Study on the Capacity Fading Effect of Low-Rate Charging on Lithium-Ion Batteries in Low-Temperature Environment
Abstract
1. Introduction
- Lithium-ion battery aging experiments and experiment platform construction. Design of the aging paths and experimental plans of the lithium-ion battery, and explanation of the flow of the cycle experiment and parameter performance test experiment;
- Analysis of the parameter characteristics of lithium-ion batteries under a low-temperature and low-rate cycling path. The aging of the battery is described in terms of each parameter such as capacity, direct current internal resistance (DCIR), electrochemical impedance and energy storage characteristics;
- Analysis of aging characteristics of lithium-ion batteries under a low-temperature, low-rate cycling path. The aging process of lithium-ion batteries will be further analyzed in terms of lithium precipitation during the aging process, and the capacity fade mechanism of power batteries will be summarized.
2. Lithium-Ion Battery Experimental Platform Construction and Aging Path Design
2.1. Experimental Battery and Test Equipment
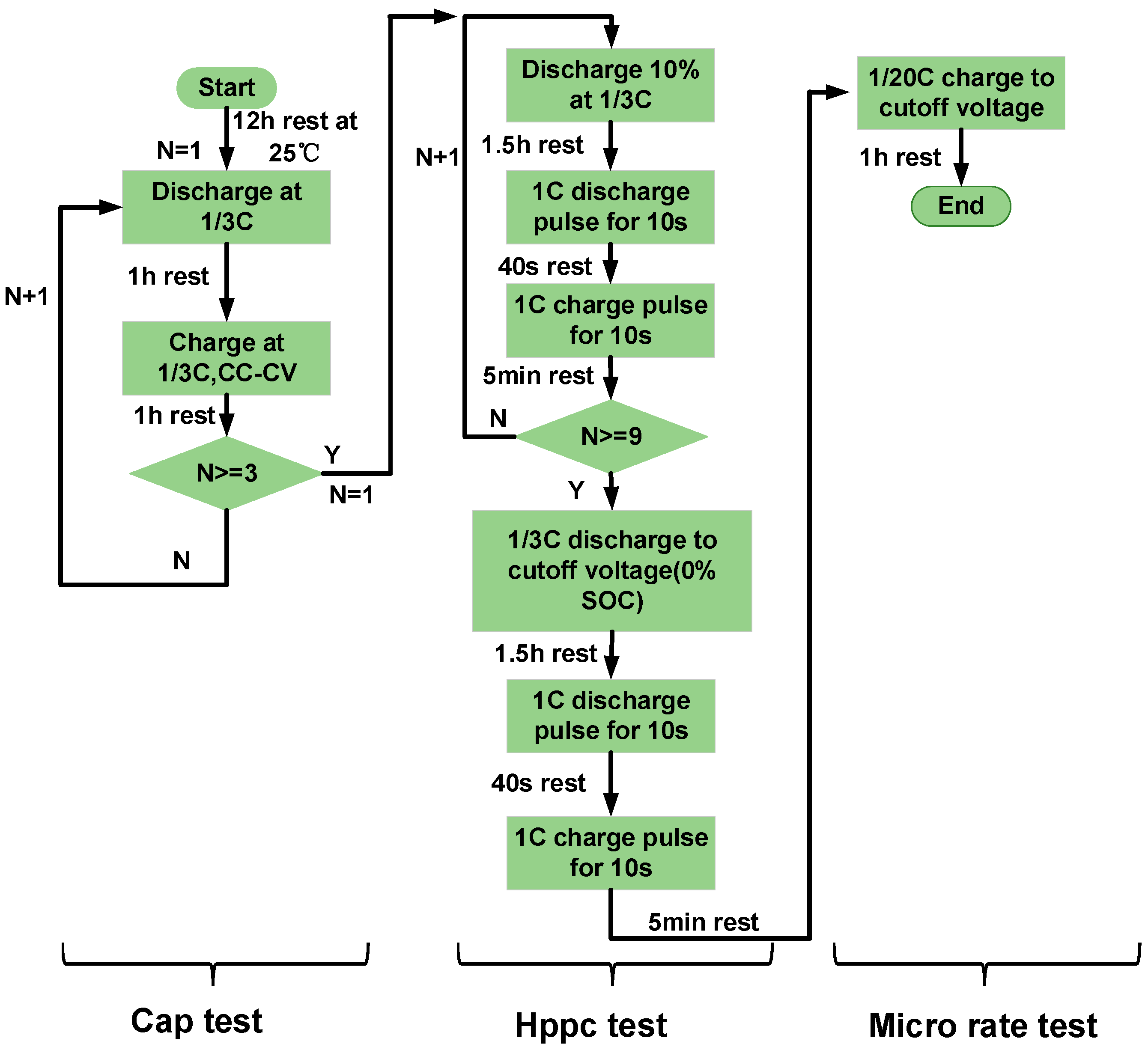
2.2. Design of Lithium-Ion Battery Aging Cycle Experimental Scheme
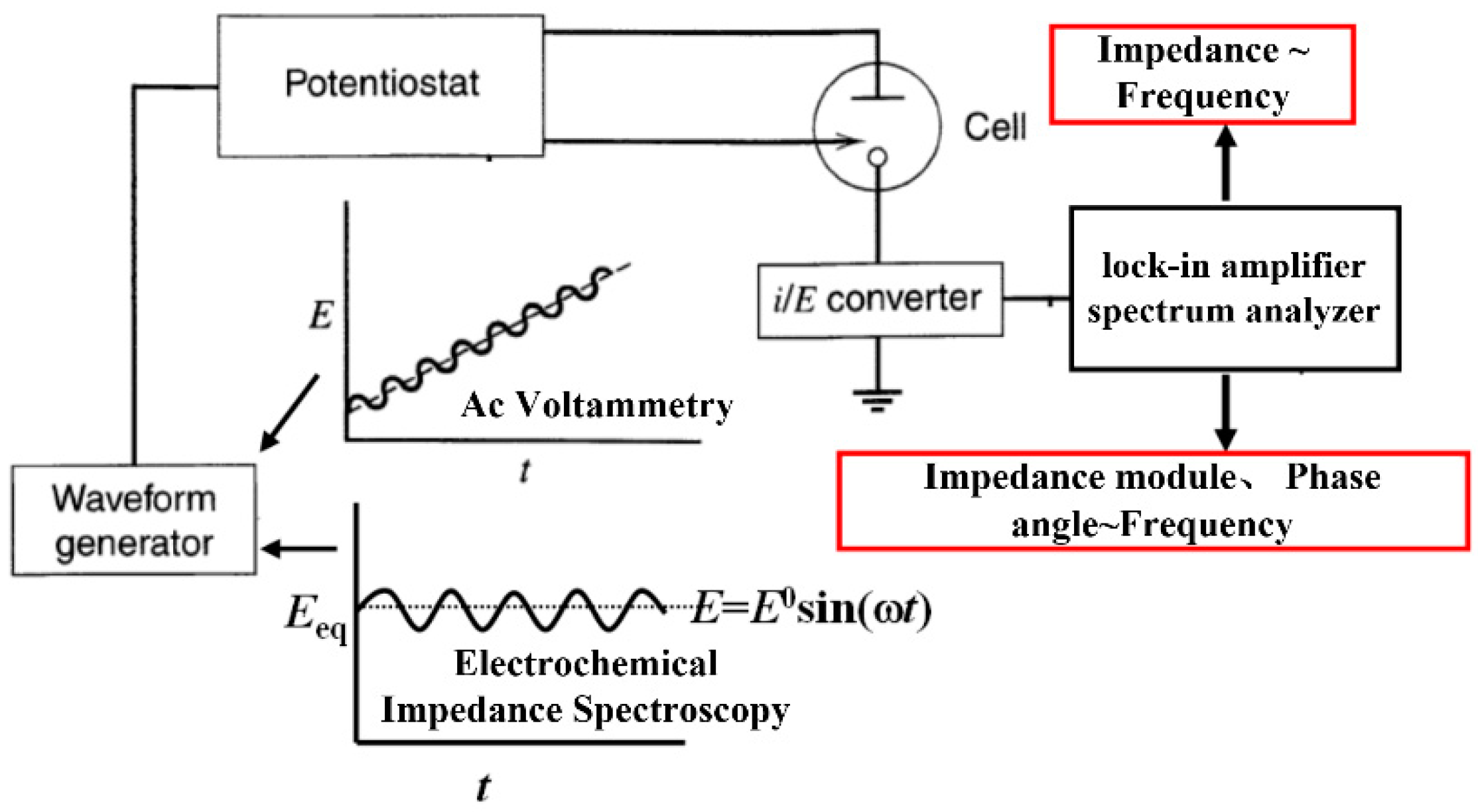
- Generate a small amplitude sinusoidal potential signal by a waveform generator;
- The signal is applied to the experimental battery through the control and adjustment of the potentiostatic instrument;
- Convert the output current/potential signal;
- The converted signal outputs its impedance and its modulus or phase angle via a lock-in amplifier or spectrum analyzer.
3. Analysis of the Impact of Low-Temperature Low-Rate Charging on the Parameters of Lithium-Ion Batteries
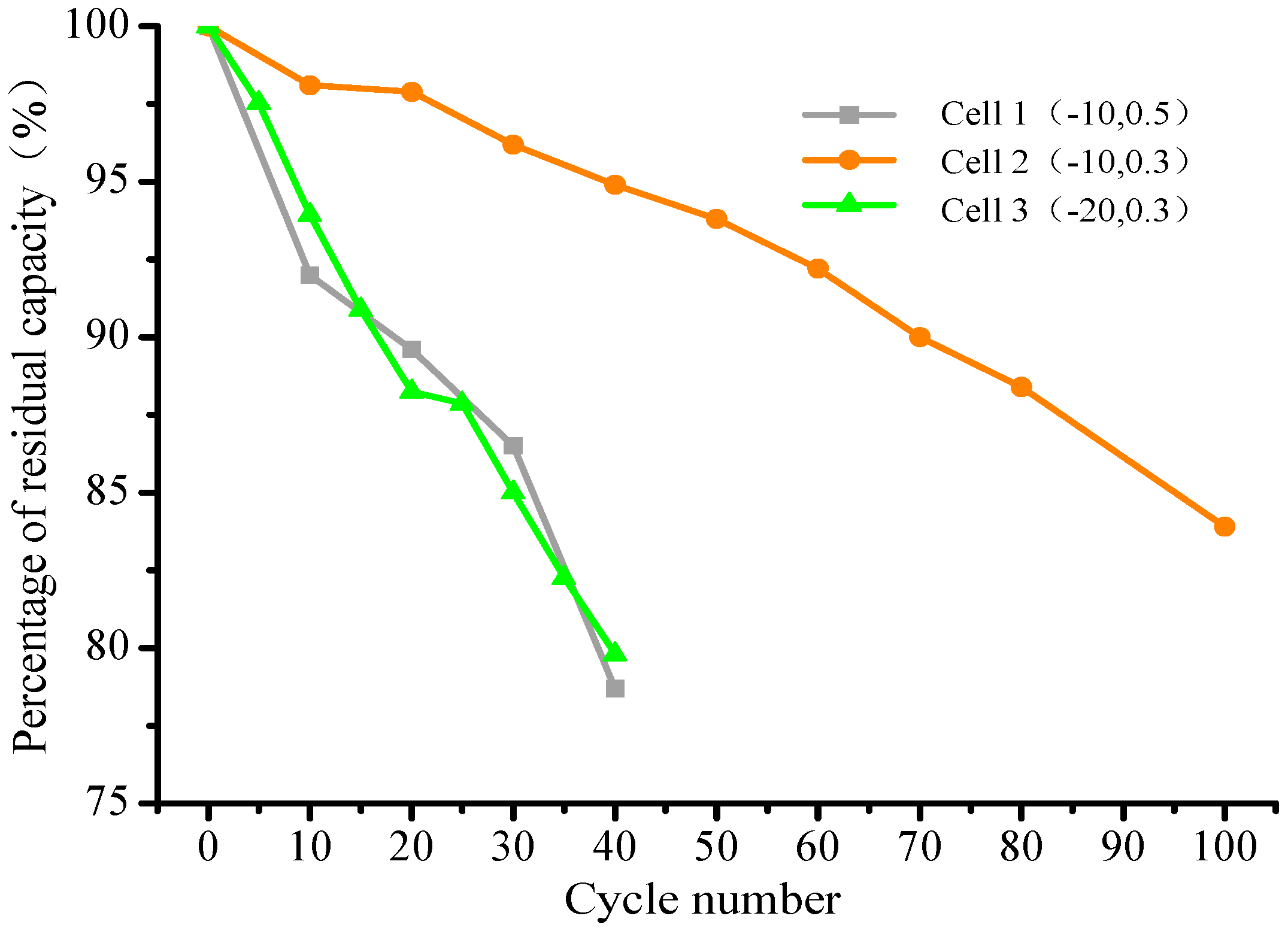
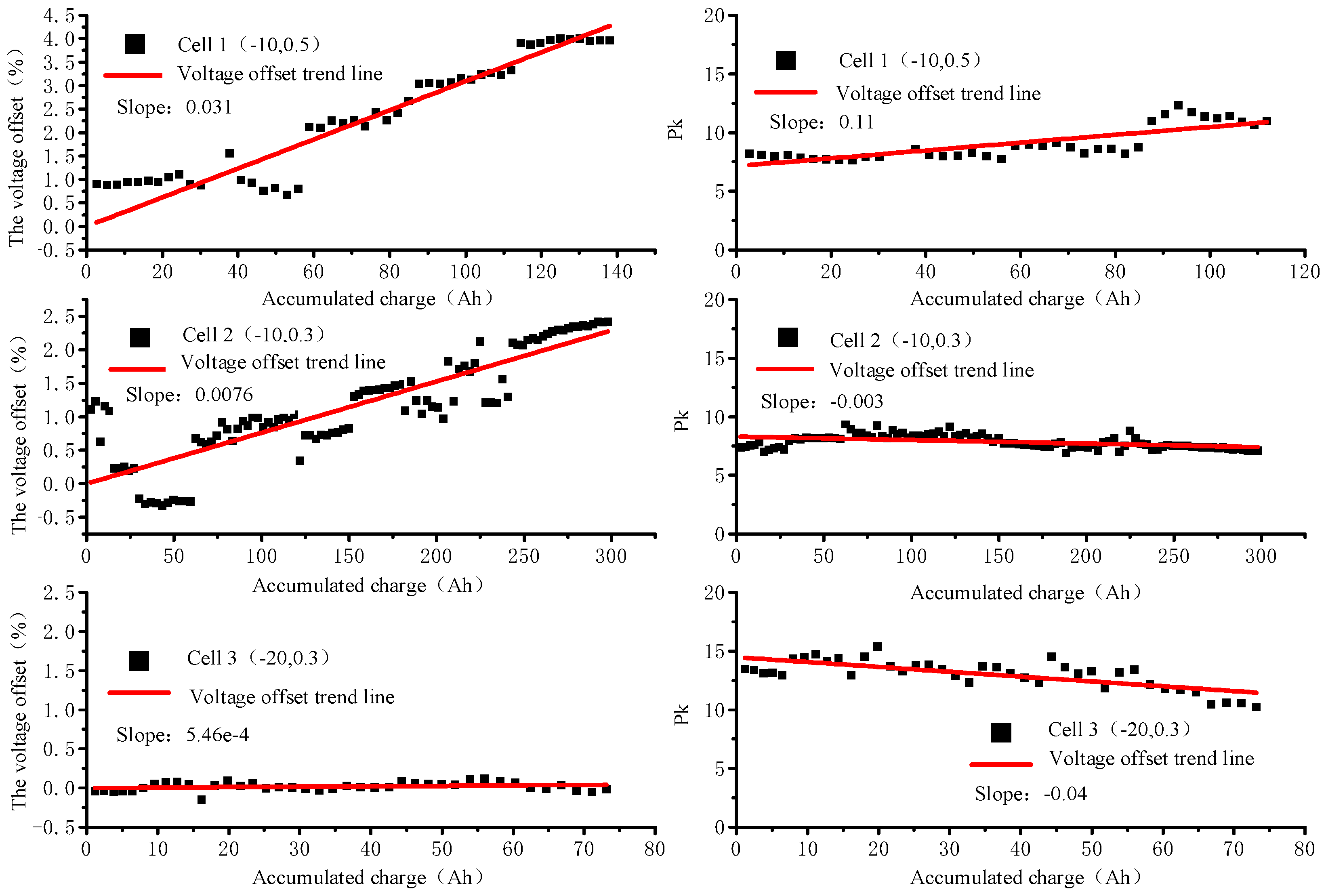
3.1. Capacity Fading Analysis
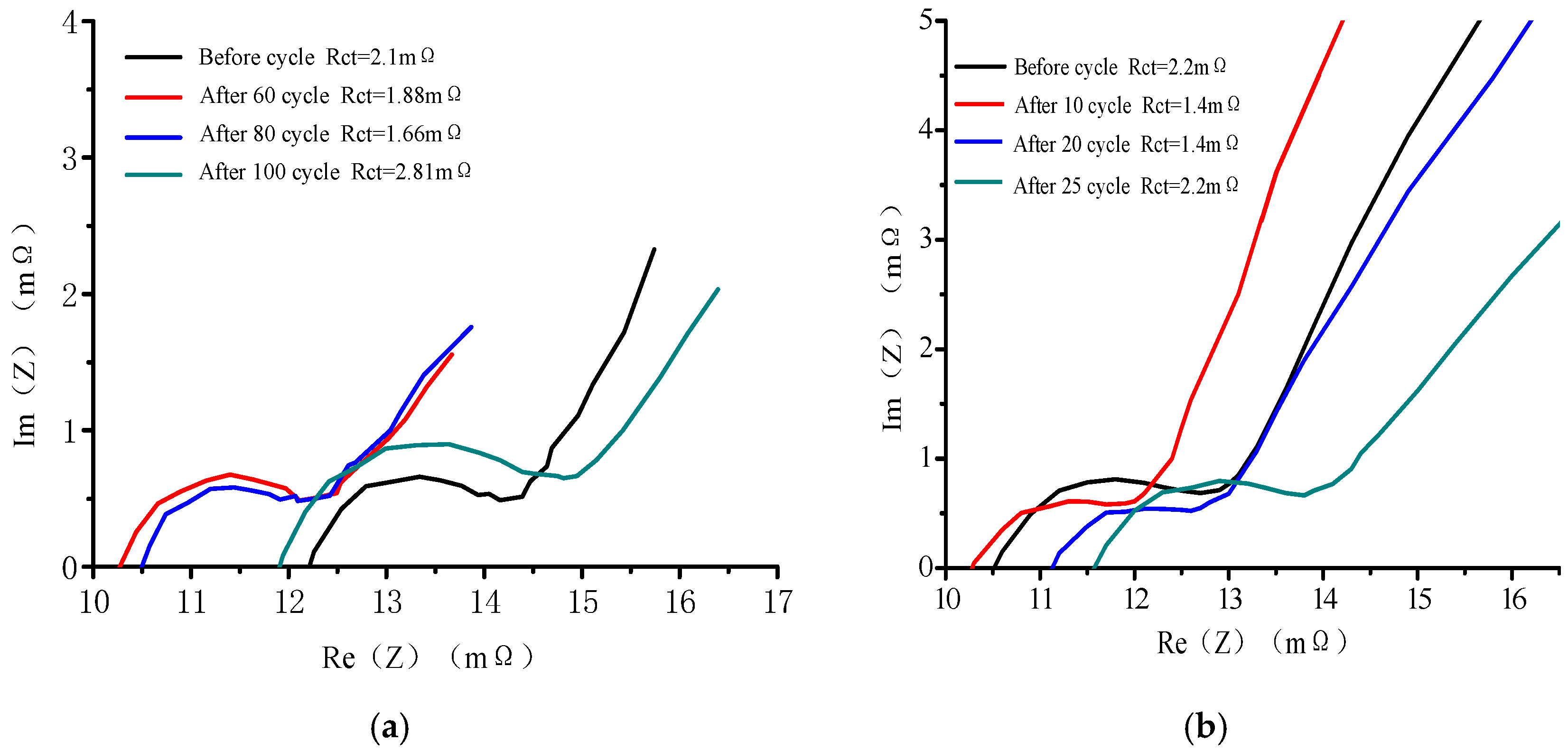
3.2. Impedance Analysis
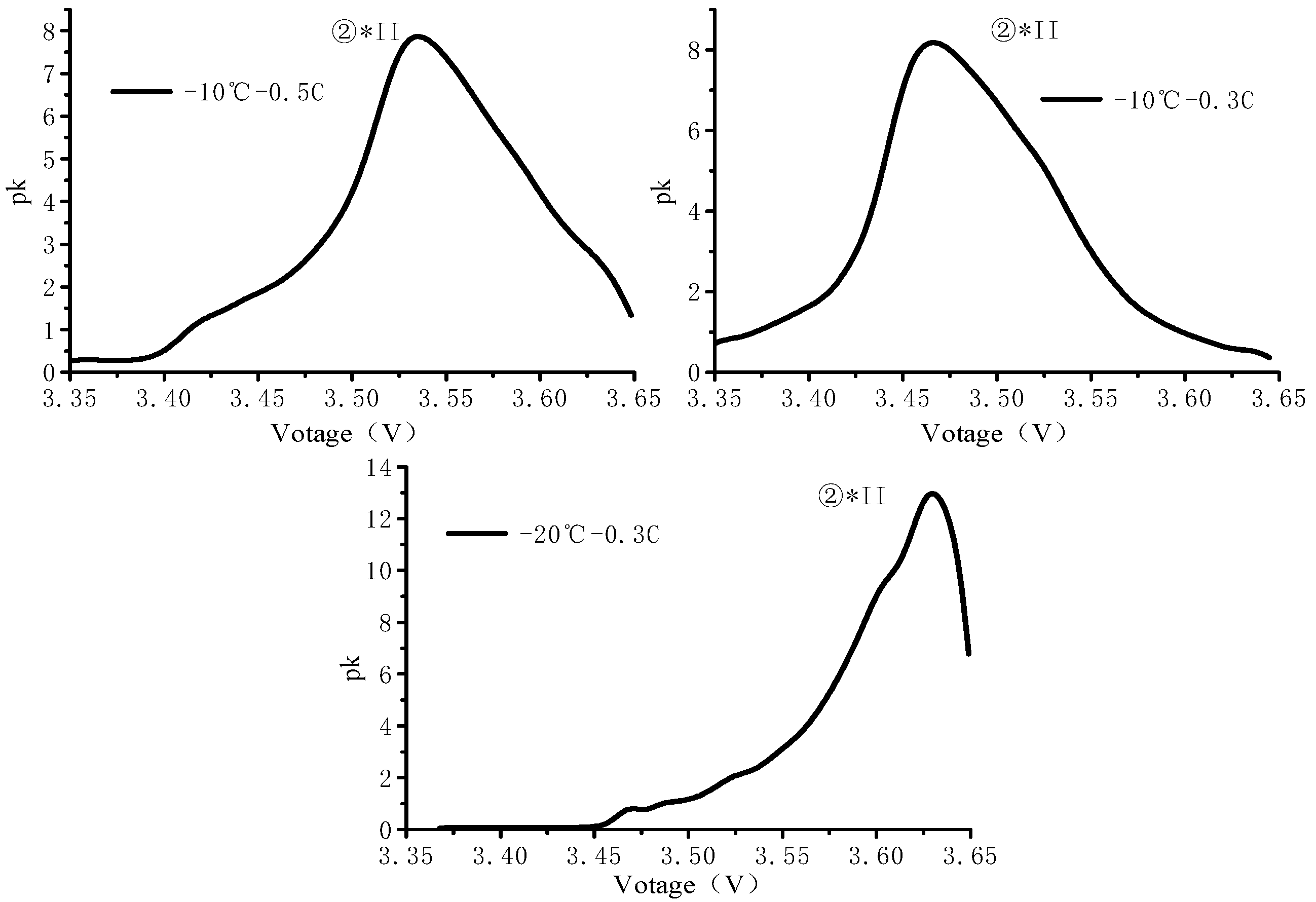
3.3. Cyclic Process PDF Analysis
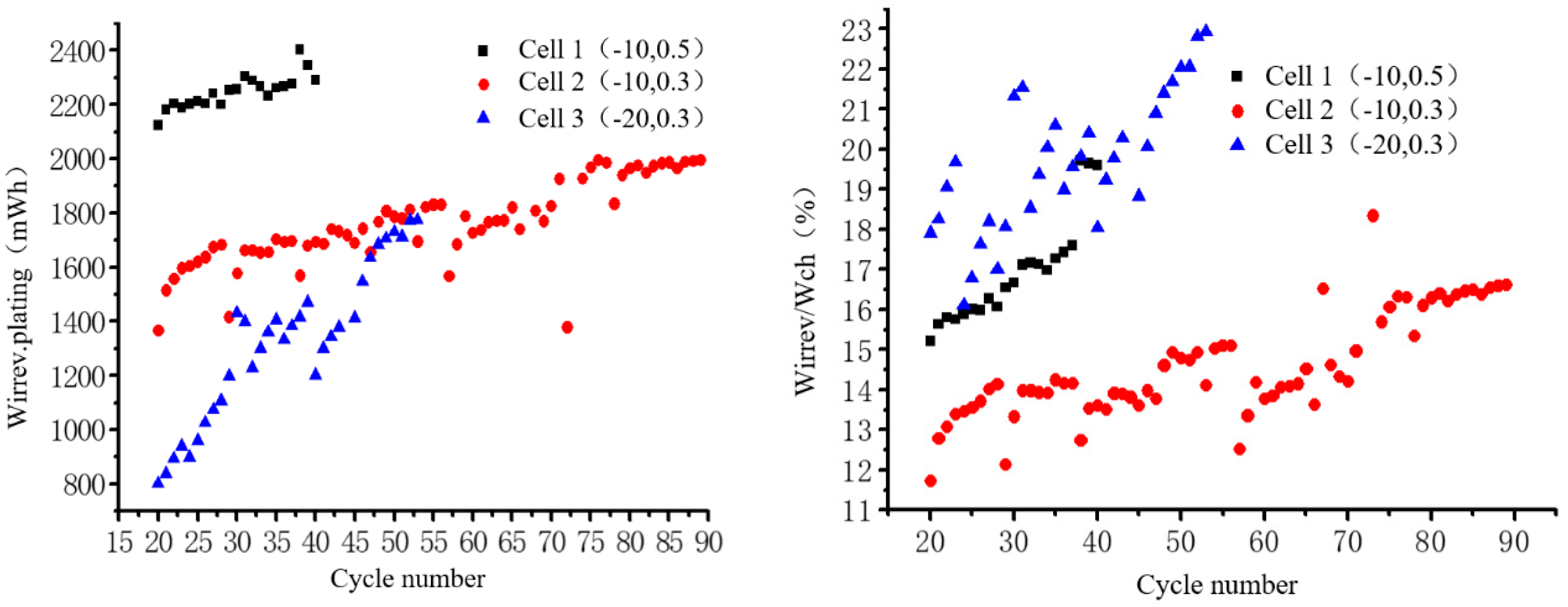
4. Lithium Precipitation Analysis of Low-Temperature Low-Rate Charge-State Lithium-Ion Batteries
5. Conclusions
- According to the analysis of impedance parameters, the influence of the contact between the SEI membrane of the electrode or the particles of the active material on the impedance of the commercial battery is weakened at the beginning of the cycle. As the cycle progresses, the SEI film thickens and the impedance gradually increases. The change in the real-part impedance and charge transfer impedance Rct at sub-zero temperature indicates that the thickening of the negative SEI film occurs earlier than the aging of the positive electrode material;
- The PDF peak characteristics of the cycling process indicate that the sub-zero temperature has a serious polarization effect on the battery cycle, and the effect of increasing the rate at the sub-zero temperature is mainly reflected in slowing down the phase transition reaction;
- The change in irreversible energy Wirrev.plating indicates that the irreversible part of the lithium plating consumes the dendrite growth on the surface of the SEI membrane at the sub-zero temperature. Increasing the rate will aggravate the formation of lithium dendrites, while decreasing the temperature will accelerate the thickening speed of SEI films. The change in the ratio of binding energy Wirrev.plating/Wch further indicates that the degradation of the cathode embedded/delithium is caused by the thickening of SEI films.
Author Contributions
Funding
Conflicts of Interest
Nomenclatures
| SEI | solid electrolyte interphase |
| SOC | state of charge |
| SOH | state of health |
| C-rate/C | the measurement of the charge or discharge current with respect to its nominal capacity. e.g., 2 C = 2 × Cn(A) |
| RPT | reference performance test |
| HPPC | hybrid pulse power characterization |
| Cn | nominal capacity of battery |
| CC-CV | constant-current and constant-voltage |
| DCIR | Direct Current Internal Resistance |
| Probability Density Function | |
| EIS | Electrochemical Impedance Spectra |
References
- Shang, Y.L.; Cui, N.X.; Duan, B.; Zhang, C. Analysis and optimization of star-structured switched-capacitor equalizers for series-connected battery strings. IEEE Trans. Power Electron. 2018, 33, 9631–9646. [Google Scholar] [CrossRef]
- Liu, K.L.; Li, Y.; Hu, X.S.; Lucu, M.; Widanage, W.D. Gaussian process regression with automatic relevance determination kernel for calendar aging prediction of lithium-ion batteries. IEEE Trans. Ind. Inf. 2020, 16, 3767–3777. [Google Scholar] [CrossRef]
- Xiong, R.; Li, L.L.; Yu, Q.Q.; Jin, Q.; Yang, R. A set membership theory based parameter and state of charge co-estimation method for all-climate batteries. J. Clean. Prod. 2020, 249, 119380. [Google Scholar] [CrossRef]
- Wu, S.J.; Xiong, R.; Li, H.L.; Nian, V.; Ma, S. The state of the art on preheating lithium-ion batteries in cold weather. J. Energy Storage 2020, 27, 101059. [Google Scholar] [CrossRef]
- Zhang, S.S.; Xu, K.; Jow, T.R. The low temperature performance of Li-ion batteries. J. Power Sources 2003, 115, 137–140. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, Y.; Wang, C.Y. Li-Ion Cell Operation at Low Temperatures. J. Electrochem. Soc. 2013, 160, A636–A649. [Google Scholar] [CrossRef]
- Ma, S.; Jiang, M.; Tao, P.; Song, C.; Wu, J.; Wang, J.; Deng, T.; Shang, W. Temperature effect and thermal impact in lithium-ion batteries: A review. Prog. Nat. Sci. Mater. Int. 2018, 28, 653–666. [Google Scholar] [CrossRef]
- Liu, D.; Zhu, W.; Kim, C.; Cho, M.; Guerfi, A.; Delp, S.A.; Allen, J.L.; Jow, T.R.; Zaghib, K. High-energy lithium-ion battery using substituted LiCoPO4: From coin type to 1Ah cell. J. Power Sources 2018, 388, 52–56. [Google Scholar] [CrossRef]
- Xiong, R.; Li, L.; Tian, J. Towards a smarter battery management system: A critical review on battery state of health monitoring methods. J. Power Sources 2018, 405, 18–29. [Google Scholar] [CrossRef]
- Jaguemont, J.; Boulon, L.; Venet, P.; Dubé, Y.; Sari, A. Lithium-Ion Battery Aging Experiments at Subzero Temperatures and Model Development for Capacity Fade Estimation. IEEE Trans. Veh. Technol. 2016, 65, 4328–4343. [Google Scholar] [CrossRef]
- Li, Y.; Qian, K.; He, Y.B.; Kaneti, Y.V.; Liu, D.; Luo, D.; Li, H.; Li, B.; Kang, F. Study on the reversible capacity loss of layered oxide cathode during low-temperature operation. J. Power Sources 2017, 342, 24–30. [Google Scholar] [CrossRef]
- Gao, F.; Tang, Z. Kinetic behavior of LiFePO4/C cathode material for lithium-ion batteries. Electrochim. Acta 2008, 53, 5071–5075. [Google Scholar] [CrossRef]
- Maruyama, J.; Maruyama, S.; Fukuhara, T.; Chashiro, K.; Uyama, H. Ordered mesoporous structure by graphitized carbon nanowall assembly. Carbon 2017, 126, 452–455. [Google Scholar] [CrossRef]
- Maruyama, S.; Fukutsuka, T.; Miyazaki, K.; Abe, Y.; Yoshizawa, N.; Abe, T. Lithium-ion intercalation and deintercalation behaviors of graphitized carbon nanospheres. J. Mater. Chem. A 2018, 6, 1128–1137. [Google Scholar] [CrossRef]
- Kabir, M.M.; Demirocak, D.E. Degradation mechanisms in Li-ion batteries: A state-of-the-art review. Int. J. Energy Res. 2017, 41, 1963–1986. [Google Scholar] [CrossRef]
- Li, X.; Meng, X.; Choe, S.Y.; Joe, W.T. Modeling and analysis of LiFePO4/Carbon battery considering two-phase transition during galvanostatic charging/discharging. Electrochim. Acta 2015, 155, 447–457. [Google Scholar] [CrossRef]
- Li, Z.; Huang, J.; Liaw, B.Y.; Metzler, V.; Zhang, J. A review of lithium deposition in lithium-ion and lithium metal secondary batteries. J. Power Sources 2014, 254, 168–182. [Google Scholar] [CrossRef]
- Burow, D.; Sergeeva, K.; Calles, S.; Schorb, K.; Boerger, A.; Roth, C.; Heitjans, P. Inhomogeneous degradation of graphite anodes in automotive lithium ion batteries under low-temperature pulse cycling conditions. J. Power Sources 2016, 307, 806–814. [Google Scholar] [CrossRef]
- Wu, C.M.; Chang, C.C.; Avdeev, M.; Pan, P.I.; Li, W.H. In operando detection of lithium diffusion behaviors at low temperature in 18650 Li-ion battery anode. Phys. B Condens. Matter 2018, 551, 305–308. [Google Scholar] [CrossRef]
- Petzl, M.; Kasper, M.; Danzer, M.A. Lithium plating in a commercial lithium-ion battery–A low-temperature aging study. J. Power Sources 2015, 275, 799–807. [Google Scholar] [CrossRef]
- Lu, L.; Han, X.; Li, J.; Hua, J.; Ouyang, M. A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources 2013, 226, 272–288. [Google Scholar] [CrossRef]
- Nazari, A.; Kavian, S.; Nazari, A. Lithium-Ion Batteries’ Energy Efficiency Prediction Using Physics-Based and State-of-the-Art Artificial Neural Network-Based Models. J. Energy Resour. Technol. 2020, 142, 10. [Google Scholar] [CrossRef]
- Farhad, S.; Nazari, A. Introducing the energy efficiency map of lithium-ion batteries. Int. J. Energy Res. 2019, 43, 931–944. [Google Scholar] [CrossRef]
- Bitzer, B.; Gruhle, A. A new method for detecting lithium plating by measuring the cell thickness. J. Power Sources 2014, 262, 297–302. [Google Scholar] [CrossRef]
- Uhlmann, C.; Illig, J.; Ender, M.; Schuster, R.; Ivers-Tiffée, E. In situ detection of lithium metal plating on graphite in experimental cells. J. Power Sources 2015, 279, 428–438. [Google Scholar] [CrossRef]
- Schindler, S.; Bauer, M.; Petzl, M.; Danzer, M.A. Voltage relaxation and impedance spectroscopy as in-operando methods for the detection of lithium plating on graphitic anodes in commercial lithium-ion cells. J. Power Sources 2016, 304, 170–180. [Google Scholar] [CrossRef]
- Jian-bo, Z.H.; Lai-suo, S.U.; Xin-yu, L.I.; Hao, G.E.; Ya-kun, Z.H.; Zhe, L.I. Lithium plating identification from degradation behaviors of lithium-ion cells. J. Electrochem. 2016, 22, 607–616. [Google Scholar]
- Smart, M.C.; Ratnakumar, B.V. Effects of Electrolyte Composition on Lithium Plating in Lithium-Ion Cells. J. Electrochem. Soc. 2011, 158, A379. [Google Scholar] [CrossRef]
- Liu, Q.; Du, C.; Shen, B.; Zuo, P.; Cheng, X.; Ma, Y.; Yin, G.; Gao, Y. Understanding undesirable anode lithium plating issues in lithium-ion batteries. RSC Adv. 2016, 6, 88683–88700. [Google Scholar] [CrossRef]
- Petzl, M.; Danzer, M.A. Nondestructive detection, characterization, and quantification of lithium plating in commercial lithium-ion batteries. J. Power Sources 2014, 254, 80–87. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, J.; He, X.; Gao, J.; Li, J.; Wan, C.; Jiang, C. Electrochemical impedance spectroscopy (EIS) study of LiNi1/3Co1/3Mn1/3O2 for Li-ion batteries. Int. J. Electrochem. Sci. 2012, 7, 345–353. [Google Scholar]
- Feng, X.; Li, J.; Ouyang, M.; Lu, L.; Li, J.; He, X. Using probability density function to evaluate the state of health of lithium-ion batteries. J. Power Sources 2013, 232, 209–218. [Google Scholar] [CrossRef]




| Parameter/Unit | Value |
|---|---|
| Operating voltage/V | 3.2 |
| Nominal capacity/A•h | 5.0 |
| Charge cut-off voltage/V | 3.65 |
| Discharge cut-off voltage/V | 2.5 |
| Max. continuous charge current/A | 5 |
| Max. continuous discharge current/A | 12.5 |
| Battery Number | Test Conditions |
|---|---|
| 1 | −10 °C, 0.5 C |
| 2 | −10 °C, 0.3 C |
| 3 | −20 °C, 0.3 C |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Wang, W.; Sun, Y.; Wen, T.; Chen, J.; Du, J. Study on the Capacity Fading Effect of Low-Rate Charging on Lithium-Ion Batteries in Low-Temperature Environment. World Electr. Veh. J. 2020, 11, 55. https://doi.org/10.3390/wevj11030055
Wu X, Wang W, Sun Y, Wen T, Chen J, Du J. Study on the Capacity Fading Effect of Low-Rate Charging on Lithium-Ion Batteries in Low-Temperature Environment. World Electric Vehicle Journal. 2020; 11(3):55. https://doi.org/10.3390/wevj11030055
Chicago/Turabian StyleWu, Xiaogang, Wenbo Wang, Yizhao Sun, Tao Wen, Jizhong Chen, and Jiuyu Du. 2020. "Study on the Capacity Fading Effect of Low-Rate Charging on Lithium-Ion Batteries in Low-Temperature Environment" World Electric Vehicle Journal 11, no. 3: 55. https://doi.org/10.3390/wevj11030055
APA StyleWu, X., Wang, W., Sun, Y., Wen, T., Chen, J., & Du, J. (2020). Study on the Capacity Fading Effect of Low-Rate Charging on Lithium-Ion Batteries in Low-Temperature Environment. World Electric Vehicle Journal, 11(3), 55. https://doi.org/10.3390/wevj11030055







