Beyond the Calcium Score: What Additional Information from a CT Scan Can Assist in Cardiovascular Risk Assessment?
Abstract
:1. Current Role of CCTA in Clinical Practice and Guidelines
1.1. Chronic Coronary Syndromes
1.2. Acute Chest Pain
1.3. Plaque Burden
1.4. Plaque Morphology
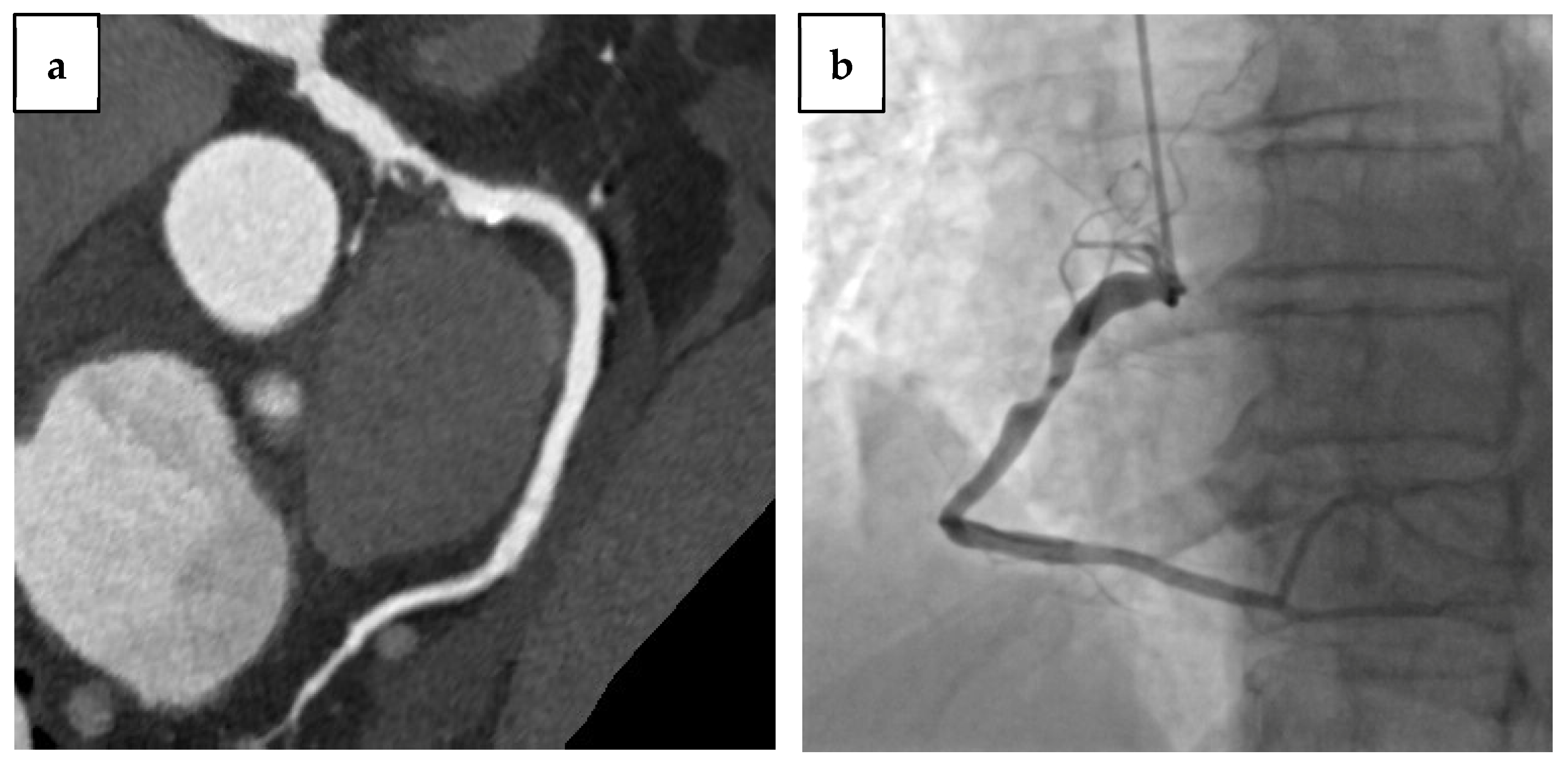
1.5. CCTA in Previous Coronary Revascularization
2. Coronary Artery Calcium Score
2.1. Imaging Acquisition, Reconstruction, and Quantification of CAC
2.2. Pathophysiological Mechanisms of Calcium Deposition
2.3. Prognostic Value of CAC and Risk Assessment
2.3.1. Asymptomatic Subjects
2.3.2. Symptomatic Patients
2.3.3. Role of CAC in Specific Subgroups
2.3.4. CAC and Progression of Coronary Atherosclerosis
2.4. Emerging Technologies
3. Epicardial Adipose Tissue
3.1. Anatomy and Functions of Epicardial Adipose Tissue
3.2. Quantification of Epicardial Adipose Tissue Using CCTA
3.3. Epicardial Fat Volume and Atherosclerosis Progression
3.4. Pericoronary Fat: Marker of Coronary Artery Stenosis Severity
3.5. Effective Therapies in the Reduction of Epicardial Fat
4. Additional CT-Measurable Parameters for Cardiovascular Risk Stratification
4.1. Aortic Calcium
4.2. Liver Fat
4.3. Myocardial Scar
5. Limits of CCTA
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ralapanawa, U.; Sivakanesan, R. Epidemiology and the Magnitude of Coronary Artery Disease and Acute Coronary Syndrome: A Narrative Review. J. Epidemiol. Glob. Health 2021, 11, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liew, S.M.; Lee, W.K.; Khoo, E.M.; Ismail, I.Z.; Ambigapathy, S.; Omar, M.; Suleiman, S.Z.; Saaban, J.; Mohd Zaidi, N.F.; Yusoff, H. Can Doctors and Patients Correctly Estimate Cardiovascular Risk? A Cross-Sectional Study in Primary Care. BMJ Open 2018, 8, e017711. [Google Scholar] [CrossRef] [Green Version]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on Cardiovascular Disease Prevention in Clinical Practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Volume-, J.E. New Insights in Cardiovascular Risk Estimation and Stratification. e-J. Cardiol. Pract. 2022, 22, 1–9. [Google Scholar]
- Fihn, S.D.; Gardin, J.M.; Abrams, J.; Berra, K.; Blankenship, J.C.; Dallas, A.P.; Douglas, P.S.; Foody, J.M.; Gerber, T.C.; Hinderliter, A.L.; et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients with Stable Ischemic Heart Disease. Circulation 2012, 126, e354–e471. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). Putting NICE Guidance into Practice Resource Impact Report: Hypercholesterolaemia and Mixed; National Institute for Health and Care Excellence (NICE): London, UK, 2016. [Google Scholar]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the Diagnosis and Management of Chronic Coronary Syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [Green Version]
- Douglas, P.S.; Hoffmann, U.; Patel, M.R.; Mark, D.B.; Al-Khalidi, H.R.; Cavanaugh, B.; Cole, J.; Dolor, R.J.; Fordyce, C.B.; Huang, M.; et al. Outcomes of Anatomical versus Functional Testing for Coronary Artery Disease. N. Engl. J. Med. 2015, 372, 1291–1300. [Google Scholar] [CrossRef] [Green Version]
- The SCOT-HEART Investigators. Coronary CT Angiography and 5-Year Risk of Myocardial Infarction. N. Engl. J. Med. 2018, 379, 924–933. [Google Scholar] [CrossRef]
- Amsterdam, E.A.; Wenger, N.K.; Brindis, R.G.; Casey, D.E.; Ganiats, T.G.; Holmes, D.R.; Jaffe, A.S.; Jneid, H.; Kelly, R.F.; Kontos, M.C.; et al. 2014 AHA/ACC Guideline for the Management of Patients with Non–ST-Elevation Acute Coronary Syndromes. J. Am. Coll. Cardiol. 2014, 64, e139–e228. [Google Scholar] [CrossRef] [Green Version]
- Collet, J.-P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef] [PubMed]
- Hadamitzky, M.; Distler, R.; Meyer, T.; Hein, F.; Kastrati, A.; Martinoff, S.; Schömig, A.; Hausleiter, J. Prognostic Value of Coronary Computed Tomographic Angiography in Comparison with Calcium Scoring and Clinical Risk Scores. Circ. Cardiovasc. Imaging 2011, 4, 16–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreini, D.; Pontone, G.; Mushtaq, S.; Bartorelli, A.L.; Bertella, E.; Antonioli, L.; Formenti, A.; Cortinovis, S.; Veglia, F.; Annoni, A.; et al. A Long-Term Prognostic Value of Coronary CT Angiography in Suspected Coronary Artery Disease. JACC Cardiovasc. Imaging 2012, 5, 690–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finck, T.; Hardenberg, J.; Will, A.; Hendrich, E.; Haller, B.; Martinoff, S.; Hausleiter, J.; Hadamitzky, M. 10-Year Follow-Up after Coronary Computed Tomography Angiography in Patients with Suspected Coronary Artery Disease. JACC Cardiovasc. Imaging 2019, 12, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Al-Mallah, M.H.; Qureshi, W.; Lin, F.Y.; Achenbach, S.; Berman, D.S.; Budoff, M.J.; Callister, T.Q.; Chang, H.-J.; Cademartiri, F.; Chinnaiyan, K.; et al. Does Coronary CT Angiography Improve Risk Stratification over Coronary Calcium Scoring in Symptomatic Patients with Suspected Coronary Artery Disease? Results from the Prospective Multicenter International CONFIRM Registry. Eur. Heart J.—Cardiovasc. Imaging 2014, 15, 267–274. [Google Scholar] [CrossRef] [Green Version]
- Bittencourt, M.S.; Hulten, E.; Ghoshhajra, B.; O’Leary, D.; Christman, M.P.; Montana, P.; Truong, Q.A.; Steigner, M.; Murthy, V.L.; Rybicki, F.J.; et al. Prognostic Value of Nonobstructive and Obstructive Coronary Artery Disease Detected by Coronary Computed Tomography Angiography to Identify Cardiovascular Events. Circ. Cardiovasc. Imaging 2014, 7, 282–291. [Google Scholar] [CrossRef] [Green Version]
- Hadamitzky, M.; Taubert, S.; Deseive, S.; Byrne, R.A.; Martinoff, S.; Schomig, A.; Hausleiter, J. Prognostic Value of Coronary Computed Tomography Angiography during 5 Years of Follow-up in Patients with Suspected Coronary Artery Disease. Eur. Heart J. 2013, 34, 3277–3285. [Google Scholar] [CrossRef] [Green Version]
- Pontone, G.; Rossi, A.; Guglielmo, M.; Dweck, M.R.; Gaemperli, O.; Nieman, K.; Pugliese, F.; Maurovich-Horvat, P.; Gimelli, A.; Cosyns, B.; et al. Clinical Applications of Cardiac Computed Tomography: A Consensus Paper of the European Association of Cardiovascular Imaging—Part I. Eur. Heart J.—Cardiovasc. Imaging 2022, 23, 299–314. [Google Scholar] [CrossRef]
- Weir-McCall, J.R.; Villines, T.C.; Shaw, L.J.; Abbara, S.; Ferencik, M.; Nieman, K.; Achenbach, S.; Nicol, E. Highlights of the Twelfth Annual Scientific Meeting of the Society of Cardiovascular Computed Tomography. J. Cardiovasc. Comput. Tomogr. 2018, 12, 3–7. [Google Scholar] [CrossRef] [Green Version]
- Min, J.K.; Shaw, L.J.; Devereux, R.B.; Okin, P.M.; Weinsaft, J.W.; Russo, D.J.; Lippolis, N.J.; Berman, D.S.; Callister, T.Q. Prognostic Value of Multidetector Coronary Computed Tomographic Angiography for Prediction of All-Cause Mortality. J. Am. Coll. Cardiol. 2007, 50, 1161–1170. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-E.; Chang, H.-J.; Sung, J.M.; Park, H.-B.; Heo, R.; Rizvi, A.; Lin, F.Y.; Kumar, A.; Hadamitzky, M.; Kim, Y.J.; et al. Effects of Statins on Coronary Atherosclerotic Plaques. JACC Cardiovasc. Imaging 2018, 11, 1475–1484. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-J.; Lin, F.Y.; Lee, S.-E.; Andreini, D.; Bax, J.; Cademartiri, F.; Chinnaiyan, K.; Chow, B.J.W.; Conte, E.; Cury, R.C.; et al. Coronary Atherosclerotic Precursors of Acute Coronary Syndromes. J. Am. Coll. Cardiol. 2018, 71, 2511–2522. [Google Scholar] [CrossRef] [PubMed]
- Maurovich-Horvat, P.; Hoffmann, U.; Vorpahl, M.; Nakano, M.; Virmani, R.; Alkadhi, H. The Napkin-Ring Sign: CT Signature of High-Risk Coronary Plaques? JACC Cardiovasc. Imaging 2010, 3, 440–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maurovich-Horvat, P.; Schlett, C.L.; Alkadhi, H.; Nakano, M.; Otsuka, F.; Stolzmann, P.; Scheffel, H.; Ferencik, M.; Kriegel, M.F.; Seifarth, H.; et al. The Napkin-Ring Sign Indicates Advanced Atherosclerotic Lesions in Coronary CT Angiography. JACC Cardiovasc. Imaging 2012, 5, 1243–1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakazato, R.; Otake, H.; Konishi, A.; Iwasaki, M.; Koo, B.-K.; Fukuya, H.; Shinke, T.; Hirata, K.-i.; Leipsic, J.; Berman, D.S.; et al. Atherosclerotic Plaque Characterization by CT Angiography for Identification of High-Risk Coronary Artery Lesions: A Comparison to Optical Coherence Tomography. Eur. Heart J.—Cardiovasc. Imaging 2015, 16, 373–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, U.; Moselewski, F.; Nieman, K.; Jang, I.-K.; Ferencik, M.; Rahman, A.M.; Cury, R.C.; Abbara, S.; Joneidi-Jafari, H.; Achenbach, S.; et al. Noninvasive Assessment of Plaque Morphology and Composition in Culprit and Stable Lesions in Acute Coronary Syndrome and Stable Lesions in Stable Angina by Multidetector Computed Tomography. J. Am. Coll. Cardiol. 2006, 47, 1655–1662. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, A.; Leipsic, J.; Øvrehus, K.A.; Gaur, S.; Bagiella, E.; Ko, B.; Dey, D.; LaRocca, G.; Jensen, J.M.; Bøtker, H.E.; et al. Lesion-Specific and Vessel-Related Determinants of Fractional Flow Reserve beyond Coronary Artery Stenosis. JACC Cardiovasc. Imaging 2018, 11, 521–530. [Google Scholar] [CrossRef]
- Motoyama, S.; Ito, H.; Sarai, M.; Kondo, T.; Kawai, H.; Nagahara, Y.; Harigaya, H.; Kan, S.; Anno, H.; Takahashi, H.; et al. Plaque Characterization by Coronary Computed Tomography Angiography and the Likelihood of Acute Coronary Events in Mid-Term Follow-Up. J. Am. Coll. Cardiol. 2015, 66, 337–346. [Google Scholar] [CrossRef] [Green Version]
- Williams, M.C.; Moss, A.J.; Dweck, M.; Adamson, P.D.; Alam, S.; Hunter, A.; Shah, A.S.V.; Pawade, T.; Weir-McCall, J.R.; Roditi, G.; et al. Coronary Artery Plaque Characteristics Associated with Adverse Outcomes in the SCOT-HEART Study. J. Am. Coll. Cardiol. 2019, 73, 291–301. [Google Scholar] [CrossRef]
- Cury, R.C.; Abbara, S.; Achenbach, S.; Agatston, A.; Berman, D.S.; Budoff, M.J.; Dill, K.E.; Jacobs, J.E.; Maroules, C.D.; Rubin, G.D.; et al. CAD-RADSTM Coronary Artery Disease—Reporting and Data System. An Expert Consensus Document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Radiology (ACR) and the North American Society for Cardiovascular Imaging (NASCI). J. Cardiovasc. Comput. Tomogr. 2016, 10, 269–281. [Google Scholar] [CrossRef] [Green Version]
- Kalisz, K.; Buethe, J.; Saboo, S.S.; Abbara, S.; Halliburton, S.; Rajiah, P. Artifacts at Cardiac CT: Physics and Solutions. RadioGraphics 2016, 36, 2064–2083. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wingert, A.; Wang, J.; Zhang, J.; Wang, X.; Sun, J.; Chen, F.; Khalid, S.G.; Jiang, J.; Zheng, D. Extraction of Coronary Atherosclerotic Plaques from Computed Tomography Imaging: A Review of Recent Methods. Front. Cardiovasc. Med. 2021, 8, 597568. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wingert, A.; Wang, X.; Zhang, J.; Sun, J.; Chen, F.; Khalid, S.G.; Gong, Y.; Xia, L.; Jiang, J.; et al. Consistency in Geometry among Coronary Atherosclerotic Plaques Extracted from Computed Tomography Angiography. Front. Physiol. 2021, 12, 715265. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Wang, J.; Hu, P. Diagnostic Performance of Computed Tomography Angiography in the Detection of Coronary Artery In-Stent Restenosis: Evidence from an Updated Meta-Analysis. Eur. Radiol. 2018, 28, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.; Ridley, L.; Dunn, D.J.; Tian, D.H.; Liou, K.; Ozdirik, J.; Cheruvu, C.; Cao, C. A Systematic Review and Meta-Analysis of Multidetector Computed Tomography in the Assessment of Coronary Artery Bypass Grafts. Int. J. Cardiol. 2016, 221, 898–905. [Google Scholar] [CrossRef]
- De Graaf, F.R.; van Velzen, J.E.; Witkowska, A.J.; Schuijf, J.D.; van der Bijl, N.; Kroft, L.J.; de Roos, A.; Reiber, J.H.C.; Bax, J.J.; de Grooth, G.J.; et al. Diagnostic Performance of 320-Slice Multidetector Computed Tomography Coronary Angiography in Patients after Coronary Artery Bypass Grafting. Eur. Radiol. 2011, 21, 2285–2296. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Bera, K.; Kikano, E.; Pierce, J.D.; Gan, J.; Rajdev, M.; Ciancibello, L.M.; Gupta, A.; Rajagopalan, S.; Gilkeson, R.C. Coronary Artery Calcium Scoring: Current Status and Future Directions. RadioGraphics 2022, 42, 947–967. [Google Scholar] [CrossRef]
- Greenland, P.; Bonow, R.O.; Brundage, B.H.; Budoff, M.J.; Eisenberg, M.J.; Grundy, S.M.; Lauer, M.S.; Post, W.S.; Raggi, P.; Redberg, R.F.; et al. ACCF/AHA 2007 Clinical Expert Consensus Document on Coronary Artery Calcium Scoring by Computed Tomography in Global Cardiovascular Risk Assessment and in Evaluation of Patients with Chest Pain. Circulation 2007, 115, 402–426. [Google Scholar] [CrossRef] [Green Version]
- Shah, N.R.; Coulter, S.A. An Evidence-Based Guide for Coronary Calcium Scoring in Asymptomatic Patients without Coronary Heart Disease. Tex. Heart Inst. J. 2012, 39, 240–242. [Google Scholar]
- McCollough, C.H.; Ulzheimer, S.; Halliburton, S.S.; Shanneik, K.; White, R.D.; Kalender, W.A. Coronary Artery Calcium: A Multi-Institutional, Multimanufacturer International Standard for Quantification at Cardiac CT. Radiology 2007, 243, 527–538. [Google Scholar] [CrossRef]
- Sandfort, V.; Bluemke, D.A. CT Calcium Scoring. History, Current Status and Outlook. Diagn. Interv. Imaging 2017, 98, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M.; Detrano, R. Quantification of Coronary Artery Calcium Using Ultrafast Computed Tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Budoff, M.J.; Nasir, K.; McClelland, R.L.; Detrano, R.; Wong, N.; Blumenthal, R.S.; Kondos, G.; Kronmal, R.A. Coronary Calcium Predicts Events Better with Absolute Calcium Scores Than Age-Sex-Race/Ethnicity Percentiles. J. Am. Coll. Cardiol. 2009, 53, 345–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrone-Filardi, P.; Achenbach, S.; Mohlenkamp, S.; Reiner, Z.; Sambuceti, G.; Schuijf, J.D.; Van der Wall, E.; Kaufmann, P.A.; Knuuti, J.; Schroeder, S.; et al. Cardiac Computed Tomography and Myocardial Perfusion Scintigraphy for Risk Stratification in Asymptomatic Individuals without Known Cardiovascular Disease: A Position Statement of the Working Group on Nuclear Cardiology and Cardiac CT of the European Soci. Eur. Heart J. 2011, 32, 1986–1993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callister, T.Q.; Cooil, B.; Raya, S.P.; Lippolis, N.J.; Russo, D.J.; Raggi, P. Coronary Artery Disease: Improved Reproducibility of Calcium Scoring with an Electron-Beam CT Volumetric Method. Radiology 1998, 208, 807–814. [Google Scholar] [CrossRef]
- Brown, E.R.; Kronmal, R.A.; Bluemke, D.A.; Guerci, A.D.; Carr, J.J.; Goldin, J.; Detrano, R. Coronary Calcium Coverage Score: Determination, Correlates, and Predictive Accuracy in the Multi-Ethnic Study of Atherosclerosis. Radiology 2008, 247, 669–675. [Google Scholar] [CrossRef] [Green Version]
- Shekar, C.; Budoff, M. Calcification of the Heart: Mechanisms and Therapeutic Avenues. Expert Rev. Cardiovasc. Ther. 2018, 16, 527–536. [Google Scholar] [CrossRef]
- Proudfoot, D.; Shanahan, C.M. Biology of Calcification in Vascular Cells: Intima versus Media. Herz 2001, 26, 245–251. [Google Scholar] [CrossRef]
- Tintut, Y.; Alfonso, Z.; Saini, T.; Radcliff, K.; Watson, K.; Boström, K.; Demer, L.L. Multilineage Potential of Cells from the Artery Wall. Circulation 2003, 108, 2505–2510. [Google Scholar] [CrossRef] [Green Version]
- Tyson, K.L.; Reynolds, J.L.; McNair, R.; Zhang, Q.; Weissberg, P.L.; Shanahan, C.M. Osteo/Chondrocytic Transcription Factors and Their Target Genes Exhibit Distinct Patterns of Expression in Human Arterial Calcification. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 489–494. [Google Scholar] [CrossRef] [Green Version]
- Nusca, A.; Viscusi, M.M.; Piccirillo, F.; De Filippis, A.; Nenna, A.; Spadaccio, C.; Nappi, F.; Chello, C.; Mangiacapra, F.; Grigioni, F.; et al. In Stent Neo-Atherosclerosis: Pathophysiology, Clinical Implications, Prevention, and Therapeutic Approaches. Life 2022, 12, 393. [Google Scholar] [CrossRef] [PubMed]
- Bear, M.; Butcher, M.; Shaughnessy, S.G. Oxidized Low-Density Lipoprotein Acts Synergistically with β-Glycerophosphate to Induce Osteoblast Differentiation in Primary Cultures of Vascular Smooth Muscle Cells. J. Cell. Biochem. 2008, 105, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, F.; Carpenito, M.; Verolino, G.; Chello, C.; Nusca, A.; Lusini, M.; Spadaccio, C.; Nappi, F.; Di Sciascio, G.; Nenna, A. Changes of the Coronary Arteries and Cardiac Microvasculature with Aging: Implications for Translational Research and Clinical Practice. Mech. Ageing Dev. 2019, 184, 111161. [Google Scholar] [CrossRef] [PubMed]
- Maher, J.E.; Raz, J.A.; Bielak, L.F.; Sheecly, P.F.; Schwartz, R.S.; Peyser, P.A. Potential of Quantity of Coronary Artery Calcification to Identify New Risk Factors for Asymptomatic Atherosclerosis. Am. J. Epidemiol. 1996, 144, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.J.; Feuerstein, I.; Wong, H.; Barko, W.; Brazaitis, M.; O’Malley, P.G. Do Conventional Risk Factors Predict Subclinical Coronary Artery Disease? Results from the Prospective Army Coronary Calcium Project. Am. Heart J. 2001, 141, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.X.; Duan, D.; O’Neill, K.D.; Moe, S.M. High Glucose Increases the Expression of Cbfa1 and BMP-2 and Enhances the Calcification of Vascular Smooth Muscle Cells. Nephrol. Dial. Transplant. 2006, 21, 3435–3442. [Google Scholar] [CrossRef] [Green Version]
- Burke, A.P.; Farb, A.; Malcom, G.T.; Liang, Y.; Smialek, J.; Virmani, R. Effect of Risk Factors on the Mechanism of Acute Thrombosis and Sudden Coronary Death in Women. Circulation 1998, 97, 2110–2116. [Google Scholar] [CrossRef] [Green Version]
- Erbel, R.; Möhlenkamp, S.; Moebus, S.; Schmermund, A.; Lehmann, N.; Stang, A.; Dragano, N.; Grönemeyer, D.; Seibel, R.; Kälsch, H.; et al. Coronary Risk Stratification, Discrimination, and Reclassification Improvement Based on Quantification of Subclinical Coronary Atherosclerosis. J. Am. Coll. Cardiol. 2010, 56, 1397–1406. [Google Scholar] [CrossRef] [Green Version]
- Vliegenthart, R.; Oudkerk, M.; Hofman, A.; Oei, H.-H.S.; van Dijck, W.; van Rooij, F.J.A.; Witteman, J.C.M. Coronary Calcification Improves Cardiovascular Risk Prediction in the Elderly. Circulation 2005, 112, 572–577. [Google Scholar] [CrossRef] [Green Version]
- Sarwar, A.; Shaw, L.J.; Shapiro, M.D.; Blankstein, R.; Hoffman, U.; Cury, R.C.; Abbara, S.; Brady, T.J.; Budoff, M.J.; Blumenthal, R.S.; et al. Diagnostic and Prognostic Value of Absence of Coronary Artery Calcification. JACC Cardiovasc. Imaging 2009, 2, 675–688. [Google Scholar] [CrossRef] [Green Version]
- Peng, A.W.; Mirbolouk, M.; Orimoloye, O.A.; Osei, A.D.; Dardari, Z.; Dzaye, O.; Budoff, M.J.; Shaw, L.; Miedema, M.D.; Rumberger, J.; et al. Long-Term All-Cause and Cause-Specific Mortality in Asymptomatic Patients with CAC ≥ 1000. JACC Cardiovasc. Imaging 2020, 13, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Greenland, P. Coronary Artery Calcium Score Combined with Framingham Score for Risk Prediction in Asymptomatic Individuals. JAMA 2004, 291, 210. [Google Scholar] [CrossRef] [PubMed]
- Arad, Y.; Goodman, K.J.; Roth, M.; Newstein, D.; Guerci, A.D. Coronary Calcification, Coronary Disease Risk Factors, C-Reactive Protein, and Atherosclerotic Cardiovascular Disease Events. J. Am. Coll. Cardiol. 2005, 46, 158–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenland, P.; Blaha, M.J.; Budoff, M.J.; Erbel, R.; Watson, K.E. Coronary Calcium Score and Cardiovascular Risk. J. Am. Coll. Cardiol. 2018, 72, 434–447. [Google Scholar] [CrossRef]
- Ajufo, E.; Ayers, C.R.; Vigen, R.; Joshi, P.H.; Rohatgi, A.; de Lemos, J.A.; Khera, A. Value of Coronary Artery Calcium Scanning in Association with the Net Benefit of Aspirin in Primary Prevention of Atherosclerotic Cardiovascular Disease. JAMA Cardiol. 2021, 6, 179. [Google Scholar] [CrossRef]
- Budoff, M.J.; Dowe, D.; Jollis, J.G.; Gitter, M.; Sutherland, J.; Halamert, E.; Scherer, M.; Bellinger, R.; Martin, A.; Benton, R.; et al. Diagnostic Performance of 64-Multidetector Row Coronary Computed Tomographic Angiography for Evaluation of Coronary Artery Stenosis in Individuals without Known Coronary Artery Disease. J. Am. Coll. Cardiol. 2008, 52, 1724–1732. [Google Scholar] [CrossRef] [Green Version]
- Meijboom, W.B.; van Mieghem, C.A.G.; Mollet, N.R.; Pugliese, F.; Weustink, A.C.; van Pelt, N.; Cademartiri, F.; Nieman, K.; Boersma, E.; de Jaegere, P.; et al. 64-Slice Computed Tomography Coronary Angiography in Patients with High, Intermediate, or Low Pretest Probability of Significant Coronary Artery Disease. J. Am. Coll. Cardiol. 2007, 50, 1469–1475. [Google Scholar] [CrossRef] [Green Version]
- Winther, S.; Schmidt, S.E.; Mayrhofer, T.; Bøtker, H.E.; Hoffmann, U.; Douglas, P.S.; Wijns, W.; Bax, J.; Nissen, L.; Lynggaard, V.; et al. Incorporating Coronary Calcification into Pre-Test Assessment of the Likelihood of Coronary Artery Disease. J. Am. Coll. Cardiol. 2020, 76, 2421–2432. [Google Scholar] [CrossRef]
- Sow, M.A.; Magne, J.; Salle, L.; Nobecourt, E.; Preux, P.-M.; Aboyans, V. Prevalence, Determinants and Prognostic Value of High Coronary Artery Calcium Score in Asymptomatic Patients with Diabetes: A Systematic Review and Meta-Analysis. J. Diabetes Complicat. 2022, 36, 108237. [Google Scholar] [CrossRef]
- Wang, F.M.; Rozanski, A.; Arnson, Y.; Budoff, M.J.; Miedema, M.D.; Nasir, K.; Shaw, L.J.; Rumberger, J.A.; Blumenthal, R.S.; Matsushita, K.; et al. Cardiovascular and All-Cause Mortality Risk by Coronary Artery Calcium Scores and Percentiles among Older Adult Males and Females. Am. J. Med. 2021, 134, 341–350. [Google Scholar] [CrossRef]
- Block, G.A.; Raggi, P.; Bellasi, A.; Kooienga, L.; Spiegel, D.M. Mortality Effect of Coronary Calcification and Phosphate Binder Choice in Incident Hemodialysis Patients. Kidney Int. 2007, 71, 438–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gassett, A.J.; Sheppard, L.; McClelland, R.L.; Olives, C.; Kronmal, R.; Blaha, M.J.; Budoff, M.; Kaufman, J.D. Risk Factors for Long-Term Coronary Artery Calcium Progression in the Multi-Ethnic Study of Atherosclerosis. J. Am. Heart Assoc. 2015, 4, e001726. [Google Scholar] [CrossRef] [PubMed]
- Schindler, T.H.; Cadenas, J.; Facta, A.D.; Li, Y.; Olschewski, M.; Sayre, J.; Goldin, J.; Schelbert, H.R. Improvement in Coronary Endothelial Function Is Independently Associated with a Slowed Progression of Coronary Artery Calcification in Type 2 Diabetes Mellitus. Eur. Heart J. 2009, 30, 3064–3073. [Google Scholar] [CrossRef] [PubMed]
- Raggi, P.; Callister, T.Q.; Shaw, L.J. Progression of Coronary Artery Calcium and Risk of First Myocardial Infarction in Patients Receiving Cholesterol-Lowering Therapy. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1272–1277. [Google Scholar] [CrossRef]
- Budoff, M.J.; Young, R.; Lopez, V.A.; Kronmal, R.A.; Nasir, K.; Blumenthal, R.S.; Detrano, R.C.; Bild, D.E.; Guerci, A.D.; Liu, K.; et al. Progression of Coronary Calcium and Incident Coronary Heart Disease Events. J. Am. Coll. Cardiol. 2013, 61, 1231–1239. [Google Scholar] [CrossRef] [Green Version]
- Radford, N.B.; DeFina, L.F.; Barlow, C.E.; Lakoski, S.G.; Leonard, D.; Paixao, A.R.M.; Khera, A.; Levine, B.D. Progression of CAC Score and Risk of Incident CVD. JACC Cardiovasc. Imaging 2016, 9, 1420–1429. [Google Scholar] [CrossRef]
- Lehmann, N.; Erbel, R.; Mahabadi, A.A.; Rauwolf, M.; Möhlenkamp, S.; Moebus, S.; Kälsch, H.; Budde, T.; Schmermund, A.; Stang, A.; et al. Value of Progression of Coronary Artery Calcification for Risk Prediction of Coronary and Cardiovascular Events. Circulation 2018, 137, 665–679. [Google Scholar] [CrossRef]
- Hecht, H.S.; Cronin, P.; Blaha, M.J.; Budoff, M.J.; Kazerooni, E.A.; Narula, J.; Yankelevitz, D.; Abbara, S. 2016 SCCT/STR Guidelines for Coronary Artery Calcium Scoring of Noncontrast Noncardiac Chest CT Scans: A Report of the Society of Cardiovascular Computed Tomography and Society of Thoracic Radiology. J. Cardiovasc. Comput. Tomogr. 2017, 11, 74–84. [Google Scholar] [CrossRef] [Green Version]
- Zang, Y.; Dai, L.; Zhang, Y.; Xia, L. One-Dimensional Simulation of Transmural Heterogeneity of Cardiac Cellular Electromechanics. Comput. Cardiol. 2011, 38, 65–68. [Google Scholar]
- Huang, A.L.; Maggiore, P.L.; Brown, R.A.; Turaga, M.; Reid, A.B.; Merkur, J.; Blanke, P.; Leipsic, J.A. CT-Derived Fractional Flow Reserve (FFR CT): From Gatekeeping to Roadmapping. Can. Assoc. Radiol. J. 2020, 71, 201–207. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Zhang, J.; Gong, Y.; Xu, L.; Liu, H.; Wei, S.; Wu, Y.; Cha, G.; Wei, H.; Mao, J.; et al. Reliable Detection of Myocardial Ischemia Using Machine Learning Based on Temporal-Spatial Characteristics of Electrocardiogram and Vectorcardiogram. Front. Physiol. 2022, 13, 854191. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Tse, G.; Korantzopoulos, P.; Letsas, K.P.; Ali-Hasan-Al-Saegh, S.; Kamel, H.; Li, G.; Lip, G.Y.H.; Liu, T. P-Wave Indices and Risk of Ischemic Stroke. Stroke 2017, 48, 2066–2072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tse, G.; Wong, C.W.; Gong, M.Q.; Meng, L.; Letsas, K.P.; Li, G.P.; Whittaker, P.; Bhardwaj, A.; Sawant, A.C.; Wu, W.K.; et al. Meta-Analysis of T-Wave Indices for Risk Stratification in Myocardial Infarction. J. Geriatr. Cardiol. 2017, 14, 776–779. [Google Scholar] [PubMed]
- Dou, J.; Xia, L.; Deng, D.; Zang, Y.; Shou, G.; Bustos, C.; Tu, W.; Liu, F.; Crozier, S. A Study of Mechanical Optimization Strategy for Cardiac Resynchronization Therapy Based on an Electromechanical Model. Comput. Math. Methods Med. 2012, 2012, 948781. [Google Scholar] [CrossRef] [Green Version]
- Geng, Y.; Wu, X.; Liu, H.; Zheng, D.; Xia, L. Index of Microcirculatory Resistance: State-of-the-Art and Potential Applications in Computational Simulation of Coronary Artery Disease. J. Zhejiang Univ. B 2022, 23, 123–140. [Google Scholar] [CrossRef]
- Geng, Y.; Liu, H.; Wang, X.; Zhang, J.; Gong, Y.; Zheng, D.; Jiang, J.; Xia, L. Effect of Microcirculatory Dysfunction on Coronary Hemodynamics: A Pilot Study Based on Computational Fluid Dynamics Simulation. Comput. Biol. Med. 2022, 146, 105583. [Google Scholar] [CrossRef]
- Yeung, C.; Baranchuk, A.; Tse, G.; Liu, T. The Importance of Measuring Coronary Blood Flow for Clinical Decision Making. Curr. Cardiol. Rev. 2019, 15, 320–321. [Google Scholar] [CrossRef]
- Rizvi, A.; Han, D.; Danad, I.; Ó Hartaigh, B.; Lee, J.H.; Gransar, H.; Stuijfzand, W.J.; Roudsari, H.M.; Park, M.W.; Szymonifka, J.; et al. Diagnostic Performance of Hybrid Cardiac Imaging Methods for Assessment of Obstructive Coronary Artery Disease Compared with Stand-Alone Coronary Computed Tomography Angiography. JACC Cardiovasc. Imaging 2018, 11, 589–599. [Google Scholar] [CrossRef]
- Hyafil, F.; Jaber, W.A.; Neglia, D. Highlights of the 14th International Conference on Nuclear Cardiology and Cardiac Computed Tomography. Eur. Heart J.—Cardiovasc. Imaging 2019, 21, 1–9. [Google Scholar] [CrossRef]
- Le Jemtel, T.H.; Samson, R.; Ayinapudi, K.; Singh, T.; Oparil, S. Epicardial Adipose Tissue and Cardiovascular Disease. Curr. Hypertens. Rep. 2019, 21, 36. [Google Scholar] [CrossRef]
- Sacks, H.S.; Fain, J.N. Human Epicardial Adipose Tissue: A Review. Am. Heart J. 2007, 153, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G.; Corradi, D.; Sharma, A.M. Epicardial Adipose Tissue: Anatomic, Biomolecular and Clinical Relationships with the Heart. Nat. Clin. Pract. Cardiovasc. Med. 2005, 2, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulos, A.S.; Sanna, F.; Sabharwal, N.; Thomas, S.; Oikonomou, E.K.; Herdman, L.; Margaritis, M.; Shirodaria, C.; Kampoli, A.-M.; Akoumianakis, I.; et al. Detecting Human Coronary Inflammation by Imaging Perivascular Fat. Sci. Transl. Med. 2017, 9, eaal2658. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, T.; Zhang, L.; Zalewski, A.; Mannion, J.D.; Diehl, J.T.; Arafat, H.; Sarov-Blat, L.; O’Brien, S.; Keiper, E.A.; Johnson, A.G.; et al. Human Epicardial Adipose Tissue Is a Source of Inflammatory Mediators. Circulation 2003, 108, 2460–2466. [Google Scholar] [CrossRef] [Green Version]
- Rabkin, S.W. Epicardial Fat: Properties, Function and Relationship to Obesity. Obes. Rev. 2007, 8, 253–261. [Google Scholar] [CrossRef]
- Lin, A.; Dey, D.; Wong, D.T.L.; Nerlekar, N. Perivascular Adipose Tissue and Coronary Atherosclerosis: From Biology to Imaging Phenotyping. Curr. Atheroscler. Rep. 2019, 21, 47. [Google Scholar] [CrossRef]
- Chechi, K.; Richard, D. Thermogenic Potential and Physiological Relevance of Human Epicardial Adipose Tissue. Int. J. Obes. Suppl. 2015, 5, S28–S34. [Google Scholar] [CrossRef] [Green Version]
- Hirata, Y.; Tabata, M.; Kurobe, H.; Motoki, T.; Akaike, M.; Nishio, C.; Higashida, M.; Mikasa, H.; Nakaya, Y.; Takanashi, S.; et al. Coronary Atherosclerosis Is Associated with Macrophage Polarization in Epicardial Adipose Tissue. J. Am. Coll. Cardiol. 2011, 58, 248–255. [Google Scholar] [CrossRef] [Green Version]
- Demir, B.; Demir, E.; Acıksarı, G.; Uygun, T.; Utku, I.K.; Gedikbasi, A.; Caglar, I.M.; Pirhan, O.; Tureli, H.O.; Oflar, E.; et al. The Association between the Epicardial Adipose Tissue Thickness and Oxidative Stress Parameters in Isolated Metabolic Syndrome Patients: A Multimarker Approach. Int. J. Endocrinol. 2014, 2014, 954045. [Google Scholar] [CrossRef]
- Baker, A.R.; Harte, A.L.; Howell, N.; Pritlove, D.C.; Ranasinghe, A.M.; da Silva, N.F.; Youssef, E.M.; Khunti, K.; Davies, M.J.; Bonser, R.S.; et al. Epicardial Adipose Tissue as a Source of Nuclear Factor-ΚB and c-Jun N-Terminal Kinase Mediated Inflammation in Patients with Coronary Artery Disease. J. Clin. Endocrinol. Metab. 2009, 94, 261–267. [Google Scholar] [CrossRef] [Green Version]
- Nusca, A.; Piccirillo, F.; Bernardini, F.; De Filippis, A.; Coletti, F.; Mangiacapra, F.; Ricottini, E.; Melfi, R.; Gallo, P.; Cammalleri, V.; et al. Glycaemic Control in Patients Undergoing Percutaneous Coronary Intervention: What Is the Role for the Novel Antidiabetic Agents? A Comprehensive Review of Basic Science and Clinical Data. Int. J. Mol. Sci. 2022, 23, 7261. [Google Scholar] [CrossRef] [PubMed]
- Montazerifar, F.; Bolouri, A.; Paghalea, R.S.; Mahani, M.K.; Karajibani, M. Obesity, Serum Resistin and Leptin Levels Linked to Coronary Artery Disease. Arq. Bras. Cardiol. 2016, 107, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Yafei, S.; Elsewy, F.; Youssef, E.; Ayman, M.; Elshafei, M.; Abayazeed, R. Echocardiographic Association of Epicardial Fat with Carotid Intima–Media Thickness in Patients with Type 2 Diabetes. Diabetes Vasc. Dis. Res. 2019, 16, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Militello, C.; Rundo, L.; Toia, P.; Conti, V.; Russo, G.; Filorizzo, C.; Maffei, E.; Cademartiri, F.; La Grutta, L.; Midiri, M.; et al. A Semi-Automatic Approach for Epicardial Adipose Tissue Segmentation and Quantification on Cardiac CT Scans. Comput. Biol. Med. 2019, 114, 103424. [Google Scholar] [CrossRef] [PubMed]
- Mancio, J.; Azevedo, D.; Saraiva, F.; Azevedo, A.I.; Pires-Morais, G.; Leite-Moreira, A.; Falcao-Pires, I.; Lunet, N.; Bettencourt, N. Epicardial Adipose Tissue Volume Assessed by Computed Tomography and Coronary Artery Disease: A Systematic Review and Meta-Analysis. Eur. Heart J.—Cardiovasc. Imaging 2018, 19, 490–497. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.-D.; Lee, W.-J.; Shih, F.-Y.; Huang, C.-H.; Chang, Y.-C.; Chen, W.-J.; Lee, Y.-T.; Chen, M.-F. Relations of Epicardial Adipose Tissue Measured by Multidetector Computed Tomography to Components of the Metabolic Syndrome Are Region-Specific and Independent of Anthropometric Indexes and Intraabdominal Visceral Fat. J. Clin. Endocrinol. Metab. 2009, 94, 662–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Grutta, L.; Toia, P.; Farruggia, A.; Albano, D.; Grassedonio, E.; Palmeri, A.; Maffei, E.; Galia, M.; Vitabile, S.; Cademartiri, F.; et al. Quantification of Epicardial Adipose Tissue in Coronary Calcium Score and CT Coronary Angiography Image Data Sets: Comparison of Attenuation Values, Thickness and Volumes. Br. J. Radiol. 2016, 89, 20150773. [Google Scholar] [CrossRef] [Green Version]
- Spearman, J.V.; Renker, M.; Schoepf, U.J.; Krazinski, A.W.; Herbert, T.L.; De Cecco, C.N.; Nietert, P.J.; Meinel, F.G. Prognostic Value of Epicardial Fat Volume Measurements by Computed Tomography: A Systematic Review of the Literature. Eur. Radiol. 2015, 25, 3372–3381. [Google Scholar] [CrossRef]
- Bastarrika, G.; Broncano, J.; Schoepf, U.J.; Schwarz, F.; Lee, Y.S.; Abro, J.A.; Costello, P.; Zwerner, P.L. Relationship between Coronary Artery Disease and Epicardial Adipose Tissue Quantification at Cardiac CT. Acad. Radiol. 2010, 17, 727–734. [Google Scholar] [CrossRef]
- Yu, W.; Liu, B.; Zhang, F.; Wang, J.; Shao, X.; Yang, X.; Shi, Y.; Wang, B.; Xu, Y.; Wang, Y. Association of Epicardial Fat Volume with Increased Risk of Obstructive Coronary Artery Disease in Chinese Patients with Suspected Coronary Artery Disease. J. Am. Heart Assoc. 2021, 10, e018080. [Google Scholar] [CrossRef]
- Gitsioudis, G.; Schmahl, C.; Missiou, A.; Voss, A.; Schüssler, A.; Abdel-Aty, H.; Buss, S.J.; Mueller, D.; Vembar, M.; Bryant, M.; et al. Epicardial Adipose Tissue Is Associated with Plaque Burden and Composition and Provides Incremental Value for the Prediction of Cardiac Outcome. A Clinical Cardiac Computed Tomography Angiography Study. PLoS ONE 2016, 11, e0155120. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, N.; McLean, D.S.; Janik, M.; Arepalli, C.D.; Stillman, A.E.; Raggi, P. Epicardial Adipose Tissue and Coronary Artery Plaque Characteristics. Atherosclerosis 2010, 210, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Suzuki, Y.; Ehara, M.; Matsuo, H.; Teramoto, T.; Terashima, M.; Nasu, K.; Kinoshita, Y.; Tsuchikane, E.; Suzuki, T.; et al. Impact of Epicardial Fat Volume on Coronary Artery Disease in Symptomatic Patients with a Zero Calcium Score. Int. J. Cardiol. 2013, 167, 2852–2858. [Google Scholar] [CrossRef] [PubMed]
- Nerlekar, N.; Brown, A.J.; Muthalaly, R.G.; Talman, A.; Hettige, T.; Cameron, J.D.; Wong, D.T.L. Association of Epicardial Adipose Tissue and High-Risk Plaque Characteristics: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2017, 6, e006379. [Google Scholar] [CrossRef]
- Otsuka, K.; Ishikawa, H.; Yamaura, H.; Shirasawa, K.; Kasayuki, N. Epicardial Adipose Tissue Volume Is Associated with Low-Attenuation Plaque Volume in Subjects with or without Increased Visceral Fat: A 3-Vessel Coronary Artery Analysis with CT Angiography. Eur. Heart J. 2021, 42. [Google Scholar] [CrossRef]
- Yamashita, K.; Yamamoto, M.H.; Igawa, W.; Ono, M.; Kido, T.; Ebara, S.; Okabe, T.; Saito, S.; Amemiya, K.; Isomura, N.; et al. Association of Epicardial Adipose Tissue Volume and Total Coronary Plaque Burden in Patients with Coronary Artery Disease. Int. Heart J. 2018, 59, 1219–1226. [Google Scholar] [CrossRef] [Green Version]
- Iwasaki, K.; Matsumoto, T.; Aono, H.; Furukawa, H.; Samukawa, M. Relationship between Epicardial Fat Measured by 64-Multidetector Computed Tomography and Coronary Artery Disease. Clin. Cardiol. 2011, 34, 166–171. [Google Scholar] [CrossRef]
- Cosson, E.; Nguyen, M.T.; Rezgani, I.; Berkane, N.; Pinto, S.; Bihan, H.; Tatulashvili, S.; Taher, M.; Sal, M.; Soussan, M.; et al. Epicardial Adipose Tissue Volume and Myocardial Ischemia in Asymptomatic People Living with Diabetes: A Cross-Sectional Study. Cardiovasc. Diabetol. 2021, 20, 224. [Google Scholar] [CrossRef]
- Franssens, B.T.; Nathoe, H.M.; Visseren, F.L.J.; van der Graaf, Y.; Leiner, T.; Algra, A.; van der Graaf, Y.; Grobbee, D.E.; Rutten, G.E.H.M.; Visseren, F.L.J.; et al. Relation of Epicardial Adipose Tissue Radiodensity to Coronary Artery Calcium on Cardiac Computed Tomography in Patients at High Risk for Cardiovascular Disease. Am. J. Cardiol. 2017, 119, 1359–1365. [Google Scholar] [CrossRef]
- Goeller, M.; Achenbach, S.; Marwan, M.; Doris, M.K.; Cadet, S.; Commandeur, F.; Chen, X.; Slomka, P.J.; Gransar, H.; Cao, J.J.; et al. Epicardial Adipose Tissue Density and Volume Are Related to Subclinical Atherosclerosis, Inflammation and Major Adverse Cardiac Events in Asymptomatic Subjects. J. Cardiovasc. Comput. Tomogr. 2018, 12, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Eisenberg, E.; McElhinney, P.A.; Commandeur, F.; Chen, X.; Cadet, S.; Goeller, M.; Razipour, A.; Gransar, H.; Cantu, S.; Miller, R.J.H.; et al. Deep Learning–Based Quantification of Epicardial Adipose Tissue Volume and Attenuation Predicts Major Adverse Cardiovascular Events in Asymptomatic Subjects. Circ. Cardiovasc. Imaging 2020, 13, e009829. [Google Scholar] [CrossRef] [PubMed]
- Fuller, B.; Garland, J.; Anne, S.; Beh, R.; McNevin, D.; Tse, R. Increased Epicardial Fat Thickness in Sudden Death From Stable Coronary Artery Atherosclerosis. Am. J. Forensic Med. Pathol. 2017, 38, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Mahabadi, A.A.; Berg, M.H.; Lehmann, N.; Kälsch, H.; Bauer, M.; Kara, K.; Dragano, N.; Moebus, S.; Jöckel, K.-H.; Erbel, R.; et al. Association of Epicardial Fat with Cardiovascular Risk Factors and Incident Myocardial Infarction in the General Population. J. Am. Coll. Cardiol. 2013, 61, 1388–1395. [Google Scholar] [CrossRef] [PubMed]
- Gorter, P.M.; de Vos, A.M.; van der Graaf, Y.; Stella, P.R.; Doevendans, P.A.; Meijs, M.F.L.; Prokop, M.; Visseren, F.L.J. Relation of Epicardial and Pericoronary Fat to Coronary Atherosclerosis and Coronary Artery Calcium in Patients Undergoing Coronary Angiography. Am. J. Cardiol. 2008, 102, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Balcer, B.; Dykun, I.; Schlosser, T.; Forsting, M.; Rassaf, T.; Mahabadi, A.A. Pericoronary Fat Volume but Not Attenuation Differentiates Culprit Lesions in Patients with Myocardial Infarction. Atherosclerosis 2018, 276, 182–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, R.; van Assen, M.; Ties, D.; Pelgrim, G.J.; van Dijk, R.; Sidorenkov, G.; van Ooijen, P.M.A.; van der Harst, P.; Vliegenthart, R. Focal Pericoronary Adipose Tissue Attenuation Is Related to Plaque Presence, Plaque Type, and Stenosis Severity in Coronary CTA. Eur. Radiol. 2021, 31, 7251–7261. [Google Scholar] [CrossRef]
- Nogic, J.; Kim, J.; Layland, J.; Chan, J.; Cheng, K.; Wong, D.; Brown, A. TCT-241 Pericoronary Adipose Tissue Is a Predictor of In-Stent Restenosis and Stent Failure in Patients Undergoing Coronary Artery Stent Insertion. J. Am. Coll. Cardiol. 2021, 78, B98. [Google Scholar] [CrossRef]
- Kang, J.; Kim, Y.-C.; Park, J.J.; Kim, S.; Kang, S.-H.; Cho, Y.J.; Yoon, Y.E.; Oh, I.-Y.; Yoon, C.-H.; Suh, J.-W.; et al. Increased Epicardial Adipose Tissue Thickness Is a Predictor of New-Onset Diabetes Mellitus in Patients with Coronary Artery Disease Treated with High-Intensity Statins. Cardiovasc. Diabetol. 2018, 17, 10. [Google Scholar] [CrossRef] [Green Version]
- Raggi, P.; Gadiyaram, V.; Zhang, C.; Chen, Z.; Lopaschuk, G.; Stillman, A.E. Statins Reduce Epicardial Adipose Tissue Attenuation Independent of Lipid Lowering: A Potential Pleiotropic Effect. J. Am. Heart Assoc. 2019, 8, e013104. [Google Scholar] [CrossRef] [Green Version]
- Ziyrek, M.; Kahraman, S.; Ozdemir, E.; Dogan, A. Metformin Monotherapy Significantly Decreases Epicardial Adipose Tissue Thickness in Newly Diagnosed Type 2 Diabetes Patients. Rev. Port. Cardiol. 2019, 38, 419–423. [Google Scholar] [CrossRef]
- Iacobellis, G.; Villasante Fricke, A.C. Effects of Semaglutide Versus Dulaglutide on Epicardial Fat Thickness in Subjects with Type 2 Diabetes and Obesity. J. Endocr. Soc. 2020, 4, bvz042. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G.; Mohseni, M.; Bianco, S.D.; Banga, P.K. Liraglutide Causes Large and Rapid Epicardial Fat Reduction. Obesity 2017, 25, 311–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, T.; Aizawa, Y.; Yuasa, S.; Kishi, S.; Fuse, K.; Fujita, S.; Ikeda, Y.; Kitazawa, H.; Takahashi, M.; Sato, M.; et al. The Effect of Dapagliflozin Treatment on Epicardial Adipose Tissue Volume. Cardiovasc. Diabetol. 2018, 17, 6. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.Y.; Cremer, P.C.; Schoenhagen, P. Thoracic Aortic Calcification. JACC Cardiovasc. Imaging 2018, 11, 1012–1026. [Google Scholar] [CrossRef]
- Budoff, M.J.; Nasir, K.; Katz, R.; Takasu, J.; Carr, J.J.; Wong, N.D.; Allison, M.; Lima, J.A.C.; Detrano, R.; Blumenthal, R.S.; et al. Thoracic Aortic Calcification and Coronary Heart Disease Events: The Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 2011, 215, 196–202. [Google Scholar] [CrossRef] [Green Version]
- Allison, M.A.; Hsi, S.; Wassel, C.L.; Morgan, C.; Ix, J.H.; Wright, C.M.; Criqui, M.H. Calcified Atherosclerosis in Different Vascular Beds and the Risk of Mortality. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 140–146. [Google Scholar] [CrossRef] [Green Version]
- Rivera, J.J.; Nasir, K.; Katz, R.; Takasu, J.; Allison, M.; Wong, N.D.; Barr, R.G.; Carr, J.J.; Blumenthal, R.S.; Budoff, M.J. Relationship of Thoracic Aortic Calcium to Coronary Calcium and Its Progression (from the Multi-Ethnic Study of Atherosclerosis [MESA]). Am. J. Cardiol. 2009, 103, 1562–1567. [Google Scholar] [CrossRef]
- El-Saed, A.; Sekikawa, A.; Edmundowicz, D.; Evans, R.W.; Sutton-Tyrrell, K.; Kadowaki, T.; Choo, J.; Takamiya, T.; Kuller, L.H. Coronary Calcification Is More Predictive of Carotid Intimal Medial Thickness in Black Compared to White Middle Aged Men. Atherosclerosis 2008, 196, 913–918. [Google Scholar] [CrossRef] [Green Version]
- Kodama, Y.; Ng, C.S.; Wu, T.T.; Ayers, G.D.; Curley, S.A.; Abdalla, E.K.; Vauthey, J.N.; Charnsangavej, C. Comparison of CT Methods for Determining the Fat Content of the Liver. Am. J. Roentgenol. 2007, 188, 1307–1312. [Google Scholar] [CrossRef] [Green Version]
- Cademartiri, F.; Sverzellati, N.; Guaricci, A.I.; Maffei, E. Fat and Cardiovascular Risk: The Role of Cardiac CT. Eur. Heart J.—Cardiovasc. Imaging 2016, 17, 1368–1369. [Google Scholar] [CrossRef]
- Bos, D.; Leening, M.J.G. Leveraging the Coronary Calcium Scan beyond the Coronary Calcium Score. Eur. Radiol. 2018, 28, 3082–3087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yki-Järvinen, H. Non-Alcoholic Fatty Liver Disease as a Cause and a Consequence of Metabolic Syndrome. Lancet Diabetes Endocrinol. 2014, 2, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Stahl, E.P.; Dhindsa, D.S.; Lee, S.K.; Sandesara, P.B.; Chalasani, N.P.; Sperling, L.S. Nonalcoholic Fatty Liver Disease and the Heart. J. Am. Coll. Cardiol. 2019, 73, 948–963. [Google Scholar] [CrossRef] [PubMed]
- Sung, K.-C.; Wild, S.H.; Kwag, H.J.; Byrne, C.D. Fatty Liver, Insulin Resistance, and Features of Metabolic Syndrome. Diabetes Care 2012, 35, 2359–2364. [Google Scholar] [CrossRef] [Green Version]
- Puchner, S.B.; Lu, M.T.; Mayrhofer, T.; Liu, T.; Pursnani, A.; Ghoshhajra, B.B.; Truong, Q.A.; Wiviott, S.D.; Fleg, J.L.; Hoffmann, U.; et al. High-Risk Coronary Plaque at Coronary CT Angiography Is Associated with Nonalcoholic Fatty Liver Disease, Independent of Coronary Plaque and Stenosis Burden: Results from the ROMICAT II Trial. Radiology 2015, 274, 693–701. [Google Scholar] [CrossRef] [Green Version]
- Wolff, L.; Bos, D.; Murad, S.D.; Franco, O.H.; Krestin, G.P.; Hofman, A.; Vernooij, M.W.; van der Lugt, A. Liver Fat Is Related to Cardiovascular Risk Factors and Subclinical Vascular Disease: The Rotterdam Study. Eur. Heart J.—Cardiovasc. Imaging 2016, 17, 1361–1367. [Google Scholar] [CrossRef] [Green Version]
- Kotronen, A.; Yki-Järvinen, H. Fatty Liver. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 27–38. [Google Scholar] [CrossRef]
- Al Rifai, M.; Silverman, M.G.; Nasir, K.; Budoff, M.J.; Blankstein, R.; Szklo, M.; Katz, R.; Blumenthal, R.S.; Blaha, M.J. The Association of Nonalcoholic Fatty Liver Disease, Obesity, and Metabolic Syndrome, with Systemic Inflammation and Subclinical Atherosclerosis: The Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 2015, 239, 629–633. [Google Scholar] [CrossRef] [Green Version]
- Kannel, W.B.; Abbott, R.D. Incidence and Prognosis of Unrecognized Myocardial Infarction. N. Engl. J. Med. 1984, 311, 1144–1147. [Google Scholar] [CrossRef]
- Madaj, P.; Budoff, M.J. Risk Stratification of Non-Contrast CT beyond the Coronary Calcium Scan. J. Cardiovasc. Comput. Tomogr. 2012, 6, 301–307. [Google Scholar] [CrossRef] [Green Version]
- Kimura, F.; Matsuo, Y.; Nakajima, T.; Nishikawa, T.; Kawamura, S.; Sannohe, S.; Hagiwara, N.; Sakai, F. Myocardial Fat at Cardiac Imaging: How Can We Differentiate Pathologic from Physiologic Fatty Infiltration? RadioGraphics 2010, 30, 1587–1602. [Google Scholar] [CrossRef] [PubMed]
- Zafar, H.M.; Litt, H.I.; Torigian, D.A. CT Imaging Features and Frequency of Left Ventricular Myocardial Fat in Patients with CT Findings of Chronic Left Ventricular Myocardial Infarction. Clin. Radiol. 2008, 63, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, Y.; Kitagawa, K.; Chino, S.; Ishida, M.; Matsuoka, K.; Tanigawa, T.; Nakamura, T.; Hirano, T.; Takeda, K.; Sakuma, H. Adipose Tissue Detected by Multislice Computed Tomography in Patients after Myocardial Infarction. JACC Cardiovasc. Imaging 2009, 2, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Nieman, K.; Cury, R.C.; Ferencik, M.; Nomura, C.H.; Abbara, S.; Hoffmann, U.; Gold, H.K.; Jang, I.-K.; Brady, T.J. Differentiation of Recent and Chronic Myocardial Infarction by Cardiac Computed Tomography. Am. J. Cardiol. 2006, 98, 303–308. [Google Scholar] [CrossRef]
- Gupta, M.; Kadakia, J.; Hacioglu, Y.; Ahmadi, N.; Patel, A.; Choi, T.; Yamada, G.; Budoff, M. Non-Contrast Cardiac Computed Tomography Can Accurately Detect Chronic Myocardial Infarction: Validation Study. J. Nucl. Cardiol. 2011, 18, 96–103. [Google Scholar] [CrossRef] [Green Version]
- Ko, S.M.; Hwang, S.H.; Lee, H.-J. Role of Cardiac Computed Tomography in the Diagnosis of Left Ventricular Myocardial Diseases. J. Cardiovasc. Imaging 2019, 27, 73. [Google Scholar] [CrossRef] [Green Version]
- Lardo, A.C.; Cordeiro, M.A.S.; Silva, C.; Amado, L.C.; George, R.T.; Saliaris, A.P.; Schuleri, K.H.; Fernandes, V.R.; Zviman, M.; Nazarian, S.; et al. Contrast-Enhanced Multidetector Computed Tomography Viability Imaging after Myocardial Infarction. Circulation 2006, 113, 394–404. [Google Scholar] [CrossRef] [Green Version]
- Mahnken, A.H.; Koos, R.; Katoh, M.; Wildberger, J.E.; Spuentrup, E.; Buecker, A.; Günther, R.W.; Kühl, H.P. Assessment of Myocardial Viability in Reperfused Acute Myocardial Infarction Using 16-Slice Computed Tomography in Comparison to Magnetic Resonance Imaging. J. Am. Coll. Cardiol. 2005, 45, 2042–2047. [Google Scholar] [CrossRef] [Green Version]
- Gerber, B.L.; Belge, B.; Legros, G.J.; Lim, P.; Poncelet, A.; Pasquet, A.; Gisellu, G.; Coche, E.; Vanoverschelde, J.-L.J. Characterization of Acute and Chronic Myocardial Infarcts by Multidetector Computed Tomography. Circulation 2006, 113, 823–833. [Google Scholar] [CrossRef] [Green Version]
- Palmisano, A.; Vignale, D.; Tadic, M.; Moroni, F.; De Stefano, D.; Gatti, M.; Boccia, E.; Faletti, R.; Oppizzi, M.; Peretto, G.; et al. Myocardial Late Contrast Enhancement CT in Troponin-Positive Acute Chest Pain Syndrome. Radiology 2022, 302, 545–553. [Google Scholar] [CrossRef]
- Bouleti, C.; Baudry, G.; Iung, B.; Arangalage, D.; Abtan, J.; Ducrocq, G.; Steg, P.-G.; Vahanian, A.; Henry-Feugeas, M.-C.; Pasi, N.; et al. Usefulness of Late Iodine Enhancement on Spectral CT in Acute Myocarditis. JACC Cardiovasc. Imaging 2017, 10, 826–827. [Google Scholar] [CrossRef] [PubMed]
- Esposito, A.; Palmisano, A.; Antunes, S.; Maccabelli, G.; Colantoni, C.; Rancoita, P.M.V.; Baratto, F.; Di Serio, C.; Rizzo, G.; De Cobelli, F.; et al. Cardiac CT with Delayed Enhancement in the Characterization of Ventricular Tachycardia Structural Substrate. JACC Cardiovasc. Imaging 2016, 9, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Treibel, T.A.; Fontana, M.; Steeden, J.A.; Nasis, A.; Yeung, J.; White, S.K.; Sivarajan, S.; Punwani, S.; Pugliese, F.; Taylor, S.A.; et al. Automatic Quantification of the Myocardial Extracellular Volume by Cardiac Computed Tomography: Synthetic ECV by CCT. J. Cardiovasc. Comput. Tomogr. 2017, 11, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Im, D.J.; Youn, J.-C.; Chang, S.; Suh, Y.J.; Hong, Y.J.; Kim, Y.J.; Hur, J.; Choi, B.W. Myocardial Extracellular Volume Fraction with Dual-Energy Equilibrium Contrast-Enhanced Cardiac CT in Nonischemic Cardiomyopathy: A Prospective Comparison with Cardiac MR Imaging. Radiology 2016, 280, 49–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Treibel, T.A.; Bandula, S.; Fontana, M.; White, S.K.; Gilbertson, J.A.; Herrey, A.S.; Gillmore, J.D.; Punwani, S.; Hawkins, P.N.; Taylor, S.A.; et al. Extracellular Volume Quantification by Dynamic Equilibrium Cardiac Computed Tomography in Cardiac Amyloidosis. J. Cardiovasc. Comput. Tomogr. 2015, 9, 585–592. [Google Scholar] [CrossRef] [Green Version]
- Richards, C.E.; Obaid, D.R. Low-Dose Radiation Advances in Coronary Computed Tomography Angiography in the Diagnosis of Coronary Artery Disease. Curr. Cardiol. Rev. 2019, 15, 304–315. [Google Scholar] [CrossRef]




| Chronic Coronary Syndromes (CCS) | |
| ESC 2019 | AHA/ACC 2012 |
| Non-invasive functional imaging for myocardial ischemic or CCTA is recommended as the initial test to diagnose CAD in symptomatic patients in whom obstructive CAD cannot be excluded by clinical assessment alone (I-B) | CCTA can be useful as a first-line test for risk assessment in patients with SIHD who are unable to exercise to an adequate workload regardless of interpretability of ECG (IIa-C) |
| It is recommended that selection of the initial non-invasive diagnostic test is done based on the clinical likelihood of CAD and other patient characteristics that influence test performance, local expertise, and the availability of tests. (I-C) | CCTA may be reasonable for risk assessment in patients with SIHD who are able to exercise to an adequate workload but have an uninterpretable ECG (IIb-B) |
| CCTA should be considered as an alternative to invasive angiography if another non-invasive test is equivocal or non-diagnostic. (IIa-C) | CCTA can be useful for risk assessment in patients with SIHD who have an indeterminate result from functional testing (IIa-C) |
| Acute Coronary Syndromes (ACS) | |
| ESC 2020 (NSTEMI) | AHA/ACC 2021 (chest pain) |
| CCTA is recommended as an alternative to ICA to exclude ACS when there is a low-to-intermediate likelihood of CAD and when cardiac troponin and/or ECG are normal or inconclusive (I-A) | For intermediate-risk patients with acute chest pain and no known CAD eligible for diagnostic testing after a negative or inconclusive evaluation for ACS, CCTA is useful for exclusion of atherosclerotic plaque and obstructive CAD (I-A) |
| In patients with no recurrence of chest pain, normal ECG findings, and normal levels of cardiac troponin (preferably high sensitivity), but still with a suspected ACS, a non-invasive stress test (preferably with imaging) for inducible ischaemia or CCTA is recommended before deciding on an invasive approach. (I-B) | For intermediate-risk patients with acute chest pain with evidence of previous mildly abnormal stress test results, CCTA is reasonable for diagnosing obstructive CAD (IIa-C) |
| CardiovascularPrevention | |
| ESC 2021 | AHA/ACC 2019 |
| Coronary artery calcium (CAC) scoring can reclassify CVD risk in addition to conventional risk factors, and may be considered in men and women with calculated risks around decision thresholds | In intermediate-risk (7.5–20% 10-year ASCVD risk) adults or selected borderline-risk (5–7.5% 10-year ASCVD risk) adults in whom a coronary artery calcium score is measured for the purpose of making a treatment decision: -CAC scoring is zero → it is reasonable to withhold statin therapy and reassess in 5 to 10 years, as long as higher-risk conditions are absent (e.g., diabetes, family history of premature CAD, cigarette smoking); -CAC score is 1 to 99 → it is reasonable to initiate statin therapy for patients >55 years of age; -CAC score is 100 or higher → it is reasonable to initiate statin therapy (IIa-B) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernardini, F.; Gelfusa, M.; Celeski, M.; Coletti, F.; Nusca, A.; De Stefano, D.; Piccirillo, F.; Mangiacapra, F.; Gallo, P.; Cammalleri, V.; et al. Beyond the Calcium Score: What Additional Information from a CT Scan Can Assist in Cardiovascular Risk Assessment? Appl. Sci. 2023, 13, 241. https://doi.org/10.3390/app13010241
Bernardini F, Gelfusa M, Celeski M, Coletti F, Nusca A, De Stefano D, Piccirillo F, Mangiacapra F, Gallo P, Cammalleri V, et al. Beyond the Calcium Score: What Additional Information from a CT Scan Can Assist in Cardiovascular Risk Assessment? Applied Sciences. 2023; 13(1):241. https://doi.org/10.3390/app13010241
Chicago/Turabian StyleBernardini, Federico, Martina Gelfusa, Mihail Celeski, Federica Coletti, Annunziata Nusca, Domenico De Stefano, Francesco Piccirillo, Fabio Mangiacapra, Paolo Gallo, Valeria Cammalleri, and et al. 2023. "Beyond the Calcium Score: What Additional Information from a CT Scan Can Assist in Cardiovascular Risk Assessment?" Applied Sciences 13, no. 1: 241. https://doi.org/10.3390/app13010241
APA StyleBernardini, F., Gelfusa, M., Celeski, M., Coletti, F., Nusca, A., De Stefano, D., Piccirillo, F., Mangiacapra, F., Gallo, P., Cammalleri, V., Cocco, N., Rinaldi, R., Quattrocchi, C. C., Ussia, G. P., & Grigioni, F. (2023). Beyond the Calcium Score: What Additional Information from a CT Scan Can Assist in Cardiovascular Risk Assessment? Applied Sciences, 13(1), 241. https://doi.org/10.3390/app13010241








