synapses with flat and convex curvatures increased [84]. However, gallic acid was studied in vivo before at 100 mg/kg bw [15]. However, gallic acid in OPE acts perfectly compared to pure gallic acid. By analyzing the in silico, in vitro, and in vivo results, we can conclude that crude OPE can used as a treatment for Alzheimer’s more effectively than gallic acid and donepezil alone.
4. Material and Methods
4.1. Plant Materials
Orange fruits were collected from an Egyptian market in April 2019. Clean orange peels were dried on a well-ventilated burner at 50 degrees Celsius, then ground into a powder using a mechanical grinder and frozen at −20 degrees Celsius for later use.
4.2. Preparation of Orange Peel Extract
Conventional Extraction (CE)
Approximately 20 g of dried powdered orange peel was added to 200 mL of distilled water and left to ferment at 37 degrees Celsius for three days. According to Awad et al. [85], the extract was filtered, condensed using a rotary machine, lyophilized using a freeze-dryer, and then stored at −20 °C until further analysis was performed.
4.3. Phytochemical Analysis
4.3.1. Total Phenolic Content (TPC)
Using the Folin–Ciocalteu spectrophotometric method, the total phenolic content (TPC) of orange peels extract was calculated, and the results were presented as microgram gallic acid equivalents (GAE) per milligram (mg) of dry extract [86].
4.3.2. Total Flavonoid Assay (TFC)
The aluminum chloride colorimetric method was used to determine the total flavonoid concentration [87]. The total flavonoid concentration was reported as μg quercetin (Q) equivalent per milligram (mg) of dry extract
4.4. HPLC Polyphenol Identification of Orange Peels Extract
Orange peel extract was subjected to HPLC for identification of its polyphenols and flavonoids, according to Tomaino et al. [88], using an Agilent 1260 series. Results for the major phenolic components were reported in units of micrograms per 100 g of extract.
4.5. Characterization of Orange Peels Extract
4.5.1. Antioxidant Activity
The antioxidant activity of the OPE was assayed using DPPH, ABTS, and hydroxyl radical scavenging assays. The DPPH% free-radical assay was carried out according to Bandoniene et al. [89]. For the ABTS method, the ability to scavenge free ABTS radicals was applied based on a published protocol Re et al. [90]. Scavenging tests for hydroxyl radicals were performed using the Fenton reaction according to Ozyürek et al. [91].
4.5.2. Anticholinesterase Potentials
In vitro anticholinesterase potentials of citrus peel extract and gallic acid was carried out according to Abd El-Aziz et al. [23]. All the reactions were performed in six replicates.
4.6. Molecular Docking Studies
The most common polyphenol through HPLC analysis were further subjected to molecular docking. Donepezil served as the reference standard. All docked ligands were drawn as 3D structures in ACD/ChemSketch. The ligand was docked into AChE’s stable binding pocket. Protein Data Bank provided the 3D structure of AchE (PDB code: 4EY7) [92] and molecular docking studies were performed using AutoDockTools-1.5.6rc3, Cygwin 64 Terminal, and Chimera 1.8.1.
4.7. In Vivo Experiment of OPE Anti-Alzheimer Potentials
4.7.1. Experimental Design
Thirty-six mature male albino rats (weighing 100–110 g) were obtained from the National Research Center in Alexandria (Egypt). To develop learning and memory deficits, male albino rats were intraperitoneally (ip) injected at 70 mg/kg body weight with aluminum chloride (AlCl3·6H2O), dissolved in physiological saline solution, once a day for 6 weeks [93]. Normal animals separately received saline as vehicles. The rats were randomly divided into six groups as follows:
Control group (n = 6) received saline solution (0.5 mL/day, i.p) for only 6 weeks. Induced group (n = 6) received AlCl3·6H2O (70 mg/kg, ip) dissolved in saline for 6 weeks. Gallic acid protective group (n = 6) received gallic acid (50 mg/kg, oral) dissolved in normal saline, followed by AlCl3·6H2O (70 mg/kg, ip) after 60 min for 6 weeks [94]. Orange peel protective group (n = 6) received OPE (100 mg/kg, oral) dissolved in normal saline, followed by AlCl3·6H2O (70 mg/kg, ip) after 60 min for 6 weeks. Orange peel protective group (n = 6) received OPE (200 mg/kg, oral) dissolved in normal saline, followed by AlCl3·6H2O (70 mg/kg, ip) after 60 min for 6 weeks. Donepezil (Aricept)-treated group (n = 6) received Donepezil (2.25 mg/kg, oral) dissolved in saline, followed by AlCl3·6H2O (70 mg/kg, ip) after 60 min. Donepezil, a centrally acting cholinesterase inhibitor, was used as a positive control. Donepezil hydrochloride was obtained as 10 mg tablets and administered in doses of 2.25 mg/kg based on previous study of Haug et al. [95] (Aricept ®, Pfizer Egypt, S.A.E. Cairo, A.R.E. Under authority of Pfizer INC. USA).
After six-week treatment, rats were subjected for body weight then scarified. Brain tissue was homogenized at 10% 0.1 M phosphate buffer saline (PBS) at a pH of 7.4, and the mixture was centrifuged at 10,000× g for 20 min at 4 degrees Celsius. Protein level was measured by the method of Bradford et al. [96] for the supernatant. The standard was 1 mg/mL of bovine serum albumin (BSA). Furthermore, samples from brain hypothalamus and hippocampus washed by normal saline and stored in −80 °C for total RNA isolation and quantitative RT-PCR analysis. Electron microscope samples were prepared from brain hippocampal specimens according to Pejman et al. [97]. Briefly, semi-thin sections were made from the blocks. After toluidine blue observation, ultrathin brain slices (70–80 nm thick) were cut using a diamond knife, placed on 200 mesh copper grids, and stained for 15 min with 4% aqueous uranyl acetate, then for 8 min with Reynolds’ lead citrate. Transmission electron microscopy was used to examine the specimens (JEOL—JSM-1400 PLUS).
4.7.2. Biochemical Parameters in Brain Homogenate
Malondialdehyde (MDA), a prominent byproduct of lipid peroxidation, was quantified spectrophotometrically by the thiobarbituric acid (TBA) reaction [98]. The level of Nitric oxide (NO) was determined using the Montgomery and Dymock [99] method. Reduced glutathione (GSH) content was determined as described previously [100]. The results of MDA, NO, and GSH were expressed as micromolars per gram of protein. The activity of Glutathione peroxidase (GPx) was measured as described previously [101]. The Glutathione S-transferases (GSTs) activity was determined as described previously [102]. The enzyme activity of GPx and GST were expressed as units per gram of protein. The activity of Superoxide dismutase (SOD) was assayed according to Marklund et al. [103]. The levels of total cholesterol and phospholipids were measured using an assay kit (Biosystems S.A., Spain and Bio-diagnostics, Egypt) (Biosystems S.A., Spain and Bio-diagnostic, Egypt) according to the manufacturer’s protocols. Acetylcholinesterase (AChE) activity was assayed as described by Ellman et al. [104]. The amount of acetylthiocholine iodide in micromoles hydrolyzed per minute per milligram of protein was used as one unit of AChE activity. AChE specific activity is measured in terms of units per gram of protein. The amyloid beta peptide 42 (Aβ42) level was measured in brain homogenate using the quantitative sandwich enzyme immunoassay technique (rat Aβ peptide 1–42 ELISA Kit CUSABIO, China) according to the manufacturer’s instructions.
4.7.3. Hippocampal and Hypothalamic Tissue Separation and Total RNA Purification for qRT-PCR Analysis
Each rat was decapitated, and then their hippocampi and hypothalamuses were swiftly removed and frozen at −80 degrees Celsius for later use. Single-step RNA isolation using acid guanidinium thiocyanate–phenol–chloroform extraction was used for the manual extraction of RNA [105]. In order to perform quantitative real-time PCR, synthesized cDNA was combined with Power SYBR Green PCR Master Mix (Applied Biosystems) and a gene-specific primer (Bioneer, Daejeon, Korea), as seen in Table 6. Different real-time PCR reactions were performed for the target and GPDH (Rotor—Gene Q, QIAGEN Hilden, Germany). The conditions used were as follows: A denaturation step at 95 °C for 10 min was followed by 40 cycles of annealing at Tm [°C] for 15 s, followed by an extension step at 72 °C for 60 s. Step One Software calculated the detection threshold cycle number (Life Technologies). Each target’s mRNA expression was normalized to the expression of the GPDH gene and expressed as a fold change from the controls. There were three technical replicates for each sample. Using the the 2−∆∆Ct technique, the fold changes in gene expression were measured [106].

Table 6.
Primers nucleotides sequence used in this study.
4.8. Statistical Analysis of the Data
IBM SPSS version 16.0 was used for data analysis, which involved entering the data into a computer and processing it [107]. We used mean and standard deviation to characterize the quantitative data. The F-test (ANOVA) and Post Hoc test (Tukey) were used to compare the groups, while the paired t-test was employed to examine the two paired data. All results were considered statistically significant at the 5% level [108].
4.9. Ethical Approval
The procedures and protocols were used in the present research were approved by the Ethical Committee of Alexandria University (Protocol approval number: AU 04190727201). Animals were housed in a well-ventilated room on wood shavings polypropylene animal cages, four per cage, with free access to water and a standard chow diet. They were kept under standard environmental conditions of 22–25 °C, 12 h light/dark cycle. All the animals’ procedures and care were performed in accordance with the Guide for the Care and Use of Laboratory Animals [8].
5. Conclusions
It is commonly known that nutrition has a crucial influence in the onset and progression of non-communicable diseases. Orange peel extract (OPE) has many applications in food and disease treatment. In this study, in vitro and in silico analysis of OPE and derived gallic acid toward acetylcholinesterase showed an interested result. Moreover, OPE protects brain tissue by decreasing oxidative stress. The anti-Alzheimer’s disease properties of OPE include the inhibition of brain acetylcholinesterase activity and gene expression. Moreover, there was evidence of a decrease in Aβ42 levels and APP, BACE1 gene expression, and, furthermore, an increase in BCL-2 and PSEN-2 gene expression. Orange peel extract treatment against an aluminum chloride-induced AD model exhibited a promising results in the level of measured biochemical, molecular, and structural observations. Therefore, OPE may be formulated in certain kinds of dietary supplements, which may help in case of AD and other neurodegenerative disorders.
Author Contributions
Conceptualization, N.M.A.E.-A., M.G.S., H.S.A. and S.A.E.-S.; methodology, N.M.A.E.-A., M.G.S., M.R.E. and A.N.B.; validation, N.M.A.E.-A., M.R.E. and M.G.S.; investigation, N.M.A.E.-A., M.G.S., T.A., M.R.E., H.S.A. and A.N.B.; data curation, N.M.A.E.-A., M.G.S., M.R.E., T.A., S.A.E.-S. and A.N.B.; writing—original draft preparation, N.M.A.E.-A., M.G.S., M.R.E., T.A., S.A.E.-S. and A.N.B. All authors have read and agreed to the published version of the manuscript.
Funding
The Researchers Supporting Project number (RSPD2023R641), King Saud University, Riyadh, Saudi Arabia, funded this research.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of the City of Scientific Research and Technological Applications (Protocol approval number: IACUCs/IACUC# 33-1Z-0521) and date of approval: 10 October 2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors extend their appreciation to Researchers Supporting Project number (RSPD2023R641), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tang, Y.; Zhang, D.; Gong, X.; Zheng, J. A mechanistic survey of Alzheimer’s disease. Biophys. Chem. 2022, 281, 106735. [Google Scholar] [CrossRef]
- Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2021, 17, 327–406. [Google Scholar]
- World Alzheimer Report. Life after Diagnosis: Navigating Treatment, Care and Support, 2022. Available online: https://www.alzint.org/u/World-Alzheimer-Report-2022.pdf (accessed on 18 November 2022).
- Akwa, Y. Steroids and Alzheimer’s Disease: Changes Associated with Pathology and Therapeutic Potential. Int. J. Mol. Sci. 2022, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, K.; Liu, F.; Gong, C.X. Tau and neurodegenerative disease: The story so far. Nat. Rev. Neurol. 2015, 12, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Du, H. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer’s disease. Nat. Med. 2008, 14, 1097–1105. [Google Scholar] [CrossRef]
- Tilokani, L.; Nagashima, S.; Paupe, V.; Prudent, J. Mitochondrial dynamics: Overview of molecular mechanisms. Essays Biochem. 2018, 62, 341–360. [Google Scholar]
- Khan, K.A.; Kumar, N.; Nayak, P.G.; Nampoothiri, M.; Shenoy, R.R.; Krishnadas, N.; Rao, M.; Mudgal, J. Impact of caffeic acid on aluminium chloride-induced dementia in rats. J. Pharm. Pharmacol. 2013, 65, 1745–1752. [Google Scholar] [CrossRef]
- Nie, J. Exposure to aluminum in daily life and Alzheimer’s disease. Adv. Exp. Med. Biol. 2018, 1091, 99–111. [Google Scholar]
- Kawahara, M.; Kato-Negishi, M. Link between aluminum and the pathogenesis of Alzheimer’s disease: The integration of the aluminum and amyloid cascade hypotheses. Int. J. Alzheimers Dis. 2011, 2011, 276393. [Google Scholar] [CrossRef]
- McLachlan, D.R. Aluminium in Neurological and Neurodegenerative Disease. Mol. Neurobiol. 2019, 56, 1531–1538. [Google Scholar] [CrossRef]
- Cummings, J.; Aisen, P.; Apostolova, L.G.; Atri, A.; Salloway, S.; Weiner, M. Aducanumab: Appropriate use recommendations. J. Prev. Alzheimer’s Dis. 2021, 4, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Raschetti, R.; Albanese, E.; Vanacore, N.; Maggini, M. Cholinesterase inhibitors in mild cognitive impairment: A systematic review of randomised trials. PLoS Med. 2007, 4, e338. [Google Scholar] [CrossRef] [PubMed]
- Colizzi, C. The protective effects of polyphenols on Alzheimer’s disease: A systematic review. Review Article. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2019, 5, 184–196. [Google Scholar] [CrossRef]
- Ogunlade, B.; Adelakun, S.A.; Agie, J.A. Nutritional supplementation of gallic acid ameliorates Alzheimer-type hippocampal neurodegeneration and cognitive impairment induced by aluminum chloride exposure in adult Wistar rats. Drug Chem. Toxicol. 2020, 45, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Nour, Y.; Majed, J.; Nawal, A.; Chantal, M. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar]
- Montone, A.M.I.; Papaianni, M.; Malvano, F.; Capuano, F.; Capparelli, R.; Albanese, D. Lactoferrin, Quercetin, and Hydroxyapatite Act Synergistically against Pseudomonas fluorescens. Int. J. Mol. Sci. 2021, 22, 9247. [Google Scholar] [CrossRef]
- Awad, A.M.; Kumar, P.; Ismail-Fitry, M.R.; Jusoh, S.; Aziz, M.F.; Sazili, A.Q. Overview of plant extracts as natural preservatives in meat. J. Food Process. Preserv. 2022, 46, 8. [Google Scholar] [CrossRef]
- Jalilzadeh-Afshari, A.; Fadaei, V. Characterization of flavored milk containing bitter orange peel extract and Gaz-angubin. Food Sci. Nutr. 2020, 9, 164–171. [Google Scholar] [CrossRef]
- Shehata, M.G.; Awad, T.S.; Asker, D.; El Sohaimy, S.A.; Abd El- Aziz, N.M.; Youssef, M.M. Antioxidant and antimicrobial activities and UPLC-ESI-MS/MS polyphenolic profile of sweet orange peel extracts. Curr. Res. Nutr. Food Sci. 2021, 4, 326–335. [Google Scholar] [CrossRef]
- Rafiq, S. Citrus peel as a source of functional ingredient: A review. J. Saudi Soc. Agric. Sci. 2018, 17, 351–358. [Google Scholar] [CrossRef]
- Escobedo-Avellaneda, Z.; Gutiérrez-Uribe, J.; Valdez-Fragoso, A.; Torres, J.A.; Welti-Chanes, J. Phytochemicals and antioxidant activity of juice, flavedo, albedo and comminuted orange. J. Funct. Foods 2014, 6, 470–481. [Google Scholar] [CrossRef]
- Abd El-Aziz, N.M.; Awad, O.M.; Shehata, M.G.; El-Sohaimy, S.A. Antioxidant and anti-acetylcholinesterase potential of artichoke phenolic compounds. Food Biosci. 2021, 41, 101006. [Google Scholar] [CrossRef]
- Makris, D.P.; Sahin, S. Polyphenolic Antioxidants from Agri-Food Waste Biomass. Antioxidants 2019, 8, 624. [Google Scholar] [CrossRef]
- Daglia, M.; Di Lorenzo, A.; Nabavi, S.F.; Talas, Z.S.; Nabavi, S.M. Polyphenols: Well beyond the antioxidant capacity: Gallic acid and related compounds as neuroprotective agents: You are what you eat! Curr. Pharm. Biotechnol. 2014, 15, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Na, J.Y.; Kim, S.; Song, K.; Kwon, J. Rutin alleviates prion Peptide-induced cell death through inhibiting apoptotic pathway activation in dopaminergic neuronal cells. Cell. Mol. Neurobiol. 2014, 34, 107. [Google Scholar] [CrossRef]
- August, P.M.; Gindri dos Santos, B. Chapter 20—Naringin and naringenin in neuroprotection and oxidative stress. In Oxidative Stress and Dietary Antioxidants in Neurological Diseases; Academic Press: Cambridge, MA, USA, 2020; pp. 309–323. [Google Scholar]
- Long, T. Ferulic Acid Exerts Neuroprotective Effects via Autophagy Induction in C. elegans and Cellular Models of Parkinson’s Disease. Oxidative Med. Cell. Longev. 2022, 2022, 3723567. [Google Scholar] [CrossRef]
- Benameur, T.; Soleti, R.; Porro, C. The Potential Neuroprotective Role of Free and Encapsulated Quercetin Mediated by miRNA against Neurological Diseases. Nutrients 2021, 13, 1318. [Google Scholar] [CrossRef] [PubMed]
- Jankobek, J.; Seruga, M.; Krivak, P. The influence of interactions among phenolic compounds on the antiradical activity of chokeberries (Aronia melanocarpa). Int. J. Food Sci. Nutr. 2011, 62, 345–352. [Google Scholar] [CrossRef]
- Altunkaya, A.; Becker, E.M.; Gokmen, V.; Skibsted, L.H. Antioxidant activity of lettuce extract (Lactuca sativa) and synergism with added phenolic antioxidants. Food Chem. 2009, 115, 163–168. [Google Scholar] [CrossRef]
- Ilyasov, I.R.; Beloborodov, V.L.; Selivanova, I.A.; Terekhov, R.P. ABTS/PP Decolorization Assay of Antioxidant Capacity Reaction Pathways. Int. J. Mol. Sci. 2020, 21, 1131. [Google Scholar] [CrossRef]
- Ismail, A.; Marjan, Z.M.; Foong, C. Total antioxidant activity and phenolic content in selected vegetables. Food Chem. 2004, 87, 581–586. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; Al Nashef, I.M. Superoxide ion: Generation and chemical implications. Chem. Rev. 2016, 116, 3029–3085. [Google Scholar] [CrossRef] [PubMed]
- Dizdaroglu, M.; Jaruga, P. Mechanisms of free radical-induced damage to DNA. Free Rad. Res. 2012, 46, 382–419. [Google Scholar] [CrossRef] [PubMed]
- Treml, J.; Šmejkal, K. Flavonoids as potent scavengers of hydroxyl radicals. Compr. Rev. Food Sci. Food Saf. 2016, 15, 720–738. [Google Scholar] [CrossRef]
- Lopa, S.S. Phytochemical Analysis and Cholinesterase Inhibitory and Antioxidant Activities of Enhydra fluctuans Relevant in the Management of Alzheimer’s Disease. Int. J. Food Sci. 2021, 2021, 8862025. [Google Scholar] [CrossRef]
- Naik, G.H. In vitro antioxidant studies and free radical reactions of triphala, an ayurvedic formulation and its constituents. Phytother. Res. 2005, 19, 582–586. [Google Scholar] [CrossRef]
- Tancheva, L. New mechanisms in preventive effect of ellagic acid on cognition in mice with Alzheimer’s disease type dementia. Bulg. Chem. Commun. 2017, 50, 20–24. [Google Scholar]
- Mohan, V.; Gibbs, A.C.; Cummings, M.D.; Jaeger, E.P.; DesJarlais, R.L. Docking: Successes and challenges. Curr. Pharm. Des. 2005, 11, 323–333. [Google Scholar] [CrossRef]
- Kumar, S.E.P. Amelioration of aluminium chloride (AlCl3) induced neurotoxicity by combination of Rivastigmine and Memantine with Artesunate in Albino wistar rats. Biomed. Pharmacol. J. 2019, 12, 703–711. [Google Scholar] [CrossRef]
- Capriello, T.; Grimaldi, M.C.; Cofone, R.; D’Aniello, S.; Ferrandino, I. Effects of aluminium and cadmium on hatching and swimming ability in developing zebrafish. Chemosphere 2019, 222, 243–249. [Google Scholar] [CrossRef]
- Inneh, C.; Eiya, B. Anticholinesterase activity and Antioxidant Effect of Vitamin E in Aluminum Chloride Induced Toxicity in Drosophila Melanogaster. FASEB J. 2022, 36. [Google Scholar] [CrossRef]
- Dey, M.; Singh, R.K. Neurotoxic effects of aluminium exposure as a potential risk factor for Alzheimer’s disease. Pharmacol. Rep. 2022, 74, 439–450. [Google Scholar] [CrossRef]
- Mori, T.; Koyama, N.; Yokoo, T.; Segawa, T.; Maeda, M.; Sawmiller, D.; Tan, J.; Town, T. Gallic acid is a dual α/β-secretase modulator that reverses cognitive impairment and remediates pathology in Alzheimer mice. J. Biol. Chem. 2020, 295, 16251–16266. [Google Scholar] [CrossRef] [PubMed]
- Ferk, F. Gallic acid improves health-associated biochemical parameters and prevents oxidative damage of DNA in type 2 diabetes patients: Results of a placebo-controlled pilot study. Mol. Nutr. Food Res. 2018, 62, 1700482. [Google Scholar] [CrossRef] [PubMed]
- Choubey, S. Medicinal importance of gallic acid and its ester derivatives: A patent review. Pharm. Pat. Anal. 2015, 4, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Hajipour, S. Effect of gallic acid on dementia type of Alzheimer’s disease in rats: Electrophysiological and histological studies. Basic Clin. Neurosci. 2016, 7, 97–106. [Google Scholar] [PubMed]
- Uddin, M.S. Revisiting the Amyloid Cascade Hypothesis: From Anti-A_ Therapeutics to Auspicious NewWays for Alzheimer’s Disease review. Int. J. Mol. Sci. 2020, 21, 5858. [Google Scholar] [CrossRef]
- Volloch, V.; Rits-Volloch, S. The Amyloid Cascade Hypothesis 2.0: On the Possibility of Once-in-a-Lifetime-Only Treatment for Prevention of Alzheimer’s Disease and for Its Potential Cure at Symptomatic Stages. J. Alzheimers Dis. Rep. 2022, 6, 369–399. [Google Scholar] [CrossRef]
- Karran, E.; De Strooper, B. The amyloid hypothesis in Alzheimer disease: New insights from new therapeutics. Nat. Rev. Drug Discov. 2022, 21, 306–318. [Google Scholar] [CrossRef]
- Walsh, D.M.; Selkoe, D.J. Oligomers on the brain: The emerging role of soluble protein aggregates in neurodegeneration. Protein Pept. Lett. 2004, 11, 213–228. [Google Scholar] [CrossRef]
- Williams, T.L.; Serpell, L.C. Membrane and surface inter.actions of Alzheimer’s Abeta peptide–insights into the mechanism of cytotoxicity. FEBS J. 2011, 278, 3905–3917. [Google Scholar] [CrossRef] [PubMed]
- Sakalauskas, A.; Ziaunys, M.; Smirnovas, V. Gallic acid oxidation products alter the formation pathway of insulin amyloid fibrils. Sci. Rep. 2020, 10, 14466. [Google Scholar] [CrossRef] [PubMed]
- Yu, M. Gallic acid disruption of Aβ1–42 aggregation rescues cognitive decline of APP/PS1 double transgenic mouse. Neurobiol. Dis. 2019, 124, 67–80. [Google Scholar] [CrossRef]
- Liu, Y. Gallic acid is the major component of grape seed extract that inhibits amyloid fibril formation. Bioorg. Med. Chem. Lett. 2013, 23, 6336–6340. [Google Scholar] [CrossRef] [PubMed]
- Ghofrani, S. Naringenin improves learning and memory in an Alzheimer’s disease rat model: Insights into the underlying mechanisms. Eur. J. Pharmacol. 2015, 764, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Hirohata, M.; Yamada, M. Ferulic acid destabilizes preformed beta-amyloid fibrils in vitro. Biochem. Biophys. Res. Commun. 2005, 336, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.Y. Ferulic Acid Ameliorates Alzheimer’s Disease-like Pathology and Repairs Cognitive Decline by Preventing Capillary Hypofunction in APP/PS1 Mice. Neurotherapeutics 2021, 18, 1064–1080. [Google Scholar] [CrossRef]
- Islam, M.S. Neuropharmacological Effects of Quercetin: A Literature-Based Review. Front Pharmacol. 2021, 12, 665031. [Google Scholar] [CrossRef]
- Zaplatic, E.; Bule, M.; Shah, S.Z.A.; Uddin, M.S.; Niaz, K. Molecular Mechanisms Underlying Protective Role of Quercetin in Attenuating Alzheimer’s Disease. Life Sci. 2019, 224, 109–119. [Google Scholar] [CrossRef]
- Rudajev, V.; Novotny, J. Cholesterol as a key player in amyloid b-mediated toxicity in Alzheimer’s disease. Front. Mol. Neurosci. 2022, 15, 937056. [Google Scholar] [CrossRef]
- Hirai, M. Preferential intercalation of human amyloid-b peptide into interbilayer region of lipid-raft membrane in macromolecular crowding environment. J. Phys. Chem. B 2018, 122, 9482–9489. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.Y.; Lee, Y.J.; Hsu, G.S.W. Aluminum overload increases oxidative stress in four functional brain areas of neonatal rats. J. Biomed. Sci. 2012, 19, 51. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Salam, O.M.; El-Shamarka, M.E.; Youness, E.R.; Shaffie, N. Inhibition of aluminum chloride-induced amyloid Aβ peptide accumulation and brain neurodegeneration by Bougainvillea spectabilis flower decoction. Iran. J. Basic Med. Sci. 2021, 24, 1437–1445. [Google Scholar] [PubMed]
- Metodiewa, D.; Kośka, C. Reactive oxygen species and reactive nitrogen species: Relevance to cyto(neuro)toxic events and neurologic disorders. An overview. Neurotox. Res. 2000, 1, 197–233. [Google Scholar] [CrossRef]
- Persson, T.; Popescu, B.O.; Cedazo-Minguez, A. Oxidative stress in Alzheimer’s disease: Why did antioxidant therapy fail? Oxidative Med. Cell. Longev. 2014, 2014, 427318. [Google Scholar] [CrossRef]
- Kaizer, R.R.; Corrêa, M.C.; Spanevello, R.M.; Morsch, V.M.; Mazzanti, C.M.; Gonçalves, J.F.; Schetinger, M.R. Acetylcholinesterase activation and enhanced lipid peroxidation after long-term exposure to low levels of aluminum on di_erent mouse brain regions. J. Inorg. Biochem. 2005, 9, 1865–1870. [Google Scholar] [CrossRef]
- Scali, C.; Casamenti, F.; Bellucci, A.; Costagli, C.; Schmidt, B.; Pepeu, G. Effect of subchronic administration of metrifonate, rivastigmine and donepezil on brain acetylcholine in aged F344 rats. J. Neural Transm. 2002, 109, 1067–1080. [Google Scholar] [CrossRef]
- Marques-Coelho, D. Differential transcript usage unravels gene expression alterations in Alzheimer’s disease human brains. NPJ Aging Mech. Dis. 2021, 7, 2. [Google Scholar] [CrossRef]
- Nguyen, K.V. β-Amyloid precursor protein (APP) and the human diseases. AIMS Neurosci. 2019, 6, 273–281. [Google Scholar] [CrossRef]
- Olsson, F. Characterization of intermediate steps in amyloid beta (Abeta) production under near-native conditions. J. Biol. Chem. 2014, 289, 1540–1550. [Google Scholar] [CrossRef]
- Schneider, L.S.; Mangialasche, F.; Andreasen, N.; Feldman, H.; Giacobini, E.; Jones, R.; Mantua, V.; Mecocci, P.; Pani, L.; Winblad, B.; et al. Clinical trials and late-stage drug development for Alzheimer’s disease: An appraisal from 1984 to 2014. J. Intern. Med. 2014, 275, 251–283. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Vassar, R.; De Strooper, B.; Hardy, J.; Willem, M.; Singh, N.; Zhou, J.; Yan, R.; Vanmechelen, E.; de Vos, A.; et al. The β-Secretase BACE1 in Alzheimer’s Disease. Biol. Psychiatry 2021, 89, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. Alzheimer prevention hopes continue to dim. Nat. Rev. Drug Discov. 2020, 19, 226. [Google Scholar] [CrossRef]
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef]
- Thenmozhi, A.J.; Raja, W.T.R.; Manivasagam, T.; Janakiraman, U.; Essa, M.M. Hesperidin ameliorates cognitive dysfunction, oxidative stress and apoptosis against aluminium chloride induced rat model of Alzheimer’s disease. Nutr. Neurosci. 2017, 20, 360–368. [Google Scholar] [CrossRef]
- Kabir, M.T. Exploring the Role of PSEN Mutations in the Pathogenesis of Alzheimer’s Disease. Neurotox. Res. 2020, 38, 833–849. [Google Scholar] [CrossRef] [PubMed]
- Elfiky, A.M.; Mahmoud, A.A.; Elreedy, H.A.; Ibrahim, K.S.; Ghazy, M.A. Quercetin stimulates the non-amyloidogenic pathway via activation of ADAM10 and ADAM17 gene expression in aluminum chloride-induced Alzheimer’s disease rat model. Life Sci. 2021, 285, 119964. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, F.; Ma, X.; Perry, G.; Zhu, X. Mitochondria dysfunction in the pathogenesis of Alzheimer’s disease: Recent advances. Mol. Neurodegener. 2020, 15, 30. [Google Scholar] [CrossRef]
- Elena, A.; Asier, R.; Maria, V.S.; Estibaliz, C.; Calos, M. Chapter 14—Polyphenols attenuate mitochondrial dysfunction induced by amyloid peptides. In Mitochondrial Physiology and Vegetal Molecules; Academic Press: Cambridge, MA, USA, 2021; pp. 317–337. [Google Scholar]
- Johnson, E.C.B. Large-scale proteomic analysis of Alzheimer’s disease brain and cerebrospinalfluid reveals early changes in energy metabolism associated with microglia and astrocyte activation. Nat. Med. 2020, 26, 769–780. [Google Scholar] [CrossRef]
- Palop, J.J.; Mucke, L. Amyloid-beta-induced neuronal dysfunction in Alzheimer’s disease: From synapses toward neural networks. Nat. Neurosci. 2010, 13, 812–818. [Google Scholar] [CrossRef]
- Rybka, V. Transmission Electron Microscopy Study of Mitochondria in Aging Brain Synapses. Antioxidants 2019, 11, 171. [Google Scholar] [CrossRef]
- Awad, O.M.E.; El-Sohaimy, S.A.; Ghareeb, D.A.; Aboulenein, A.; Saleh, S.R.; El-Aziz, N.M.A. Phytochemical Analysis and toxicity Assessment of Artichoke By-product Extract. Pak. J. Biol. Sci. 2020, 23, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.J. Colorimetry of total phenolics with phospho-molybdic phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on Superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Tomaino, A.; Martorana, M.; Arcoraci, T. Antioxidant activity and phenolic profile of pistachio (Pistacia vera L., variety Bronte) seeds and skins. Biochimie 2010, 92, 1115–1122. [Google Scholar] [CrossRef]
- Bandoniene, D.; Murkovic, M.; Pfannhauser, W.; Venskutonis, P.R.; Gruzdiene, D. Detection and activity evaluation of radical scavenging compounds by using DPPH free radical and on-line HPLC–DPPH methods. Eur. Food Res. Technol. 2002, 214, 143–147. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ozyürek, M.; Bektaşoğlu, B.; Güçlü, K.; Apak, R. Hydroxyl radical scavenging assay of phenolics and flavonoids with a modified cupric reducing antioxidant capacity (CUPRAC) method using catalase for hydrogen peroxide degradation. Anal. Chim. Acta 2008, 2, 196–206. [Google Scholar] [CrossRef]
- Cavdara, H.; Senturkb, M.; Guneyc, M.; Durdagid, S.; Kayikd, G.; Supuran, C.T.; Ekinci, D. Inhibition of acetylcholinesterase and butyrylcholinesterase with uracil derivatives: Kinetic and computational studies. J. Enzym. Inhib. Med. Chem. 2019, 34, 429–437. [Google Scholar] [CrossRef]
- Ali, A.A.; Ahmed, H.I.; Abu-Elfotuh, K. Modeling Stages Mimic Alzheimer’s Disease Induced by Different Doses of Aluminum in Rats: Focus on Progression of the Disease in Response to Time. J. Alzheimers Dis. Park. 2016, 1, 2. [Google Scholar]
- Kaur, R.; Parveen, S.; Mehan, S.; Khanna, D.; Kalra, S. Neuroprotective Effect of Ellagic Acid Against Chronically Scopolamine Induced Alzheimer’s Type Memory and Cognitive Dysfunctions: Possible Behavioural and Biochemical Evidences. Int. J. Prev. Med. 2015, 1, 45–64. [Google Scholar]
- Haug, K.H.; Myhrer, T.; Fonnum, F. The combination of donepezil and procyclidine protects against soman-induced seizures in rats. Toxicol. Appl. Pharmacol. 2007, 220, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantification of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Pejman, T.; Farzam, J.; Muhammad, S. Three-Dimensional Stochastic Characterization of Shale SEM Images. Transp. Porous Media 2015, 110, 521–531. [Google Scholar]
- Tappel, A.L.; Zalkin, H. Inhibition of lipid peroxidation in mitochondria by vitamin E. Arch. Biochem. Biophys. 1959, 80, 333. [Google Scholar] [CrossRef]
- Montgomery, H.A.C.; Dymock, J.F. The determination of nitrite in water. Analyst 1961, 86, 414–416. [Google Scholar]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Paglia, E.; Valentine, N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Med. 1967, 70, 158–169. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxidedismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Piotr, C.; Nicoletta, S. The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: Twenty-something years on. Nat. Protoc. 2006, 1, 581–585. [Google Scholar]
- Wang, W.; Barnaby, J.Y.; Tada, Y.; Li, H.; Tör, M.; Caldelari, D.; Lee, D.-U.; Fu, X.-D.; Dong, X. Timing of plant immune responses by a central circadian regulator. Nature 2011, 470, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, L.A.; Feeney, B.C. A Simple Guide to IBM SPSS Statistics for Version 20.0; Wadsworth Publishing: Belmont, CA, USA, 2013. [Google Scholar]
- Kotz, S.; Balakrishnan, N.; Read, C.B.; Vidakovic, B. Encyclopedia of Statistical Sciences, 2nd ed.; Wiley-Interscience: Hoboken, NJ, USA, 2006; ISBN 978-0-471-15044-2-9686. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).