Research Progress on Thermal Runaway Warning Methods and Fire Extinguishing Technologies for Lithium-Ion Batteries
Abstract
1. Introduction
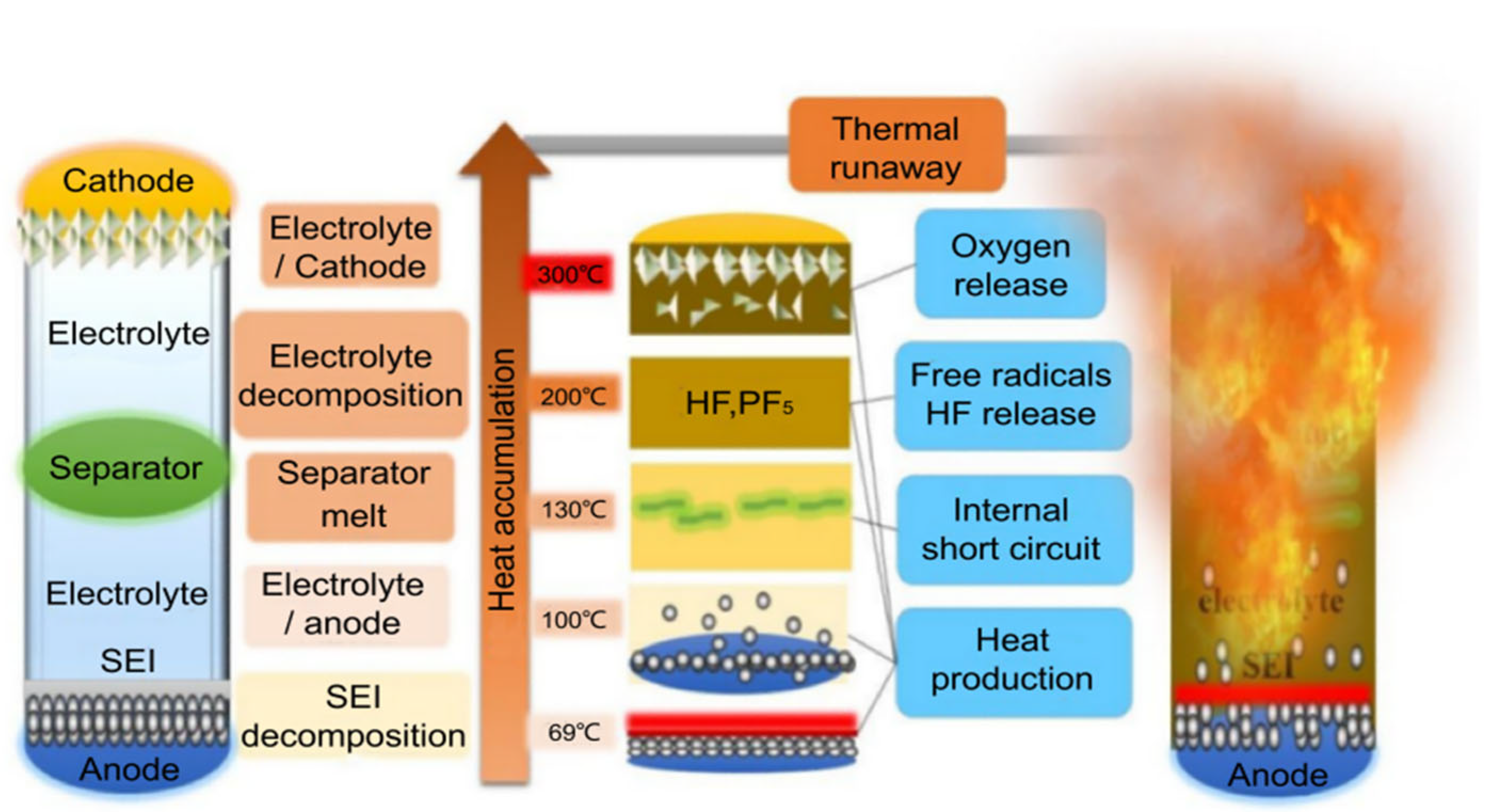
2. Thermal Runaway Mechanism and Triggers
2.1. Thermal Runaway Mechanism
2.1.1. Solid Electrolyte Interphase (SEI) Decomposition
2.1.2. Reactions Between Anode and Electrolyte
2.1.3. Separator Collapse
2.1.4. Decomposition of Cathode Materials and Reactions with Electrolyte
2.1.5. Electrolyte Decomposition Reactions
2.1.6. Reactions Between Anode and Binder Decomposition
2.2. Causes of Thermal Runaway
2.2.1. Mechanical Abuse
2.2.2. Electrical Abuse
2.2.3. Thermal Abuse
2.2.4. Internal Short Circuit
3. Thermal Runaway Warning Technology
- High sensitivity and real-time capabilities to quickly detect internal temperature changes and gas concentration fluctuations, providing timely warnings for response.
- Sensors must be stable, heat-resistant, and interference-resistant and should be strategically placed at critical battery locations for comprehensive monitoring.
- Advanced data processing and analysis capabilities, integrating sophisticated algorithms and machine learning models, are essential to extract key features and reduce false alarms and missed detections.
- The system should have strong compatibility and scalability, enabling seamless integration with the battery management system (BMS) and adaptation to technological upgrades and diverse application needs.
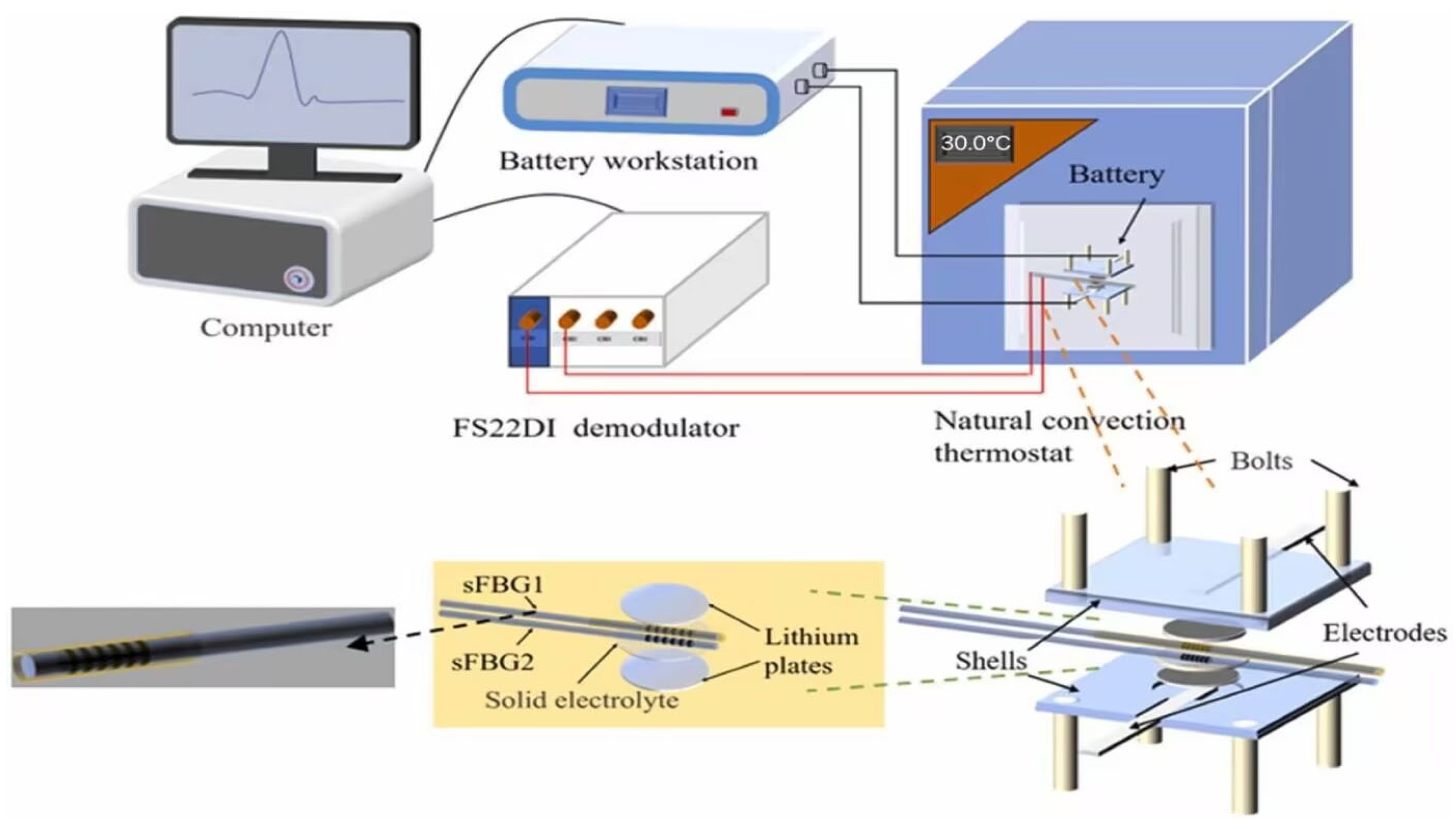
3.1. Early Warning Technology Based on Temperature Detection
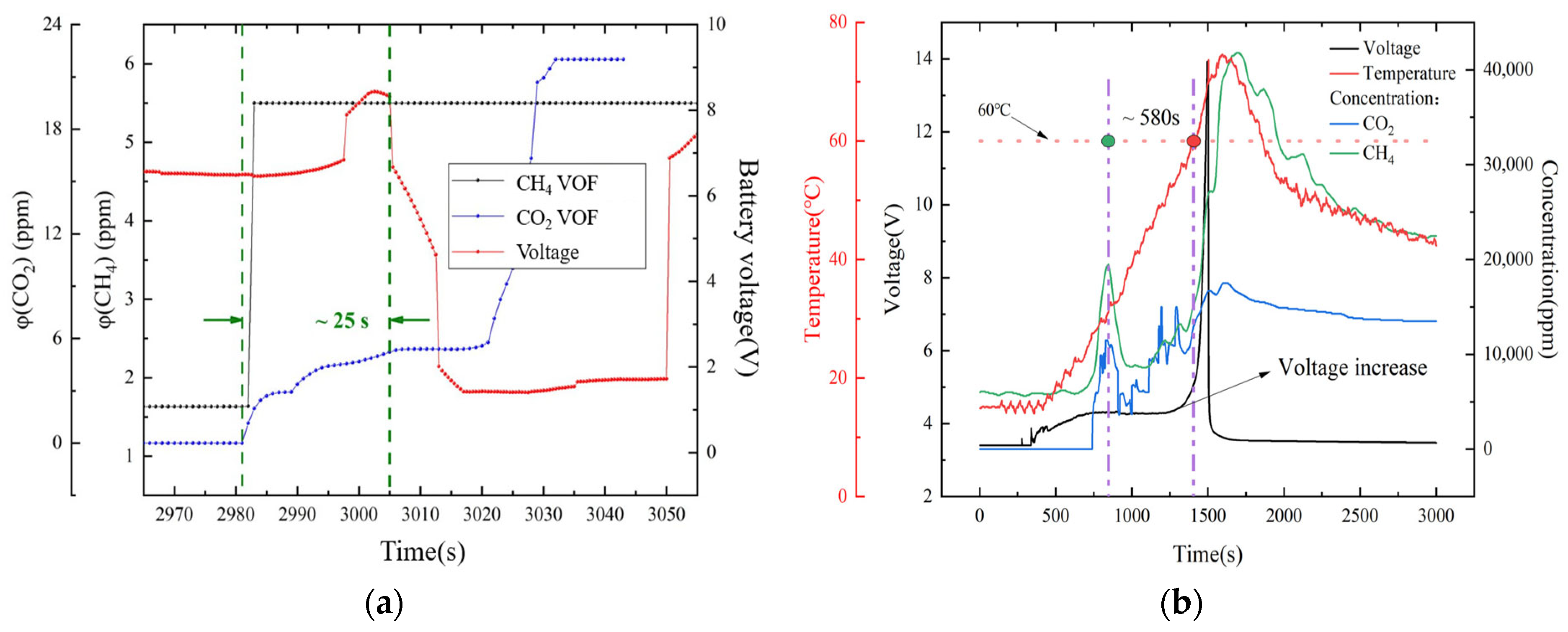
3.2. Early Warning Technology Based on Gas Detection
3.2.1. Single Gas Detection
3.2.2. Multiple Gas Detection
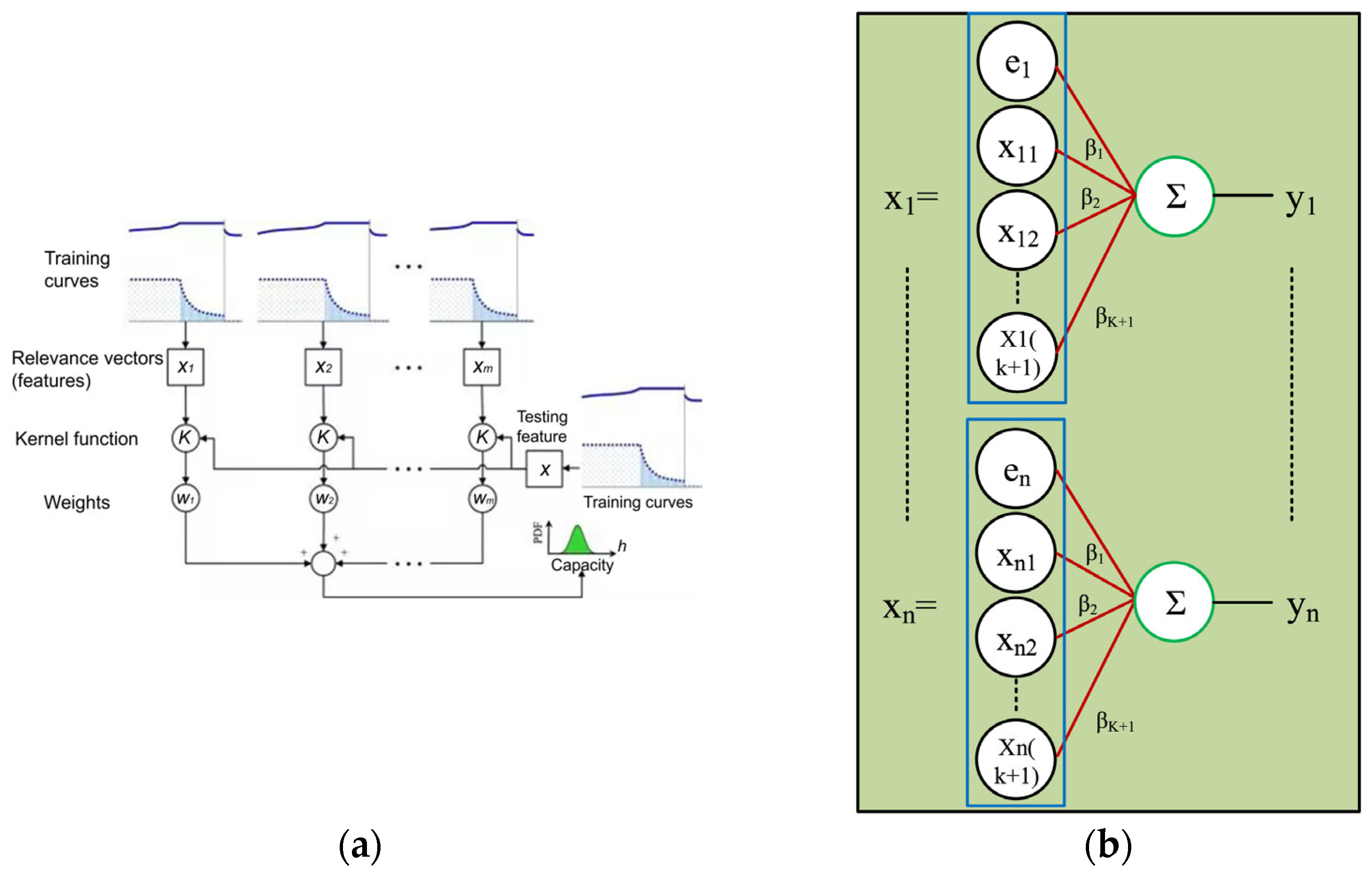
3.3. Early Warning Technology Based on Machine Learning
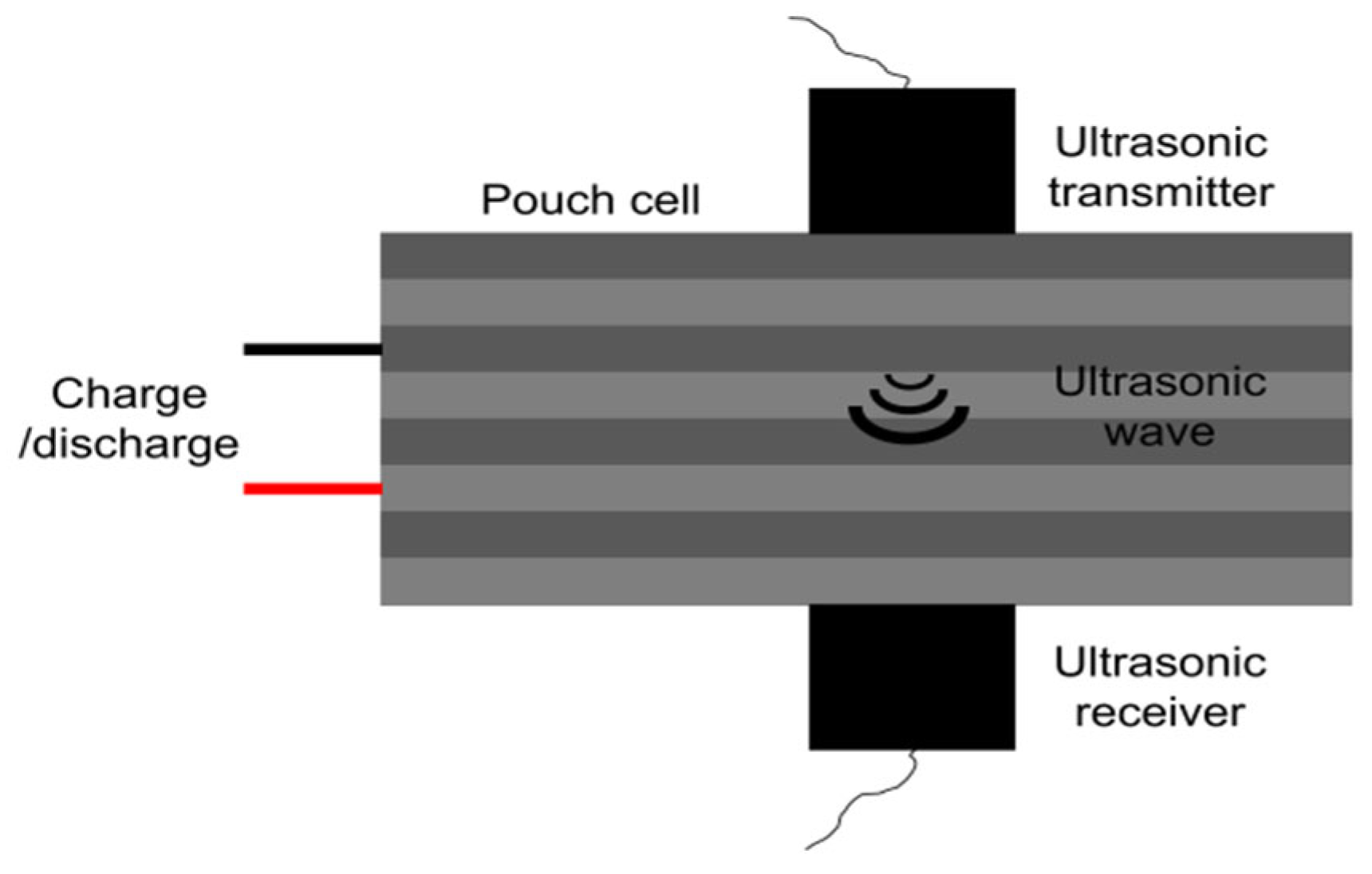
3.4. Early Warning Technology Based on Ultrasonic Detection
3.5. Other Detection and Warning Technologies
3.6. Comparison of Different Early Warning Technologies
4. Lithium-Ion Battery Fire Extinguishing Technology
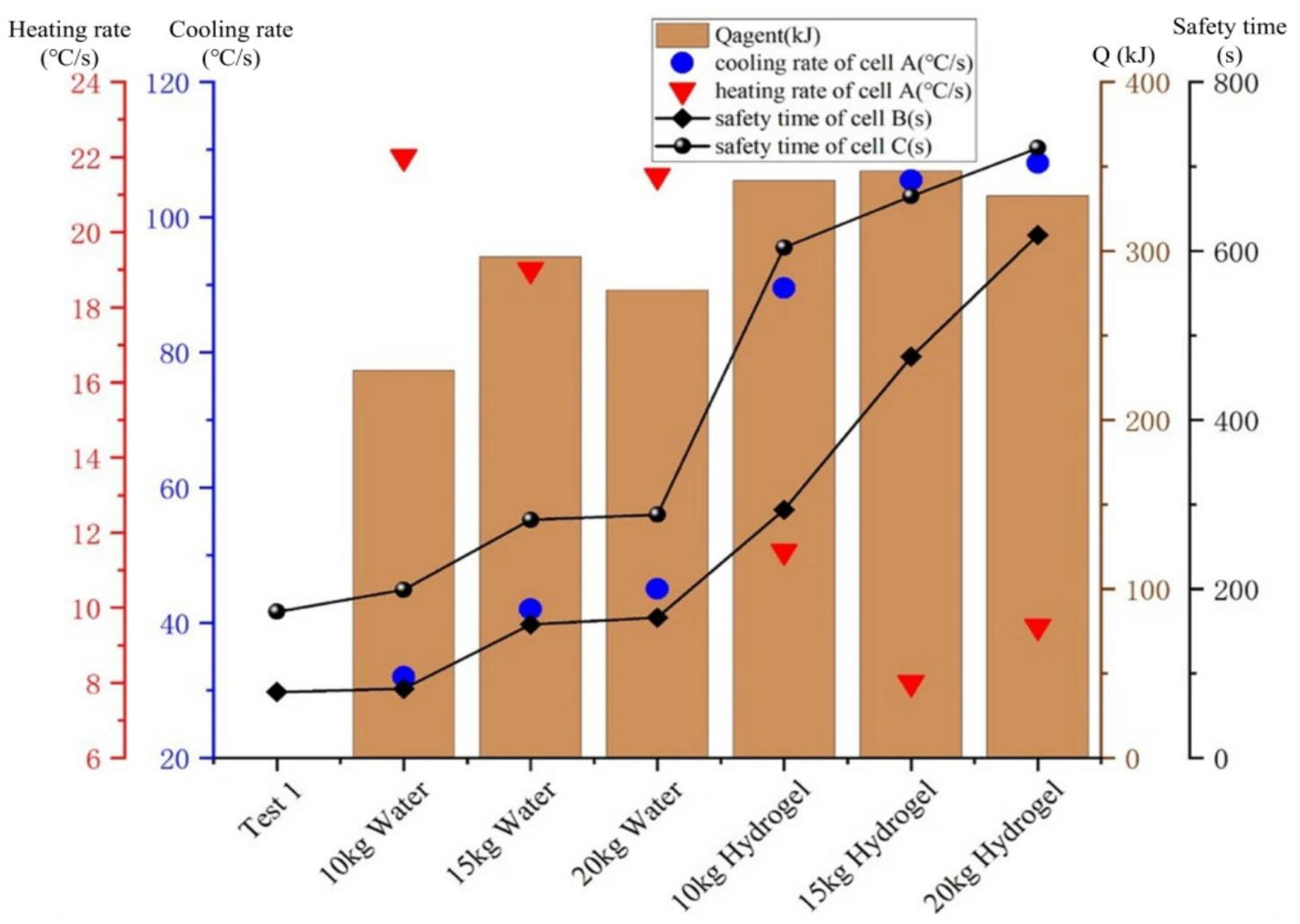
4.1. Hydrogel Fire Extinguishing Agent
4.2. Perfluorohexane Fire Extinguishing Agent
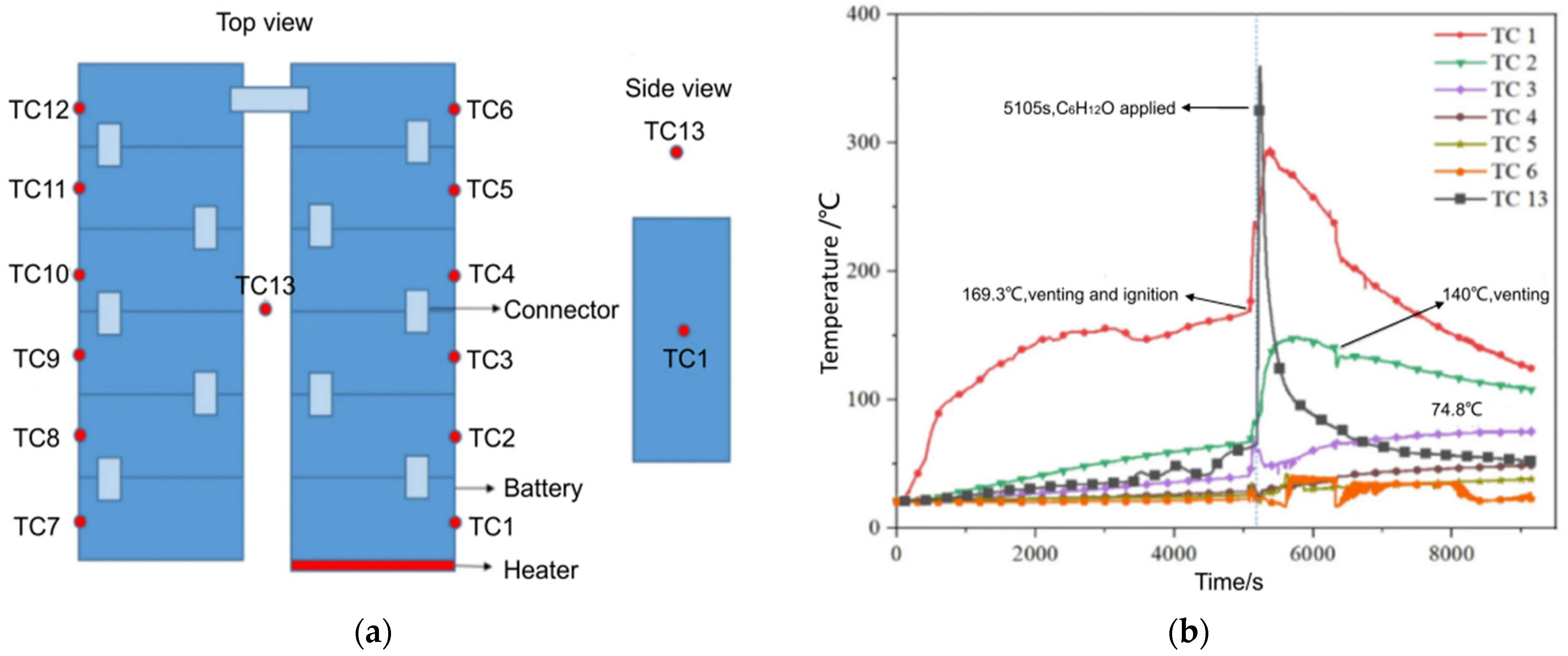
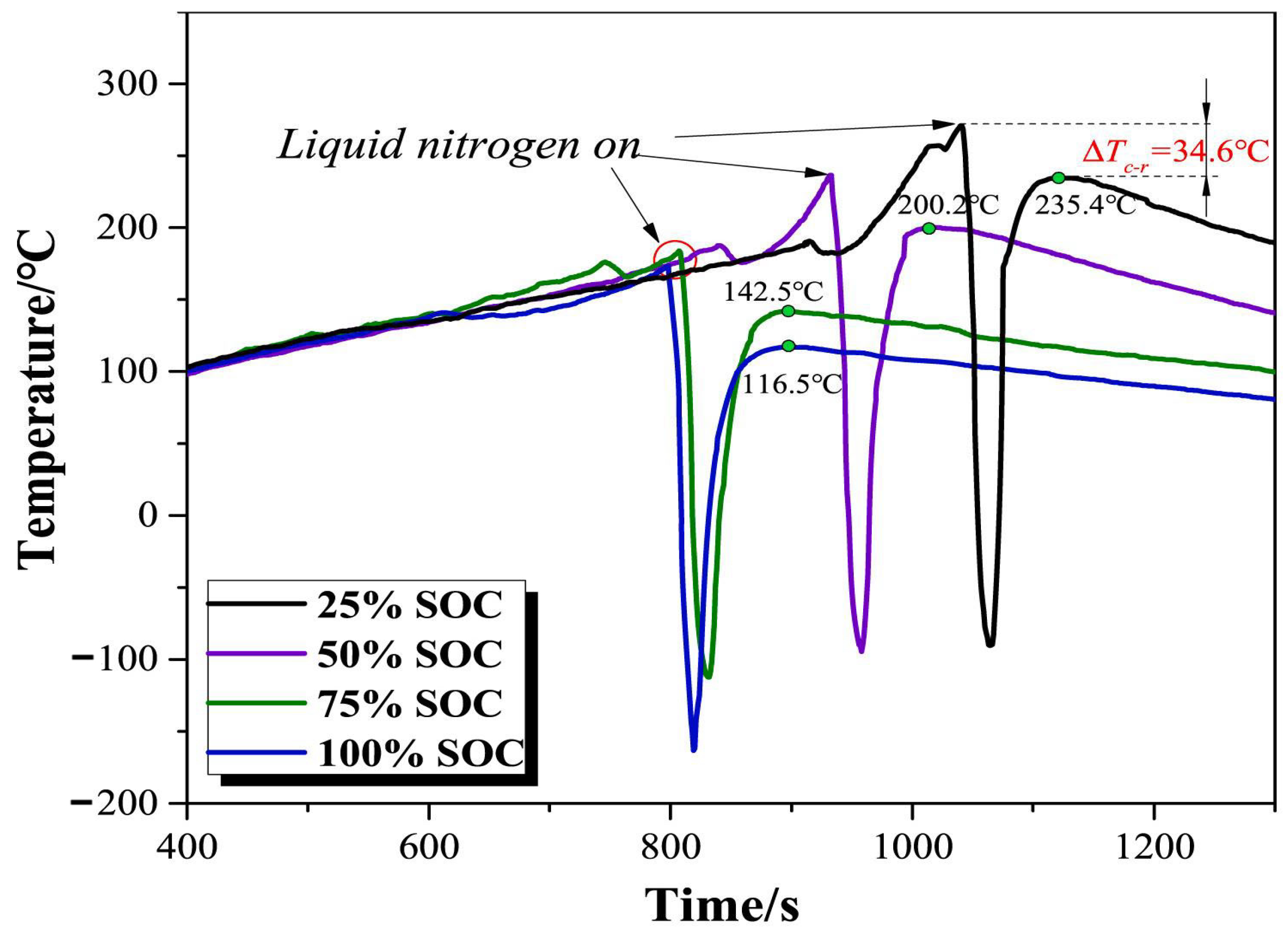
4.3. Liquid Nitrogen (LN) Fire Extinguishing Agent
4.4. Dry Powder Fire Extinguishing Agent
4.5. Aqueous Vermiculite Dispersion (AVD) Fire Extinguishing Agent
4.6. Comparison of the Advantages and Disadvantages of Fire Extinguishing Agents
5. Research on Flame Retardant Coatings
5.1. Innovation in Coating Composition
5.1.1. Polymer Matrix
5.1.2. Application of Flame Retardants
5.2. Optimization of Structural Design
5.2.1. Composite Coating Structure
5.2.2. Application of Micro/Nano Structures
6. Future Technology Outlook
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jia, Z.; Wang, Z.; Wang, Q.; Li, X.; Sun, F. Research on Thermal Runaway Mechanism and Safety Risk Control Method of Power Battery in New-Energy Vehicles. Automot. Eng. 2022, 44, 1689–1705. [Google Scholar] [CrossRef]
- Ji, C.; Wang, B.; Wang, S.; Pan, S.; Wang, D.; Qi, P.; Zhang, K. Optimization on Uniformity of Lithium-Ion Cylindrical Battery Module by Different Arrangement Strategy. Appl. Therm. Eng. 2019, 157, 113683. [Google Scholar] [CrossRef]
- He, D.; Wang, J.; Peng, Y.; Li, B.; Feng, C.; Shen, L.; Ma, S. Research Advances on Thermal Runaway Mechanism of Lithium-Ion Batteries and Safety Improvement. Sustain. Mater. Technol. 2024, 41, e01017. [Google Scholar] [CrossRef]
- Chavan, S.; Venkateswarlu, B.; Prabakaran, R.; Salman, M.; Joo, S.W.; Choi, G.S.; Kim, S.C. Thermal Runaway and Mitigation Strategies for Electric Vehicle Lithium-Ion Batteries Using Battery Cooling Approach: A Review of the Current Status and Challenges. J. Energy Storage 2023, 72, 108569. [Google Scholar] [CrossRef]
- Guo, D.; Guo, P.; Sun, L.; Xiao, P. Research on the influence of water mist on the performance of lithium iron phosphate energy storage battery modules. Fire Sci. Technol. 2022, 41, 1093–1097. [Google Scholar]
- Deng, J.; Chen, B.; Lu, J.; Chen, J.; Zhu, H.; Fang, Z. Comparative of the Fire Suppression Characteristics of Low-pressure Carbon Dioxide and Conven-tional Extinguishing Agents on Lithium-ions Batteries. High Volt. Eng. 2023, 49, 364–372. [Google Scholar] [CrossRef]
- Wang, W.; He, S.; Wang, X.; Mi, H. Influence of the water mist featured parameters on the fire extinguishing of the lithium-ion batteries. J. Saf. Environ. 2021, 21, 2505–2512. [Google Scholar] [CrossRef]
- Yuan, S.; Chang, C.; Yan, S.; Zhou, P.; Qian, X.; Yuan, M.; Liu, K. A Review of Fire-Extinguishing Agent on Suppressing Lithium-Ion Batteries Fire. J. Energy Chem. 2021, 62, 262–280. [Google Scholar] [CrossRef]
- Mallick, S.; Gayen, D. Thermal Behaviour and Thermal Runaway Propagation in Lithium-Ion Battery Systems—A Critical Review. J. Energy Storage 2023, 62, 106894. [Google Scholar] [CrossRef]
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal Runaway Mechanism of Lithium Ion Battery for Electric Vehicles: A Review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, L.; Yu, Y.; Sun, J. Progress of Enhancing the Safety of Lithium Ion Battery from the Electrolyte Aspect. Nano Energy 2019, 55, 93–114. [Google Scholar] [CrossRef]
- Feng, X.; Zheng, S.; Ren, D.; He, X.; Wang, L.; Cui, H.; Liu, X.; Jin, C.; Zhang, F.; Xu, C.; et al. Investigating the Thermal Runaway Mechanisms of Lithium-Ion Batteries Based on Thermal Analysis Database. Appl. Energy 2019, 246, 53–64. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, X.; Wang, Z.; Wang, L.; Zhang, Z.; Liu, Y.; Qiu, J.; Zhang, H.; He, X. The Significance of Enhancing the Reliability of Lithium-Ion Batteries in Reducing Electric Vehicle Field Safety Accidents. Batter. Supercaps 2025, 8, e202400355. [Google Scholar] [CrossRef]
- Hu, D.; Huang, S.; Wen, Z.; Gu, X.; Lu, J. A Review on Thermal Runaway Warning Technology for Lithium-Ion Batteries. Renew. Sustain. Energy Rev. 2024, 206, 114882. [Google Scholar] [CrossRef]
- Wang, Q.; Mao, B.; Stoliarov, S.I.; Sun, J. A Review of Lithium Ion Battery Failure Mechanisms and Fire Prevention Strategies. Prog. Energy Combust. Sci. 2019, 73, 95–131. [Google Scholar] [CrossRef]
- Lu, H.; Shen, W.; Lv, D.; Zhao, J.; Song, W.; Tan, R. Research Progress on Safety Strategies for Thermal Runaway in Lithium-Ion Batteries. Mater. Rep. 2025, 1–33. Available online: http://kns.cnki.net/kcms/detail/50.1078.TB.20240725.1554.006.html (accessed on 29 January 2025).
- Yu, S.; Mao, Y.; Xie, J.; Xu, C.; Lu, T. Thermal Runaway Chain Reaction Determination and Mechanism Model Establishment of NCA-Graphite Battery Based on the Internal Temperature. Appl. Energy 2024, 353, 122097. [Google Scholar] [CrossRef]
- Orendorff, C.J. The Role of Separators in Lithium-Ion Cell Safety. Electrochem. Soc. Interface 2012, 21, 61. [Google Scholar] [CrossRef]
- Mao, B.; Chen, H.; Cui, Z.; Wu, T.; Wang, Q. Failure Mechanism of the Lithium Ion Battery during Nail Penetration. Int. J. Heat Mass Transf. 2018, 122, 1103–1115. [Google Scholar] [CrossRef]
- Jiang, J.; Dahn, J.R. ARC Studies of the Thermal Stability of Three Different Cathode Materials: LiCoO2; Li[Ni0.1Co0.8Mn0.1]O2; and LiFePO4, in LiPF6 and LiBoB EC/DEC Electrolytes. Electrochem. Commun. 2004, 6, 39–43. [Google Scholar] [CrossRef]
- MacNeil, D.D.; Dahn, J.R. The Reaction of Charged Cathodes with Nonaqueous Solvents and Electrolytes: I. Li0.5CoO2. J. Electrochem. Soc. 2001, 148, A1205. [Google Scholar] [CrossRef]
- Arai, H.; Tsuda, M.; Saito, K.; Hayashi, M.; Sakurai, Y. Thermal Reactions Between Delithiated Lithium Nickelate and Electrolyte Solutions. J. Electrochem. Soc. 2002, 149, A401. [Google Scholar] [CrossRef]
- Lyu, P.; Liu, X.; Qu, J.; Zhao, J.; Huo, Y.; Qu, Z.; Rao, Z. Recent Advances of Thermal Safety of Lithium Ion Battery for Energy Storage. Energy Storage Mater. 2020, 31, 195–220. [Google Scholar] [CrossRef]
- Wang, H.; Tang, A.; Huang, K. Oxygen Evolution in Overcharged Lix Ni1/3 Co1/3 Mn1/3 O2 Electrode and Its Thermal Analysis Kinetics. Chin. J. Chem. 2011, 29, 1583–1588. [Google Scholar] [CrossRef]
- Martha, S.K.; Haik, O.; Zinigrad, E.; Exnar, I.; Drezen, T.; Miners, J.H.; Aurbach, D. On the Thermal Stability of Olivine Cathode Materials for Lithium-Ion Batteries. J. Electrochem. Soc. 2011, 158, A1115. [Google Scholar] [CrossRef]
- Zaghib, K.; Dubé, J.; Dallaire, A.; Galoustov, K.; Guerfi, A.; Ramanathan, M.; Benmayza, A.; Prakash, J.; Mauger, A.; Julien, C.M. Enhanced Thermal Safety and High Power Performance of Carbon-Coated LiFePO4 Olivine Cathode for Li-Ion Batteries. J. Power Sources 2012, 219, 36–44. [Google Scholar] [CrossRef]
- Yamada, A.; Chung, S.C.; Hinokuma, K. Optimized LiFePO4 for Lithium Battery Cathodes. J. Electrochem. Soc. 2001, 148, A224. [Google Scholar] [CrossRef]
- Joachin, H.; Kaun, T.D.; Zaghib, K.; Prakash, J. Electrochemical and Thermal Studies of Carbon-Coated LiFePO4 Cathode. J. Electrochem. Soc. 2009, 156, A401. [Google Scholar] [CrossRef]
- Ping, P.; Wang, Q.; Huang, P.; Li, K.; Sun, J.; Kong, D.; Chen, C. Study of the Fire Behavior of High-Energy Lithium-Ion Batteries with Full-Scale Burning Test. J. Power Sources 2015, 285, 80–89. [Google Scholar] [CrossRef]
- Yang, H.; Shen, X.-D. Dynamic TGA–FTIR Studies on the Thermal Stability of Lithium/Graphite with Electrolyte in Lithium-Ion Cell. J. Power Sources 2007, 167, 515–519. [Google Scholar] [CrossRef]
- Kawamura, T.; Kimura, A.; Egashira, M.; Okada, S.; Yamaki, J.-I. Thermal Stability of Alkyl Carbonate Mixed-Solvent Electrolytes for Lithium Ion Cells. J. Power Sources 2002, 104, 260–264. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, J.; Yao, X.; Chen, C. Thermal Stability of LiPF6/EC+DEC Electrolyte with Charged Electrodes for Lithium Ion Batteries. Thermochim. Acta 2005, 437, 12–16. [Google Scholar] [CrossRef]
- Gnanaraj, J.S.; Zinigrad, E.; Asraf, L.; Gottlieb, H.E.; Sprecher, M.; Aurbach, D.; Schmidt, M. The Use of Accelerating Rate Calorimetry (ARC) for the Study of the Thermal Reactions of Li-Ion Battery Electrolyte Solutions. J. Power Sources 2003, 119–121, 794–798. [Google Scholar] [CrossRef]
- Moshkovich, M.; Cojocaru, M.; Gottlieb, H.E.; Aurbach, D. The Study of the Anodic Stability of Alkyl Carbonate Solutions by in Situ FTIR Spectroscopy, EQCM, NMR and MS. J. Electroanal. Chem. 2001, 497, 84–96. [Google Scholar] [CrossRef]
- Jin, Y.; Zheng, Z.; Wei, D.; Jiang, X.; Lu, H.; Sun, L.; Tao, F.; Guo, D.; Liu, Y.; Gao, J.; et al. Detection of Micro-Scale Li Dendrite via H2 Gas Capture for Early Safety Warning. Joule 2020, 4, 1714–1729. [Google Scholar] [CrossRef]
- Qin, P.; Jia, Z.; Wu, J.; Jin, K.; Duan, Q.; Jiang, L.; Sun, J.; Ding, J.; Shi, C.; Wang, Q. The Thermal Runaway Analysis on LiFePO4 Electrical Energy Storage Packs with Different Venting Areas and Void Volumes. Appl. Energy 2022, 313, 118767. [Google Scholar] [CrossRef]
- Huang, Z.; Zhao, C.; Li, H.; Peng, W.; Zhang, Z.; Wang, Q. Experimental Study on Thermal Runaway and Its Propagation in the Large Format Lithium Ion Battery Module with Two Electrical Connection Modes. Energy 2020, 205, 117906. [Google Scholar] [CrossRef]
- Mourtada, S.; Tedjani, M.; Sébastien, D.; Hicham, C. Application of the Theory of Inventive Problem Solving (TRIZ) to Enhance Understanding of Li-Ion Battery Thermal Runaway in Electric Vehicle Applications. In Proceedings of the 2023 IEEE Vehicle Power and Propulsion Conference (VPPC), Milan, Italy, 24–27 October 2023; pp. 1–7. [Google Scholar]
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang, L.; Liu, J.; Li, Y.; Liang, Z.; He, X.; Li, X.; Tavajohi, N.; et al. A Review of Lithium-Ion Battery Safety Concerns: The Issues, Strategies, and Testing Standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Fernandes, Y.; Bry, A.; de Persis, S. Identification and Quantification of Gases Emitted during Abuse Tests by Overcharge of a Commercial Li-Ion Battery. J. Power Sources 2018, 389, 106–119. [Google Scholar] [CrossRef]
- Dong, T.; Peng, P.; Jiang, F. Numerical Modeling and Analysis of the Thermal Behavior of NCM Lithium-Ion Batteries Subjected to Very High C-Rate Discharge/Charge Operations. Int. J. Heat Mass Transf. 2018, 117, 261–272. [Google Scholar] [CrossRef]
- Li, W.; Xia, Y.; Chen, G.; Sahraei, E. Comparative Study of Mechanical-Electrical-Thermal Responses of Pouch, Cylindrical, and Prismatic Lithium-Ion Cells under Mechanical Abuse. Sci. China Technol. Sci. 2018, 61, 1472–1482. [Google Scholar] [CrossRef]
- Zhang, G.; Wei, X.; Tang, X.; Zhu, J.; Chen, S.; Dai, H. Internal Short Circuit Mechanisms, Experimental Approaches and Detection Methods of Lithium-Ion Batteries for Electric Vehicles: A Review. Renew. Sustain. Energy Rev. 2021, 141, 110790. [Google Scholar] [CrossRef]
- Liu, B.; Jia, Y.; Yuan, C.; Wang, L.; Gao, X.; Yin, S.; Xu, J. Safety Issues and Mechanisms of Lithium-Ion Battery Cell upon Mechanical Abusive Loading: A Review. Energy Storage Mater. 2020, 24, 85–112. [Google Scholar] [CrossRef]
- Ouyang, D.; Weng, J.; Hu, J.; Liu, J.; Chen, M.; Huang, Q.; Wang, J. Effect of High Temperature Circumstance on Lithium-Ion Battery and the Application of Phase Change Material. J. Electrochem. Soc. 2019, 166, A559. [Google Scholar] [CrossRef]
- An, Z.; Jia, L.; Ding, Y.; Dang, C.; Li, X. A Review on Lithium-Ion Power Battery Thermal Management Technologies and Thermal Safety. J. Therm. Sci. 2017, 26, 391–412. [Google Scholar] [CrossRef]
- Liu, X.; Ren, D.; Hsu, H.; Feng, X.; Xu, G.-L.; Zhuang, M.; Gao, H.; Lu, L.; Han, X.; Chu, Z.; et al. Thermal Runaway of Lithium-Ion Batteries without Internal Short Circuit. Joule 2018, 2, 2047–2064. [Google Scholar] [CrossRef]
- Lai, X.; Jin, C.; Yi, W.; Han, X.; Feng, X.; Zheng, Y.; Ouyang, M. Mechanism, Modeling, Detection, and Prevention of the Internal Short Circuit in Lithium-Ion Batteries: Recent Advances and Perspectives. Energy Storage Mater. 2021, 35, 470–499. [Google Scholar] [CrossRef]
- Grabow, J.; Klink, J.; Benger, R.; Hauer, I.; Beck, H.-P. Particle Contamination in Commercial Lithium-Ion Cells—Risk Assessment with Focus on Internal Short Circuits and Replication by Currently Discussed Trigger Methods. Batteries 2023, 9, 9. [Google Scholar] [CrossRef]
- Gulsoy, B.; Vincent, T.A.; Sansom, J.E.H.; Marco, J. In-Situ Temperature Monitoring of a Lithium-Ion Battery Using an Embedded Thermocouple for Smart Battery Applications. J. Energy Storage 2022, 54, 105260. [Google Scholar] [CrossRef]
- Liu, G.; Tan, Q.; Kou, H.; Zhang, L.; Wang, J.; Lv, W.; Dong, H.; Xiong, J. A Flexible Temperature Sensor Based on Reduced Graphene Oxide for Robot Skin Used in Internet of Things. Sensors 2018, 18, 1400. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Xu, G.; Ji, X.; Yang, Z.; Guan, C.; Chen, S.; Chen, X.; Ma, Y.; Yu, Y.; Feng, J. A Strain-Resistant Flexible Thermistor Sensor Array Based on CNT/MXene Hybrid Materials for Lithium-Ion Battery and Human Temperature Monitoring. Sens. Actuators A Phys. 2024, 368, 115059. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, K.; Chen, X.; Wang, Z.; Wang, S. The research and of explosion early warning device for type 18650 lithium ion battery fire. Fire Sci. Technol. 2018, 37, 939–942. [Google Scholar]
- Yang, L.; Li, N.; Hu, L.; Wang, S.; Wang, L.; Zhou, J.; Song, W.-L.; Sun, L.; Pan, T.-S.; Chen, H.-S.; et al. Internal Field Study of 21700 Battery Based on Long-Life Embedded Wireless Temperature Sensor. Acta Mech. Sin. 2021, 37, 895–901. [Google Scholar] [CrossRef]
- Keil, P.; Rumpf, K.; Jossen, A. Thermal Impedance Spectroscopy for Li-Ion Batteries with an IR Temperature Sensor System. In Proceedings of the 2013 World Electric Vehicle Symposium and Exhibition (EVS27), Barcelona, Spain, 17–20 November 2013; pp. 1–11. [Google Scholar]
- Sharma, A.K.; Pandey, A.K.; Kaur, B. A Review of Advancements (2007–2017) in Plasmonics-Based Optical Fiber Sensors. Opt. Fiber Technol. 2018, 43, 20–34. [Google Scholar] [CrossRef]
- Su, Y.-D.; Preger, Y.; Burroughs, H.; Sun, C.; Ohodnicki, P.R. Fiber Optic Sensing Technologies for Battery Management Systems and Energy Storage Applications. Sensors 2021, 21, 1397. [Google Scholar] [CrossRef] [PubMed]
- Parhizi, M.; Ahmed, M.B.; Jain, A. Determination of the Core Temperature of a Li-Ion Cell during Thermal Runaway. J. Power Sources 2017, 370, 27–35. [Google Scholar] [CrossRef]
- Xi, J.; Li, J.; Sun, H.; Ma, T.; Deng, L.; Liu, N.; Huang, X.; Zhang, J. In-Situ Monitoring of Internal Temperature and Strain of Solid-State Battery Based on Optical Fiber Sensors. Sens. Actuators A Phys. 2022, 347, 113888. [Google Scholar] [CrossRef]
- Feng, X.; Ren, D.; He, X.; Ouyang, M. Mitigating Thermal Runaway of Lithium-Ion Batteries. Joule 2020, 4, 743–770. [Google Scholar] [CrossRef]
- Yuan, L.; Dubaniewicz, T.; Zlochower, I.; Thomas, R.; Rayyan, N. Experimental Study on Thermal Runaway and Vented Gases of Lithium-Ion Cells. Process Saf. Environ. Prot. 2020, 144, 186–192. [Google Scholar] [CrossRef]
- Huang, Z.; Qin, P.; Shi, H.; Wu, J.; Yao, L.; Wang, Q. Study on Thermal Runaway Behavior of 86 Ah Lithium Iron Phosphate Battery Under Overheat Condition. High Volt. Eng. 2022, 48, 1185–1191. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, Y.; Tong, X.; Cheng, C.; Liu, K.; Jing, M. An automatic Alarm System for Thermal Runaway of Lithium-Ion Battery Packs Based on Gas Monitoring and Its Monitoring Method. CN Patent CN201711401706.9, 8 May 2018. [Google Scholar]
- Liao, Z.; Zhang, J.; Gan, Z.; Wang, Y.; Zhao, J.; Chen, T.; Zhang, G. Thermal Runaway Warning of Lithium-Ion Batteries Based on Photoacoustic Spectroscopy Gas Sensing Technology. Int. J. Energy Res. 2022, 46, 21694–21702. [Google Scholar] [CrossRef]
- Han, Y.; Zhao, Y.; Ming, A.; Fang, Y.; Fang, S.; Bi, S.; Chen, J.; Xu, R.; Wei, F.; Mao, C. Application of an NDIR Sensor System Developed for Early Thermal Runaway Warning of Automotive Batteries. Energies 2023, 16, 3620. [Google Scholar] [CrossRef]
- Hu, C.; Jain, G.; Schmidt, C.; Strief, C.; Sullivan, M. Online Estimation of Lithium-Ion Battery Capacity Using Sparse Bayesian Learning. J. Power Sources 2015, 289, 105–113. [Google Scholar] [CrossRef]
- Babaeiyazdi, I.; Rezaei-Zare, A.; Shokrzadeh, S. State of Charge Prediction of EV Li-Ion Batteries Using EIS: A Machine Learning Approach. Energy 2021, 223, 120116. [Google Scholar] [CrossRef]
- Sun, H.; Muralidharan, N.; Amin, R.; Rathod, V.; Ramuhalli, P.; Belharouak, I. Ultrasonic Nondestructive Diagnosis of Lithium-Ion Batteries with Multiple Frequencies. J. Power Sources 2022, 549, 232091. [Google Scholar] [CrossRef]
- Wang, Y.; Lai, X.; Chen, Q.; Han, X.; Lu, L.; Ouyang, M.; Zheng, Y. Progress and Challenges in Ultrasonic Technology for State Estimation and Defect Detection of Lithium-Ion Batteries. Energy Storage Mater. 2024, 69, 103430. [Google Scholar] [CrossRef]
- Shen, Y.; Zou, B.; Zhang, Z.; Xu, M.; Wang, S.; Li, Q.; Li, H.; Zhou, M.; Jiang, K.; Wang, K. In Situ Detection of Lithium-Ion Batteries by Ultrasonic Technologies. Energy Storage Mater. 2023, 62, 102915. [Google Scholar] [CrossRef]
- Astafev, E.A. Measurements and Analysis of Electrochemical Noise of Li-Ion Battery. Russ. J. Electrochem. 2019, 55, 488–495. [Google Scholar] [CrossRef]
- Gan, N.; Sun, Z.; Zhang, Z.; Xu, S.; Liu, P.; Qin, Z. Data-Driven Fault Diagnosis of Lithium-Ion Battery Overdischarge in Electric Vehicles. IEEE Trans. Power Electron. 2022, 37, 4575–4588. [Google Scholar] [CrossRef]
- Zhang, M.; Ouyang, M.; Lu, L.; He, X.; Feng, X.; Liu, L.; Xie, X. Battery Internal Short Circuit Detection. ECS Trans. 2017, 77, 217. [Google Scholar] [CrossRef]
- Laresgoiti, I.; Käbitz, S.; Ecker, M.; Sauer, D.U. Modeling Mechanical Degradation in Lithium Ion Batteries during Cycling: Solid Electrolyte Interphase Fracture. J. Power Sources 2015, 300, 112–122. [Google Scholar] [CrossRef]
- Cai, T.; Stefanopoulou, A.G.; Siegel, J.B. Modeling Li-Ion Battery Temperature and Expansion Force during the Early Stages of Thermal Runaway Triggered by Internal Shorts. J. Electrochem. Soc. 2019, 166, A2431–A2443. [Google Scholar] [CrossRef]
- Koch, S.; Birke, K.; Kuhn, R. Fast Thermal Runaway Detection for Lithium-Ion Cells in Large Scale Traction Batteries. Batteries 2018, 4, 16. [Google Scholar] [CrossRef]
- Luo, F.; Chu, G.; Huang, J. Fundamental scientific aspects of lithium batteries (VIII)-Anode electrode materials. Energy Storage Sci. Technol. 2014, 3, 146–163. [Google Scholar]
- Lagonotte, P.; Soulier, F.; Thomas, A.; Martemianov, S. Hybrid Impedance Spectroscopy and Transients Testing Methodology. J. Energy Storage 2023, 74, 109290. [Google Scholar] [CrossRef]
- Carkhuff, B.G.; Demirev, P.A.; Srinivasan, R. Impedance-Based Battery Management System for Safety Monitoring of Lithium-Ion Batteries. IEEE Trans. Ind. Electron. 2018, 65, 6497–6504. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, L.; Zhang, N.; Wei, Z.; Mei, W.; Duan, Q.; Sun, J.; Wang, Q. Early Warning Method for Thermal Runaway of Lithium-Ion Batteries under Thermal Abuse Condition Based on Online Electrochemical Impedance Monitoring. J. Energy Chem. 2024, 92, 74–86. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhou, X.; Peng, Y.; Li, L.; Cao, J.; Yang, L.; Cao, B. Quantitative Study on the Thermal Failure Features of Lithium Iron Phosphate Batteries under Varied Heating Powers. Appl. Therm. Eng. 2021, 185, 116346. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, X.; Zhang, Y.; Song, Z.; Wang, Z. A Capacitive Particle-Analyzing Smoke Detector for Very Early Fire Detection. Sensors 2024, 24, 1692. [Google Scholar] [CrossRef]
- Wang, W.; Liao, C.; Liu, L.; Cai, W.; Yuan, Y.; Hou, Y.; Guo, W.; Zhou, X.; Qiu, S.; Song, L.; et al. Comparable Investigation of Tervalent and Pentavalent Phosphorus Based Flame Retardants on Improving the Safety and Capacity of Lithium-Ion Batteries. J. Power Sources 2019, 420, 143–151. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, T.; Yuan, S.; Chang, C.; Wang, K.; Song, Z.; Qian, X. Patent-Based Technological Developments and Surfactants Application of Lithium-Ion Batteries Fire-Extinguishing Agent. J. Energy Chem. 2024, 88, 39–63. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, G.; Zhu, G.; Li, Z.; Yuan, D.; Jin, C. Research on fire-extinguishing performance of hydrogel fire extinguishing agent on lithium iron phosphate battery pack. China Saf. Sci. J. 2023, 33, 161–168. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, G.; Yuan, D.; Jiang, L.; Fan, Y.; Kong, D. Experimental Study on the Efficiency of Hydrogel on Suppressing Thermal Runaway Propagation of Lithium-Ion Battery. Fire Technol 2024, 1–22. [Google Scholar] [CrossRef]
- Wang, T.; Qin, K.; Zhang, P.; Pan, R. Investigation of the High Temperature Pyrolysis Behavior of Perfluorohexanone as an Environmental-Friendly Fire Extinguishing Agent. 2024. Available online: https://ssrn.com/abstract=5005708 (accessed on 29 January 2025).
- Zhang, L.; Jin, K.; Sun, J.; Wang, Q. A Review of Fire-Extinguishing Agents and Fire Suppression Strategies for Lithium-Ion Batteries Fire. Fire Technol. 2024, 60, 817–858. [Google Scholar] [CrossRef]
- Zhang, L.; Ye, F.; Li, Y.; Chen, M.; Meng, X.; Xu, J.; Sun, J.; Wang, Q. Experimental Study on the Efficiency of Dodecafluoro-2-Methylpentan-3-One on Suppressing Large-Scale Battery Module Fire. Fire Technol. 2023, 59, 1247–1267. [Google Scholar] [CrossRef]
- Shi, B.; Shen, W.; Yin, B.; Wang, Z.; Ruan, H.; Li, Z.; Huang, D. Experimental Study on Suppressing Thermal Runaway Propagation of Lithium-Ion Battery Modules by Using Liquid Nitrogen: Influence of Injection Pipe Diameter and Position. Therm. Sci. Eng. Prog. 2024, 50, 102580. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Chu, Y.; Chen, Y. Study on theoretical analysis method of thermal runaway liquid nitrogen cooling inerting of LPF batteries. Fire Sci. Technol. 2024, 43, 656–662. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, P.; Duan, Q.; Zhao, C.; Wang, Q. Experimental Investigation on the Cooling and Suppression Effects of Liquid Nitrogen on the Thermal Runaway of Lithium Ion Battery. J. Power Sources 2021, 495, 229795. [Google Scholar] [CrossRef]
- Li, X.; Zhang, M.; Zhou, Z.; Zhu, Y.; Du, K.; Zhou, X. A Novel Dry Powder Extinguishant with High Cooling Performance for Suppressing Lithium Ion Battery Fires. Case Stud. Therm. Eng. 2023, 42, 102756. [Google Scholar] [CrossRef]
- Majeed, F.; Jamal, H.; Kamran, U.; Noman, M.; Ali, M.M.; Shahzad, T.; Baig, M.M.; Akhtar, F. Review–Recent Advances in Fire-Suppressing Agents for Mitigating Lithium-Ion Battery Fires. J. Electrochem. Soc. 2024, 171, 060522. [Google Scholar] [CrossRef]
- Wang, H.; Sun, Q.; Guo, J.; Xie, S.; He, Y.; Chen, X. The Efficiency of Aqueous Vermiculite Dispersion Fire Extinguishing Agent on Suppressing Three Typical Power Batteries. J. Electrochem. Energy Convers. Storage 2020, 18, 020901. [Google Scholar] [CrossRef]
- Guo, J.; He, Y.; Wang, H.; Chen, X. Research on suppression thermal runaway of 21700 lithium-ion batteries by aqueous vermiculite dispersion agent. J. Henan Univ. Sci. Technol. (Nat. Sci.) 2021, 42, 40–47+4-5. [Google Scholar] [CrossRef]
- Sun, J.; Mao, B.; Wang, Q. Progress on the Research of Fire Behavior and Fire Protection of Lithium Ion Battery. Fire Saf. J. 2021, 120, 103119. [Google Scholar] [CrossRef]
- Gai, Q.; Zhao, T.; Ma, J.; Wang, C.; Gao, H.; Li, L. An In-Situ Bicomponent Polymeric Matrix Solid Electrolyte for Solid-State Lithium Metal Batteries with Extended Cycling-Life. J. Energy Storage 2024, 80, 110150. [Google Scholar] [CrossRef]
- Guo, K.; Xu, Y.; Luo, Y.; Wang, Y.; Li, X.; Sun, X.; Zhang, K.; Pang, Q.; Qin, A. A Review of Composite Organic-Inorganic Electrolytes for Lithium Batteries. J. Energy Storage 2024, 77, 109912. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, Y.; Wang, N.; Fu, W. Recent Progress in Flame Retardant Technology of Battery: A Review. Resour. Chem. Mater. 2023, 2, 80–99. [Google Scholar] [CrossRef]
- Liu, Q.; Deng, Q.; Zhao, R.; Cheng, W.-L.; Wang, Y.-D. A Novel Flexible Flame-Retardant Phase Change Materials with Battery Thermal Management Test. J. Energy Storage 2023, 70, 108077. [Google Scholar] [CrossRef]
- Guan, P.; Zhou, L.; Yu, Z.; Sun, Y.; Liu, Y.; Wu, F.; Jiang, Y.; Chu, D. Recent Progress of Surface Coating on Cathode Materials for High-Performance Lithium-Ion Batteries. J. Energy Chem. 2020, 43, 220–235. [Google Scholar] [CrossRef]
- Li, F.; Wu, Y.; Chou, J.; Winter, M.; Wu, N. A Mechanically Robust and Highly Ion-Conductive Polymer-Blend Coating for High-Power and Long-Life Lithium-Ion Battery Anodes. Adv. Mater. 2015, 27, 130–137. [Google Scholar] [CrossRef]
- Dube, T.; Ferg, E.E.; Niekerk, X.V.; Davoren, B. Applications of Microcapsule Technology in Lithium-Ion Batteries: A Review. S. Afr. J. Chem. 2024, 78, 136–146. [Google Scholar] [CrossRef]
- Cao, X.; Ma, C.; Luo, L.; Chen, L.; Cheng, H.; Orenstein, R.S.; Zhang, X. Nanofiber Materials for Lithium-Ion Batteries. Adv. Fiber Mater. 2023, 5, 1141–1197. [Google Scholar] [CrossRef]
- Li, Z.; Cong, J.; Ding, Y.; Yang, Y.; Huang, K.; Ge, X.; Chen, K.; Zeng, T.; Huang, Z.; Fang, C.; et al. Strategies for Intelligent Detection and Fire Suppression of Lithium-Ion Batteries. Electrochem. Energy Rev. 2024, 7, 32. [Google Scholar] [CrossRef]
- Song, X.; Yang, F.; Wang, D.; Tsui, K.-L. Combined CNN-LSTM Network for State-of-Charge Estimation of Lithium-Ion Batteries. IEEE Access 2019, 7, 88894–88902. [Google Scholar] [CrossRef]
- Wang, H.; Wang, P.; Feng, Y.; Liu, J.; Wang, J.; Hu, M.; Wei, J.; Huang, Y. Recent Advances on Self-Healing Materials and Batteries. ChemElectroChem 2019, 6, 1605–1622. [Google Scholar] [CrossRef]
- Dai, H.; Jiang, B.; Hu, X.; Lin, X.; Wei, X.; Pecht, M. Advanced Battery Management Strategies for a Sustainable Energy Future: Multilayer Design Concepts and Research Trends. Renew. Sustain. Energy Rev. 2021, 138, 110480. [Google Scholar] [CrossRef]
- Wang, L.; Han, J.; Liu, C.; Li, G. State of Charge Estimation of Lithium-Ion Based on VFFRLS-Noise Adaptive CKF Algorithm. Ind. Eng. Chem. Res. 2022, 61, 7489–7503. [Google Scholar] [CrossRef]
- Zhang, J.; Shao, D.; Jiang, L.; Zhang, G.; Wu, H.; Day, R.; Jiang, W. Advanced Thermal Management System Driven by Phase Change Materials for Power Lithium-Ion Batteries: A Review. Renew. Sustain. Energy Rev. 2022, 159, 112207. [Google Scholar] [CrossRef]


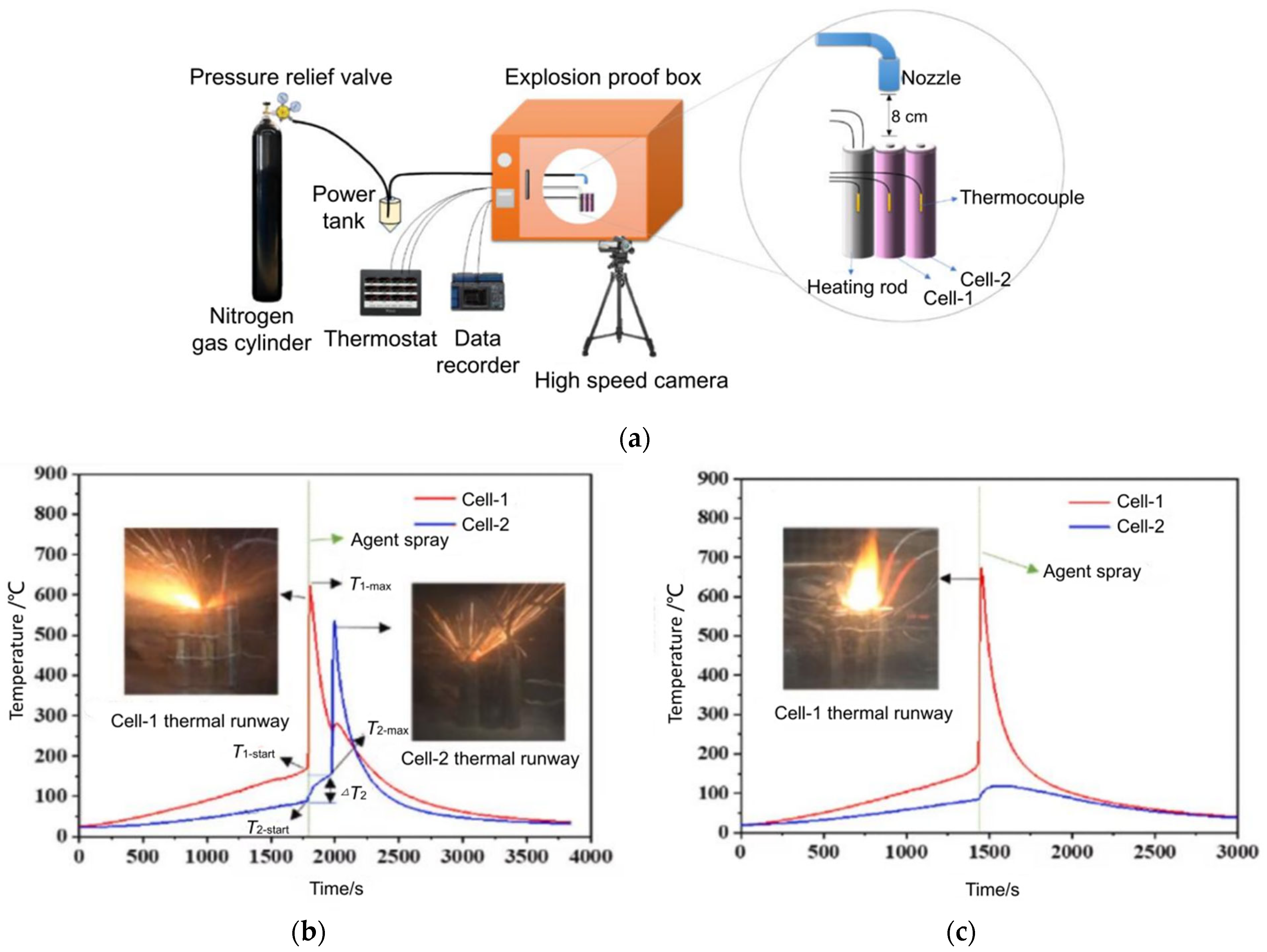
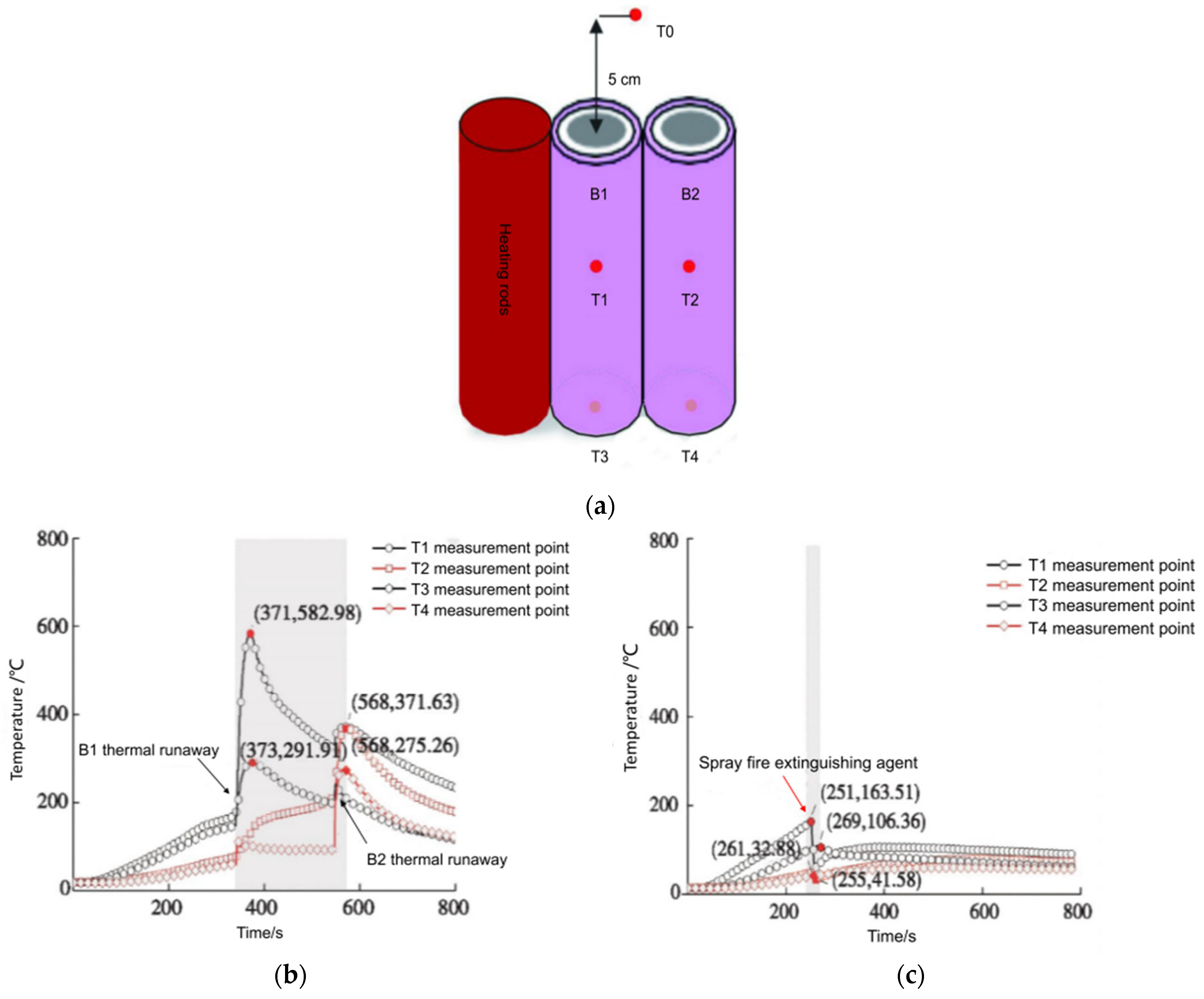
| No. | Date/Year | Description | Causes |
|---|---|---|---|
| 1 | 2019 | The McMicken energy storage facility in Arizona experienced a fire and explosion | The failure of the battery was attributed to the presence of lithium dendrites |
| 2 | 2020 | A brake failure incident involving the Tesla Model 3 resulted in a fire in China | A mechanical puncture led to a short circuit |
| 3 | 2021 | The explosion at an integrated optical storage and charging facility in China claimed the lives of three individuals | A short circuit in the battery was identified as the cause |
| 4 | 2021 | During the commissioning of the Tesla Megapack energy storage system in Australia, a fire broke out | A coolant leak was detected on the exterior of the battery compartment |
| 5 | 2022 | In California, the Vistra Energy battery pack was entirely destroyed by melting | The overheating of the battery was a result of a failure in the energy storage system |
| 6 | 2022 | An electric truck ignited during the charging process in China | A thermal runaway explosion occurred |
| 7 | 2023 | A fire and explosion occurred at a lithium-ion battery company in China | A thermal runaway event involving polymer lithium-ion batteries took place |
| Technology | Advantages | Disadvantages | Accuracy | Applicability | Cost |
|---|---|---|---|---|---|
| Early warning technology based on temperature detection | Early detection of thermal issues; simple technology | May not detect all failure types; affected by environmental factors | Moderate | Wide, especially in high-risk environments | Low |
| Early warning technology based on gas detection | Detects gas emissions indicating failure; reliable | Requires gas sensors; may be less effective in sealed systems | High | Suitable for enclosed systems | Moderate |
| Early warning technology based on machine learning | Predictive capabilities; adapts over time | Requires data and computational power; complex implementation | High | Advanced applications | High |
| Early warning technology based on ultrasonic detection | Non-invasive; detects internal structural changes | Requires specialized equipment; interpretation complexity | High | Industrial and specialized uses | High |
| Current and voltage monitoring | Immediate detection of electrical anomalies | May not detect non-electrical issues; false positives possible | Moderate | Standard in many systems | Low |
| Pressure monitoring | Detects pressure build-up indicating potential failure | Limited to systems where pressure changes are a precursor | Moderate | Niche applications | Moderate |
| Impedance monitoring | Provides state-of-health insights; non-invasive | Requires specific equipment; may need frequent calibration | High | Maintenance and lifecycle management | Moderate |
| Smoke detection | Detects smoke indicating combustion or overheating | Requires smoke sensors; may activate during external fires | High | Fire-prone environments | Moderate |
| Fire Suppressant Type | Efficiency | Safety | Environmental Impact | Application Scope | Clean up Difficulty |
|---|---|---|---|---|---|
| Water Gel Suppressant | High | High | Low | Li-ion Battery Fires | Easy |
| Perfluorohexanone Suppressant | High | Medium | Moderate (Pollution Risk) | Various Fire Types | Easy |
| Liquid Nitrogen Suppressant | High | High | Low | Various Fire Types | Moderate (Special Equipment Needed) |
| Dry Powder Suppressant | High | High | Low | Various Fire Types | Moderate (Requires Post-cleaning) |
| Vermiculite Dispersion Suppressant | Medium | High | Low | Specific Fire Types | Easy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the World Electric Vehicle Association. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, P.; Zhu, H.; Dong, X.; Hai, B. Research Progress on Thermal Runaway Warning Methods and Fire Extinguishing Technologies for Lithium-Ion Batteries. World Electr. Veh. J. 2025, 16, 81. https://doi.org/10.3390/wevj16020081
Shi P, Zhu H, Dong X, Hai B. Research Progress on Thermal Runaway Warning Methods and Fire Extinguishing Technologies for Lithium-Ion Batteries. World Electric Vehicle Journal. 2025; 16(2):81. https://doi.org/10.3390/wevj16020081
Chicago/Turabian StyleShi, Peicheng, Hailong Zhu, Xinlong Dong, and Bin Hai. 2025. "Research Progress on Thermal Runaway Warning Methods and Fire Extinguishing Technologies for Lithium-Ion Batteries" World Electric Vehicle Journal 16, no. 2: 81. https://doi.org/10.3390/wevj16020081
APA StyleShi, P., Zhu, H., Dong, X., & Hai, B. (2025). Research Progress on Thermal Runaway Warning Methods and Fire Extinguishing Technologies for Lithium-Ion Batteries. World Electric Vehicle Journal, 16(2), 81. https://doi.org/10.3390/wevj16020081






