Exploring Thermal Runaway: Role of Battery Chemistry and Testing Methodology
Abstract
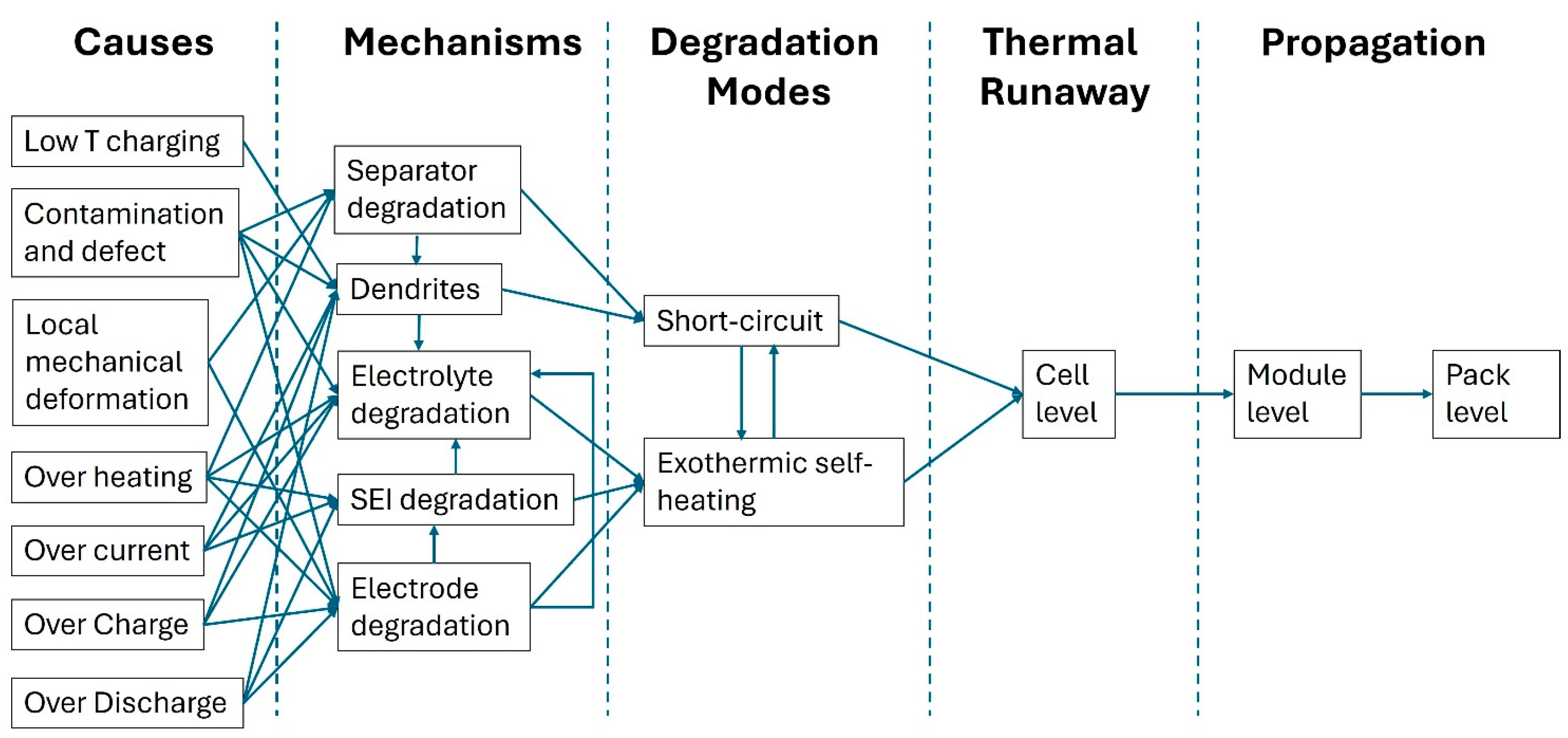
1. Introduction
2. Materials and Methods
- Constant heating (CH) with a continuously applied heating power of 27 W. The constant heating was applied until the occurrence of the thermal runaway
- Heat–wait–seek (HWS) tests with heating steps of 5 K and step time of 300 s.
3. Results and Discussion
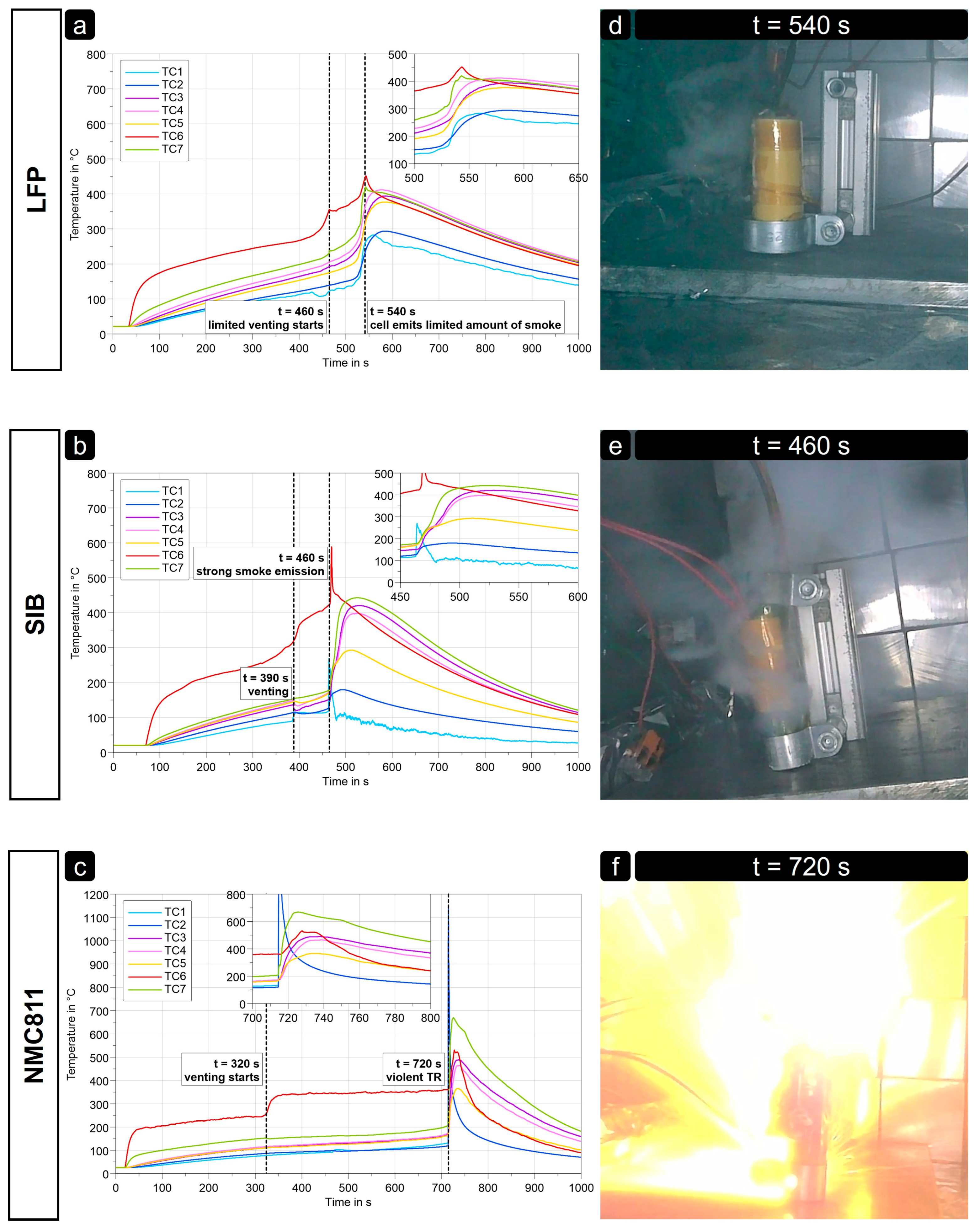
3.1. Individual Cell TR Tests
3.1.1. Cell Characteristics in Constant Heating Tests
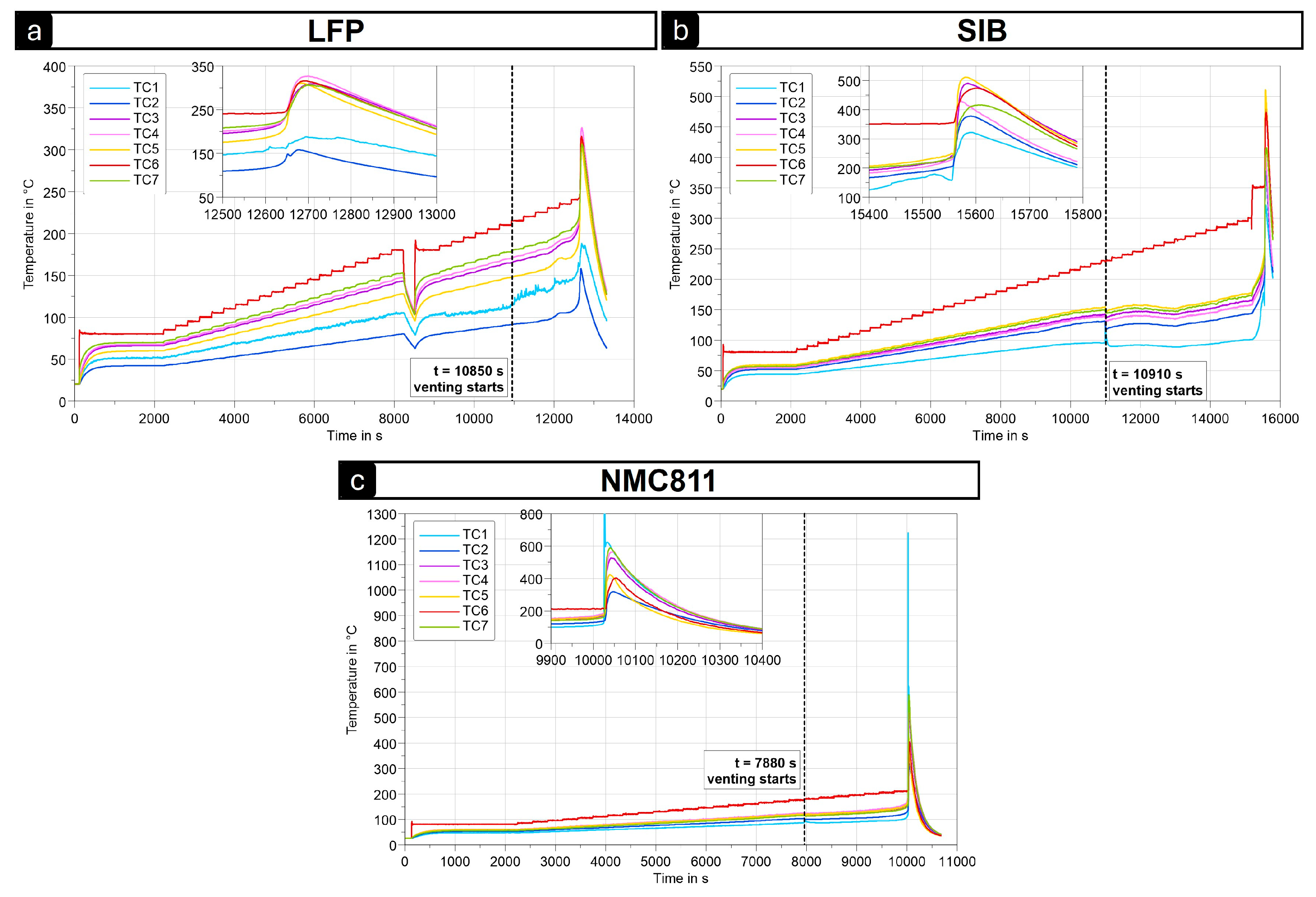
3.1.2. Cell Performance in Heat–Wait–Seek Tests
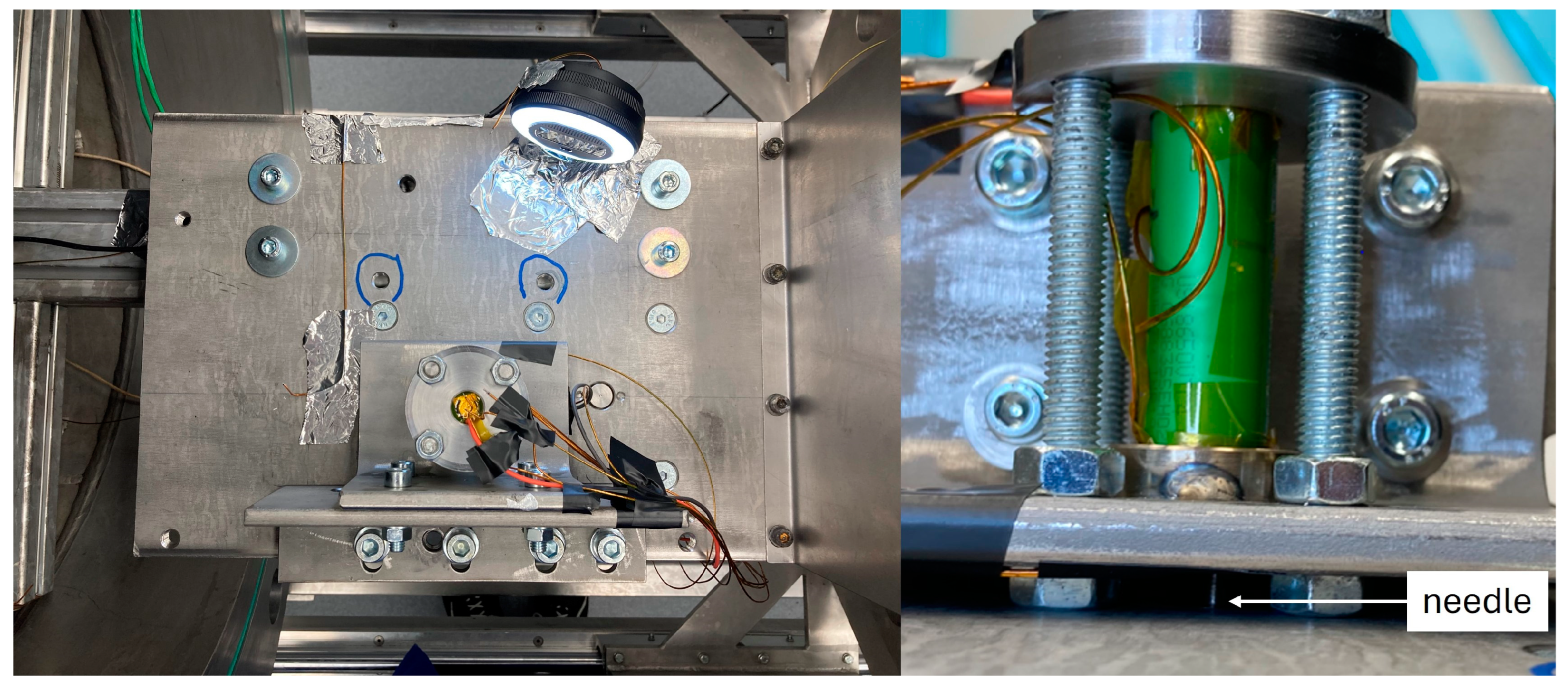
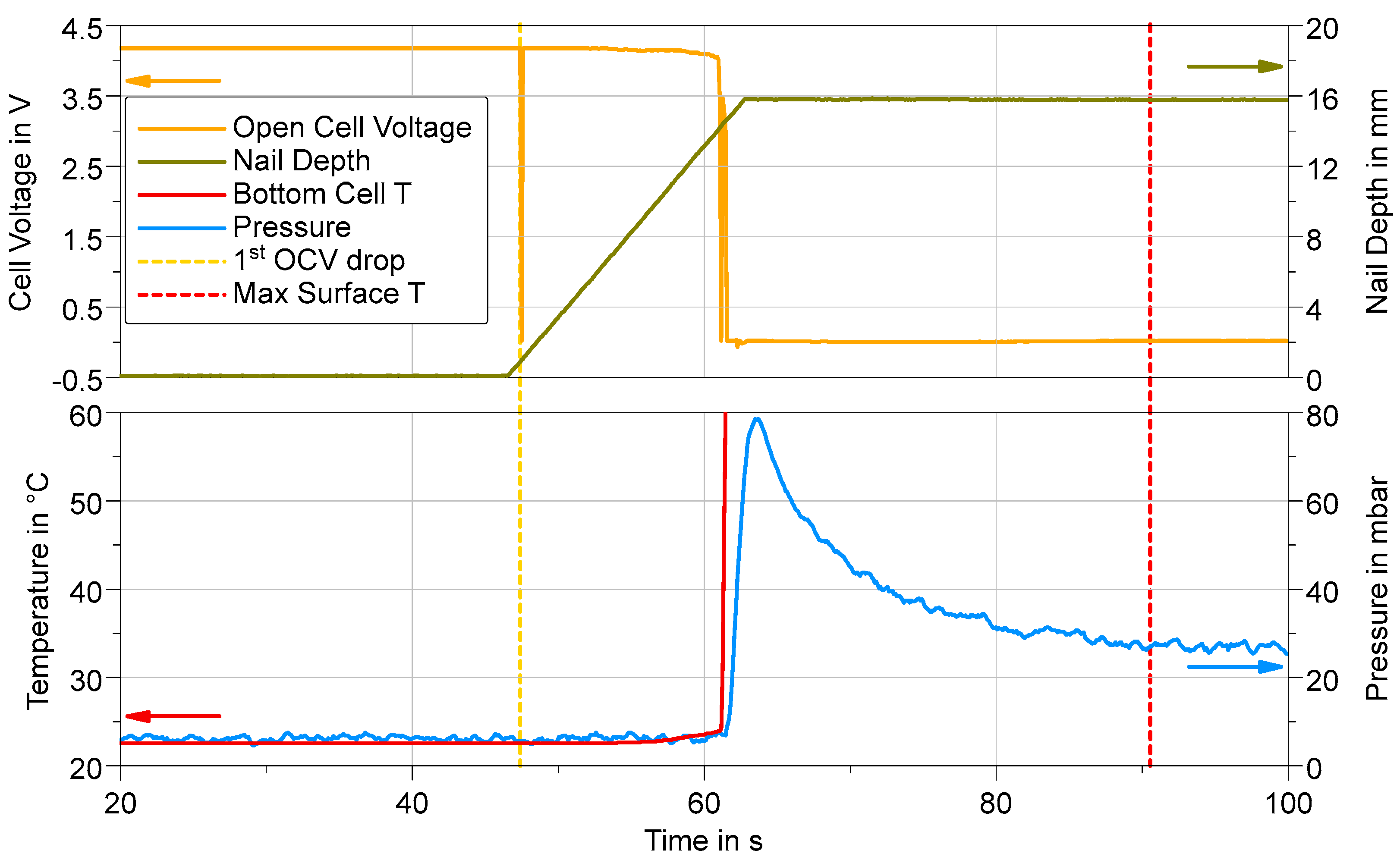
3.1.3. Cell Performance in Nail Penetration Tests
3.2. Trigger Method Performance Across Different Cell Chemistries
3.3. Chemistry Performance Across Different Trigger Methods
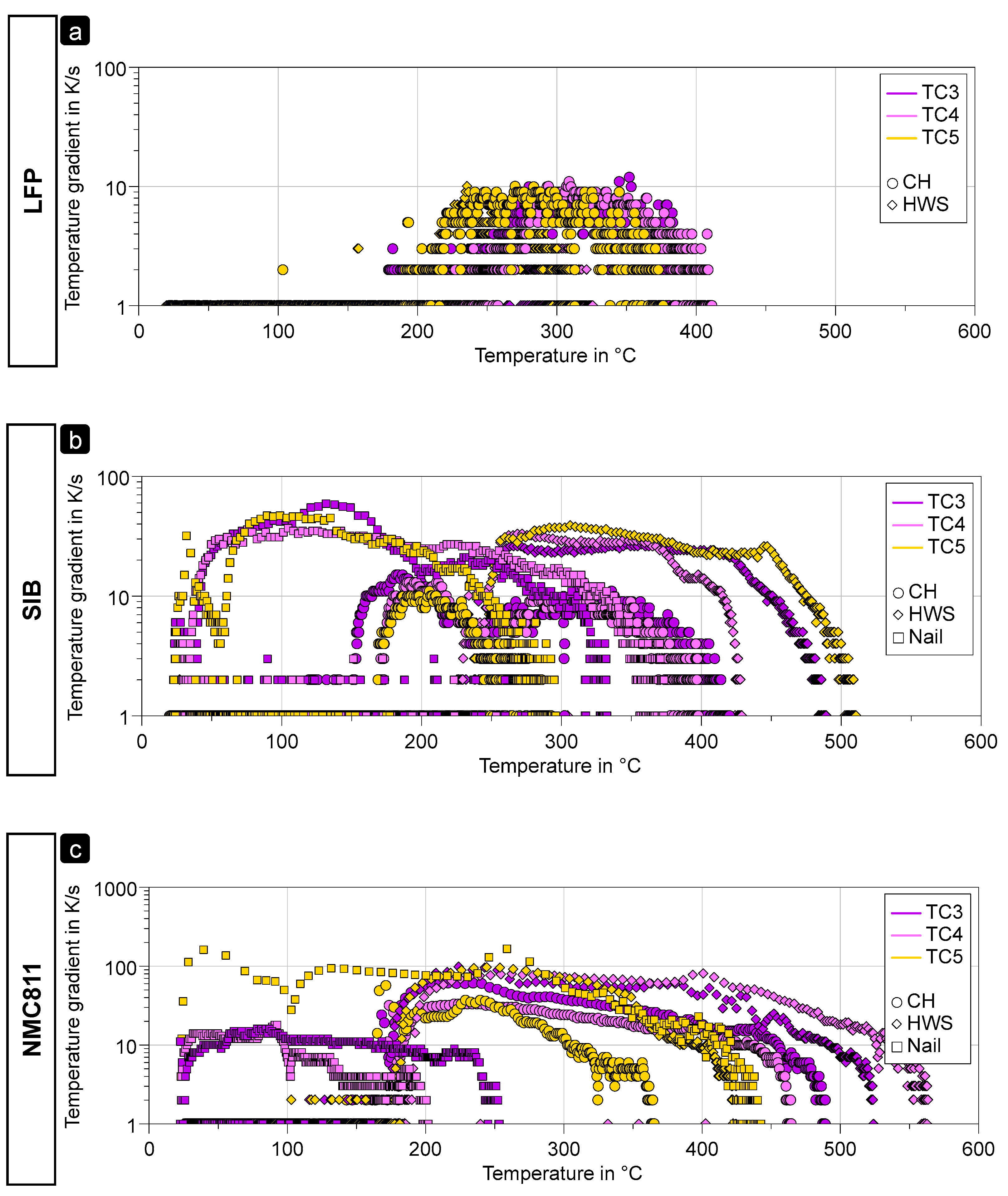
- The LFP, as a lithium metal phosphate, has a thermal stability superior to 500 °C [9] even in a fully charged state. Based on the maximum cell body temperature recorded, we can reasonably suppose that LFP did not contribute to any TR event (both CH and HWS) or very little, which agrees with the limited temperature gradient of ca. ≤10 K/s (Figure 9a and related discussion) and weight loss < 20%.
- NMC811 is described as very reactive, especially when fully charged, with thermal stability inferior to 240 °C [10], while the maximum temperature recorded of the cell body is always superior to 650 °C. Thus, combustion and/or ejection of the NMC811 during the TR events (see Figure 5f) are very likely to have occurred, in agreement with a high-temperature gradient (>60 K/s for CH and >90 K/s for HWS; see Figure 9c and related discussion) and a weight loss always superior to 60%.
- We suppose that the cathodic material of the SIB, Na0.8Ni0.32Fe0.34Mn0.34O2, has a thermal stability relatively close to that of the alkali metal oxides used in the LIB:
- ○
- With the CH trigger method, the temperature in the SIB cell is probably not sufficient to lead to any significant degradation of the cathodic material, leading to a limited temperature gradient ≤ 20 K/s (Figure 9b and related discussion) and a weight loss inferior to 20%.
- ○
- With the HWS trigger method, the SIB cell is homogeneously heated, and more energy is released during the TR event, with a temperature gradient of ≈40 K/s (Figure 9b), allowing a reaction of the cathodic metal oxide, in agreement with a weight loss > 50% and significant gas release.
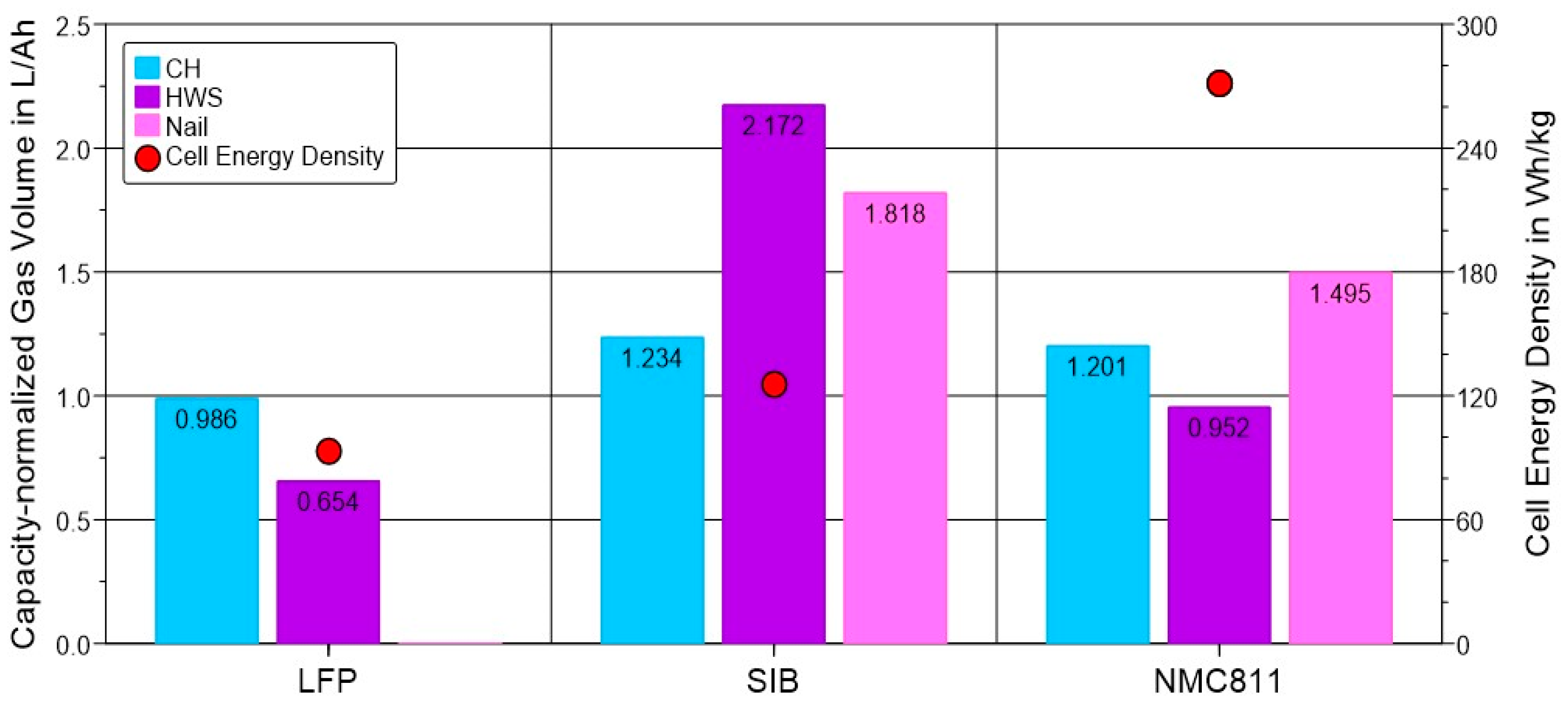
3.4. Gas Emission: Combined Impact of the Cell Chemistry and Trigger Method
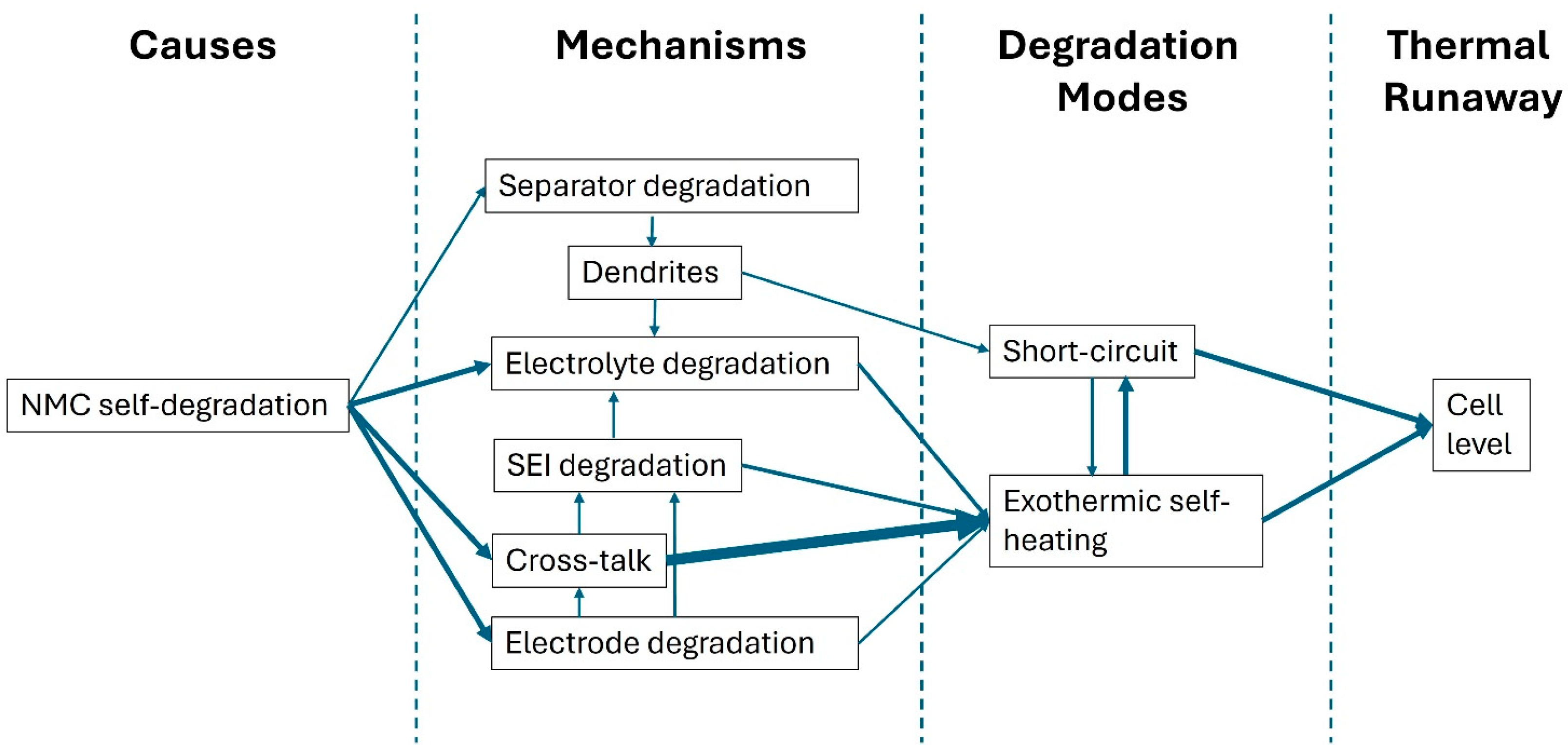
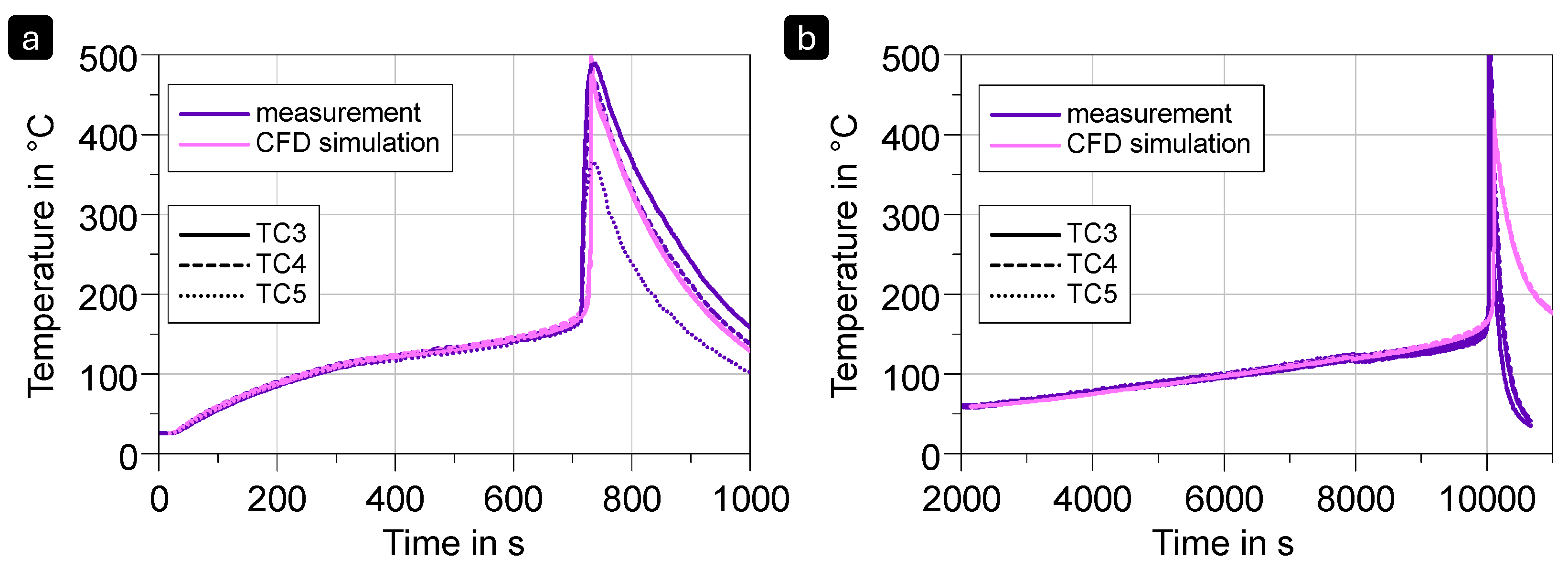
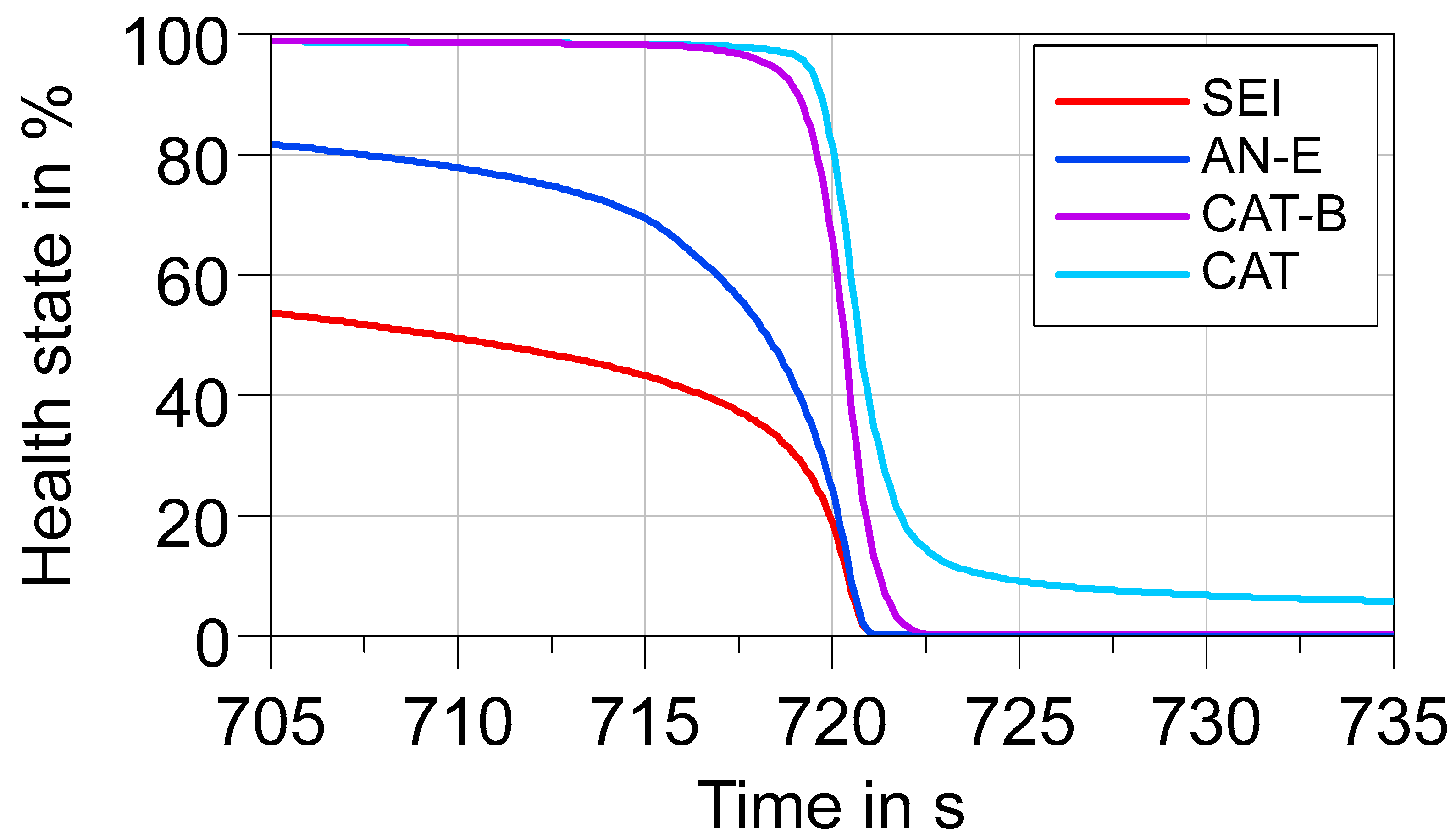
3.5. Thermal Runaway Simulation as a Basis for Propagation Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A





References
- Volta Foundation. Annual Battery Report 2023. Available online: www.volta.foundation/annual-battery-report (accessed on 31 January 2025).
- United Nations. Regulation No. 100: Uniform Provisions Concerning the Approval of Vehicles with Regard to Specific Requirements for the Electric Power Train; E/ECE/Rev. 2/Add.99/Rev.3; United Nations: Geneva, Switzerland, 2015. [Google Scholar]
- IRENA. Critical Materials: Batteries for Electric Vehicles, International Renewable Energy Agency, Abu Dhabi. 2024. Available online: https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2024/Sep/IRENA_Critical_materials_Batteries_for_EVs_2024.pdf (accessed on 31 January 2025).
- Sens, M.; Fandakov, A.; Clauß, M.; Werfel, J.; Tourlonias, P.; Prüger, M.; Özkan, E.; Danzer, C.; Kruschel, C.; Meyer, S.; et al. Towards a Sustainable Vehicle Concept, Part 1: The High-Voltage Battery—Technologies and Methods. In Proceedings of the 44th International Vienna Motor Symposium, Vienna, Austria, 26–28 April 2023. [Google Scholar]
- Tarascon, J.-M. Na-ion versus Li-ion batteries: Complementarity rather than competitiveness. Joule 2020, 4, 1616–1620. [Google Scholar]
- Tapia Ruiz, N.; Armstrong, A.R.; Alptekin, H.; Amores, M.A.; Au, H.; Barker, J.; Boston, R.; Brant, W.R.; Brittain, J.M.; Chen, Y.; et al. 2021 roadmap for sodium-ion batteries. J. Phys. Energy 2021, 3, 031503. [Google Scholar]
- Song, T.; Kishore, B.; Lakhdar, Y.; Chen, L.; Slater, P.R.; Kendrick, E. Effect of storage voltage upon sodium-ion batteries. Batteries 2024, 10, 361. [Google Scholar] [CrossRef]
- Lei, B.; Zhao, W.; Ziebert, C.; Uhlmann, N.; Rohde, M.; Seifert, H.J. Experimental analysis of thermal runaway in 18650 cylindrical Li-ion cells using an accelerating rate calorimeter. Batteries 2017, 3, 14. [Google Scholar] [CrossRef]
- Barkholtz, H.M.; Preger, Y.; Ivanov, S.; Langendorf, J.; Torres-Castro, L.; Lamb, J.; Chamala, B.; Ferreira, S.R. Multi-scale thermal stability study of commercial lithium-ion batteries as a function of cathode chemistry and state-of-charge. J. Power Sources 2019, 435, 226777. [Google Scholar]
- Tang, Z.; Feng, D.; Xu, Y.; Chen, L.; Zhang, X.; Ma, Q. Safety issues of layered nickel-based cathode materials for lithium-ion batteries: Origin, strategies and prospects. Batteries 2023, 9, 156. [Google Scholar] [CrossRef]
- Yang, X.; Wang, H.; Li, M.; Li, Y.; Li, C.; Zhang, Y.; Chen, S.; Shen, H.; Qian, F.; Feng, X.; et al. Experimental study on thermal runaway behavior of lithium-ion battery and analysis of combustible limit of gas production. Batteries 2022, 8, 250. [Google Scholar] [CrossRef]
- Jung, R.; Metzger, M.; Maglia, F.; Stinner, C.; Gasteiger, H.A. Oxygen release and its effect on the cycling stability of LiNixMnyCozO2 (NMC) cathode materials for Li-ion batteries. J. Electrochem. Soc. 2017, 164, A1361. [Google Scholar]
- Liu, X.; Ren, D.; Hsu, H.; Feng, X.; Xu, G.L.; Zhuang, M.; Gao, H.; Lu, L.; Han, X.; Chu, Z.; et al. Thermal runaway of lithium-ion batteries without internal short circuit. Joule 2018, 2, 2047. [Google Scholar]
- Tran, M.K.; Mevawalla, A.; Aziz, A.; Panchal, S.; Xie, Y.; Fowler, M. A review of lithium-ion battery thermal runaway modeling and diagnosis approaches. Processes 2022, 10, 1192. [Google Scholar] [CrossRef]
- Roth, E.P.; Crafts, C.C.; Doughty, D.H.; McBreen, J. Advanced Technology Development Program for Lithium-Ion Batteries: Thermal Abuse Performance of 18650 Li-Ion Cells. Sandia Report SAND2004-0584, 1 March 2004. Available online: https://www.osti.gov/servlets/purl/918751 (accessed on 31 January 2025).
- Tang, X.; Zhang, G.; Wang, X.; Wei, G.; Han, G.; Zhu, J.; Wei, X.; Dai, H. Investigating the critical characteristics of thermal runaway process for LiFePO4/graphite batteries by a ceased segmented method. iScience 2020, 24, 103088. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Zhang, H.; Wei, L.; Feng, X.; Xu, W. Multi-layer internal short-circuit mechanism and thermal runaway risk assessment of lithium-ion batteries using nail penetration simulation. J. Energy Storage 2024, 97, 112824. [Google Scholar] [CrossRef]
- Finegan, D.P.; Tjaden, B.; Heenan, T.M.M.; Jervis, R.; Di Michiel, M.; Rack, A.; Hinds, G.; Brett, D.J.L.; Shearing, P.R. Tracking internal temperature and structural dynamics during nail penetration of lithium-ion cells. J. Electrochem. Soc. 2017, 164, A3285. [Google Scholar] [CrossRef]
- Yokoshima, T.; Mukoyama, D.; Maeda, F.; Osaka, T.; Takazawa, K.; Egusa, S. Operando analysis of thermal runaway in lithium ion battery during nail-penetration test using an x-ray inspection system. J. Electrochem. Soc. 2017, 166, A1243. [Google Scholar] [CrossRef]
- Joshi, T.; Azam, A.; Lopez, C.; Kinyon, S.; Jeevarajan, J. Safety of lithium-ion cells and batteries at different states-of-charge. J. Electrochem. Soc. 2020, 167, 140547. [Google Scholar] [CrossRef]
- Golubkov, A.W.; Scheikl, S.; Planteu, R.; Voitic, G.; Wiltsche, H.; Snagi, C.; Fauler, G.; Thaler, A.; Hacker, V. Thermal runaway of commercial 18650 Li-ion batteries with LFP and NCA cathodes -impact of the state of charge and overcharge. RSC Adv. 2015, 5, 57171–57186. [Google Scholar]
- Kurzaski, A.; Gray, L.; Torres-Castra, L.; Hewson, J. An investigation into the effects of state of charge and heating rate on propagating thermal runaway in Li-ion batteries with experiments and simulations. Fire Saf. J. 2023, 140, 103885. [Google Scholar] [CrossRef]
- Volta Foundation. Annual Battery Report 2024. Available online: https://volta.foundation/battery-report-2024 (accessed on 31 January 2025).
- Wei, N.; Li, M. Experimental study of thermal runaway process of 256 Ah prismatic nickel-rich battery. Frontiers 2023, 11, 1230429. [Google Scholar]
- Bischof, K.; Marangon, V.; Kasper, M.; Regalado, A.A.; Wohlfahrt-Mehrens, M.; Hölzle, M.; Bresser, D.; Waldmann, T. Evaluation of commercial 18650 and 267000 sodium-ion cells and comparison with well-established lithium-ion cells. J. Power Sources 2024, 27, 100148. [Google Scholar]
- Richards, K.J.; Senecal, P.K.; Pomraning, E. CONVERGE, 3.1; Convergent Science: Madison, WI, USA, 2024. [Google Scholar]
- Hahn, A.; Müller, J.; Doose, S.; Kwade, A. Impact of silicon content mechanical abuse and thermal runaway of Li-ion battery cells. J. Power Sources 2025, 628, 235840. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 2004, 104, 4303–4417. [Google Scholar] [CrossRef]
- Desai, P.; Huang, J.; Foix, D.; Tarascon, J.-M.; Mariyappan, S. Zero volt storage of Na-ion batteries; Performances dependence on cell chemistry. J. Power Sources 2022, 551, 232177. [Google Scholar] [CrossRef]
- Rudola, A.; Rennie, A.J.R.; Shayan Meysami, S.; Lowbridge, A.; Mazzali, F.; Sayers, R.; Wright, C.J.; Barker, J. Commercialisation of high energy density sodium-ion batteries: Faradion’s journey and outlook. J. Mater. Chem A 2021, 9, 8279–8302. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Wang, H.; Wang, M. Research progress of organic liquid electrolyte for sodium ion battery. Front. Chem. 2023, 11, 1253959. [Google Scholar] [CrossRef]
- Sturm, J.; Rheinfeld, A.; Zilberman, I.; Singler, F.B.; Kosch, S.; Frie, F.; Jossen, A. Modeling and simulation of inhomogeneities in 18650 nickel-rich, silicon-graphite lithium-ion cell during fast charging. J. Power Sources 2019, 412, 204–233. [Google Scholar] [CrossRef]
- Wijeyakulasuriya, S.; Kim, J.; Probst, D.; Srivastava, K.; Yang, P.; Scarcelli, R.; Senecal, P.K. Enabling powertrain technologies for euro 7/VII vehicles with computational fluid dynamics. Trans. Eng. 2022, 9, 100127. [Google Scholar] [CrossRef]
- Kim, G.H.; Pesaran, A.; Spotnitz, R. A three-dimensional thermal abuse model of lithium-ion cells. J. Power Sources 2007, 170, 476. [Google Scholar] [CrossRef]
- Ren, D.; Liu, X.; Feng, X.; Lu, L.; Ouyang, M.; Li, J.; He, X. Model-based thermal runaway prediction of lithium-ion batteries from kinetics analysis of cell components. Appl. Energy 2018, 228, 633. [Google Scholar] [CrossRef]
- Parhizi, M.; Crompton, K.R.; Ostanek, J. Probing the role of venting and evaporative cooling in thermal runaway for small format Li-ion cells. In Proceedings of the ASME 2021 International Mechanical Engineering Congress and Exposition, Virtual, 1–5 November 2021; Volume 8A: Energy. [Google Scholar]
- Ostanek, J.; Li, W.; Mukherjee, P.P.; Crompton, K.R.; Hacker, C. Simulating onset and evolution of thermal runaway in Li-ion cells using a coupled thermal and venting model. Appl. Energy 2020, 268, 114972. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, D.; Feng, X.; Wang, L.; Ouyang, M. Thermal kinetics comparison of delithiated Li[NixCoyMn1−x−y]O2 cathodes. J. Power Sources 2021, 514, 230582. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, S.; Liu, L.; Zhao, P. Cell-to-cell variability in Li-ion battery thermal runaway: Experimental testing, statistical analysis, and kinetic modeling. J. Energy Storage 2022, 56, 106024. [Google Scholar] [CrossRef]
- Srivastava, K.; Burton, T.; Probst, D. Capturing battery thermal runaway and venting phenomena using detailed 3D CFD solutions. In Proceedings of the 2022 NASA Aerospace Battery Workshop, Huntsville, TX, USA, 15–17 November 2022. [Google Scholar]
- Hang, Y.; Mei, W.; Qin, P.; Duan, Q.; Wang, Q. Numerical modeling on thermal runaway triggered by local overheat for lithium iron phosphate battery. Appl. Therm. Eng. 2021, 192, 116928. [Google Scholar]








| Cell | Manufacturer | Description | Nominal Capacity | Nominal Voltage | Cell Mass |
|---|---|---|---|---|---|
| [mAh] | [V] | [g] | |||
| LFP | A123 | APE18650M1-A | 1100 | 3.2–3.3 | 39 |
| SIB * | HAKADI | 18650E-1500 | 1500 | 3.0 | 37 |
| NMC811 | LG Chem | INR18650-MJ15 | 3500 | 3.6 | 49 |
| Cells | Trigger Method | Vent Time (s) | TR Time (s) | Max. Tgradient (K/s) | Tmax Cell Body (°C) | Tmax Gases (°C) | Weight Loss (%) |
|---|---|---|---|---|---|---|---|
| LFP | CH | 460 | 540 | 10 | 410 | - | 14 |
| HWS | 10,850 | 12,650 | 10 | 330 | - | 19 | |
| SIB | CH | 390 | 460 | <20 | 450 | 275 | 19 |
| HWS | 10,910 | 15,560 | 40 | 500 | - | >50 | |
| Nail | - | 18 $ | 60 | 370 | 450 | - | |
| NMC | CH | 320 | 720 | 60 | 680 | >1100 | 65 |
| HWS | 7880 | 10,030 | 100 | 600 | >1200 | >60 | |
| Nail | - | 15 $ | >100 | 440 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the World Electric Vehicle Association. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sallard, S.; Nolte, O.; von Roemer, L.; Soltani, B.; Fandakov, A.; Mueller, K.; Kalogirou, M.; Sens, M. Exploring Thermal Runaway: Role of Battery Chemistry and Testing Methodology. World Electr. Veh. J. 2025, 16, 153. https://doi.org/10.3390/wevj16030153
Sallard S, Nolte O, von Roemer L, Soltani B, Fandakov A, Mueller K, Kalogirou M, Sens M. Exploring Thermal Runaway: Role of Battery Chemistry and Testing Methodology. World Electric Vehicle Journal. 2025; 16(3):153. https://doi.org/10.3390/wevj16030153
Chicago/Turabian StyleSallard, Sébastien, Oliver Nolte, Lorenz von Roemer, Brahim Soltani, Alexander Fandakov, Karsten Mueller, Maria Kalogirou, and Marc Sens. 2025. "Exploring Thermal Runaway: Role of Battery Chemistry and Testing Methodology" World Electric Vehicle Journal 16, no. 3: 153. https://doi.org/10.3390/wevj16030153
APA StyleSallard, S., Nolte, O., von Roemer, L., Soltani, B., Fandakov, A., Mueller, K., Kalogirou, M., & Sens, M. (2025). Exploring Thermal Runaway: Role of Battery Chemistry and Testing Methodology. World Electric Vehicle Journal, 16(3), 153. https://doi.org/10.3390/wevj16030153







