Walking with UAN.GO Exoskeleton: Training and Compliance in a Multiple Sclerosis Patient
Abstract
:1. Background
2. Materials and Methods
- − The first ability to develop while wearing the UAN.GO is standing up and sitting down.
- − With the warm-up, the patient learns to load his weight through the exoskeleton, and the skill to transfer it to the other leg.
- − The third moment of UAN.GO training is step: joining the acquired abilities, the patient has to stand, transfer the weight through the trunk, and move a leg to 90 degrees flexion.
- − Then, the patient is sure to walk (fourth moment of training), reaching a good coordination between the legs and arms.
- − The last point of the rehabilitation protocol is about autonomous movement.
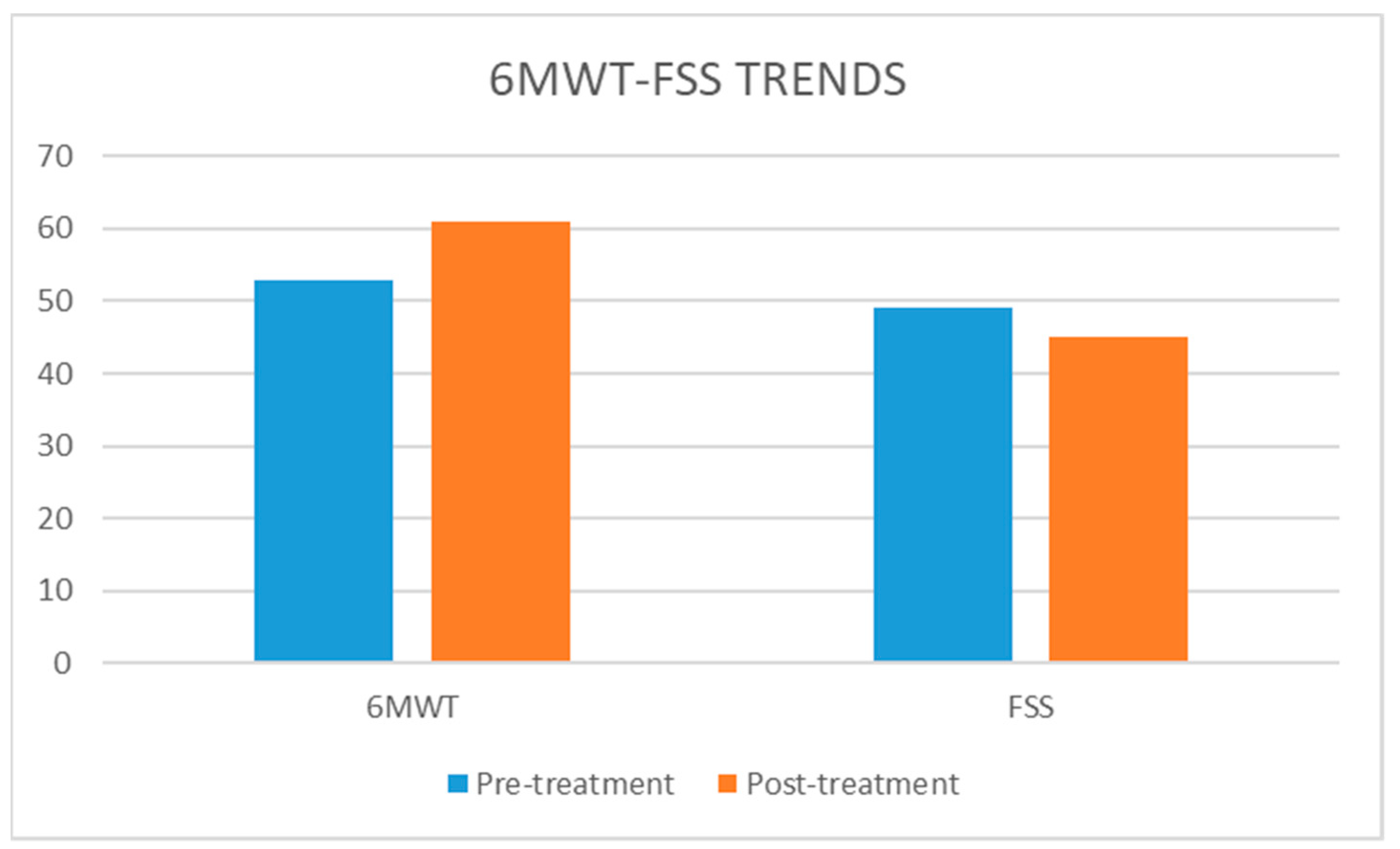
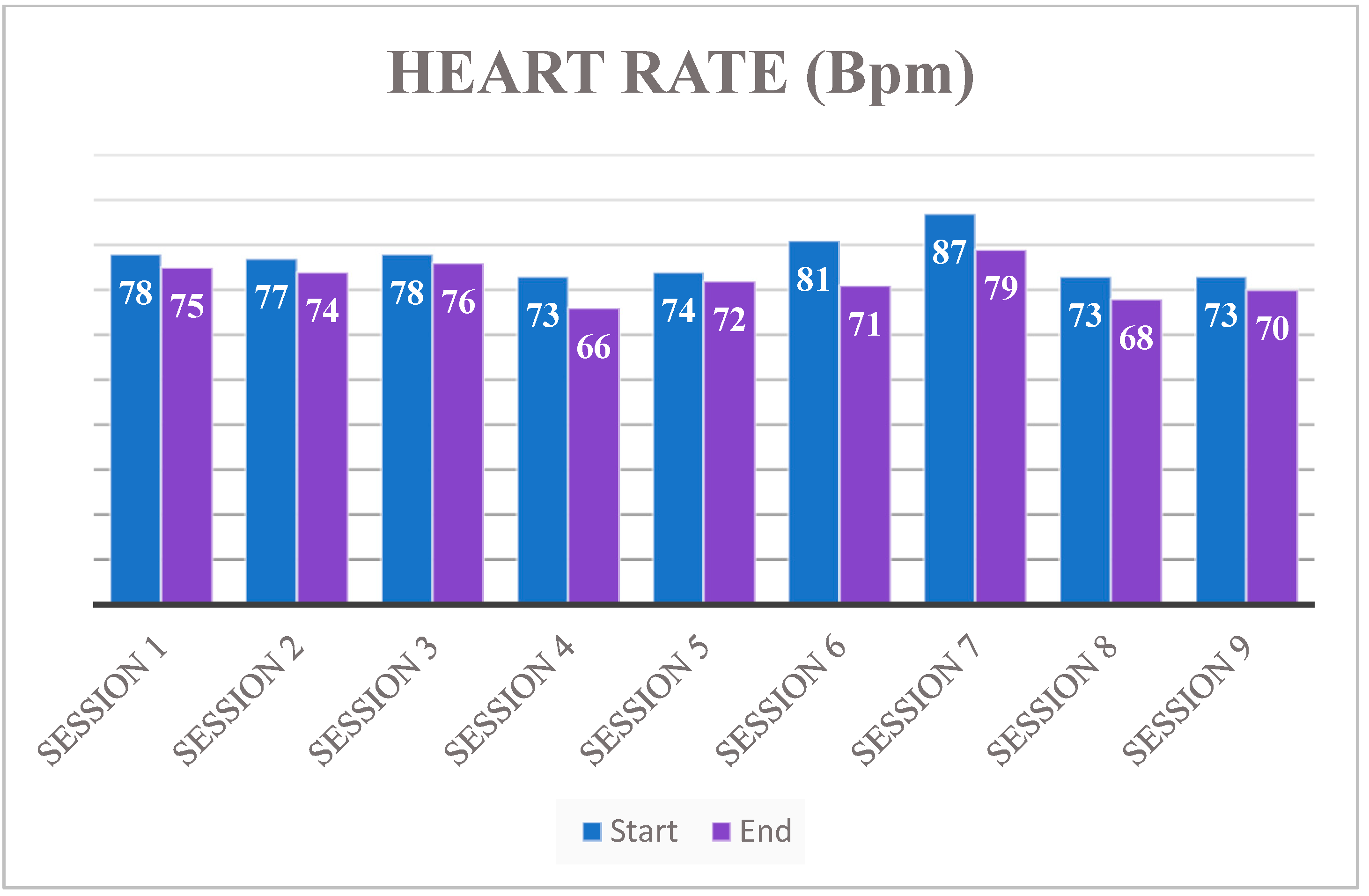
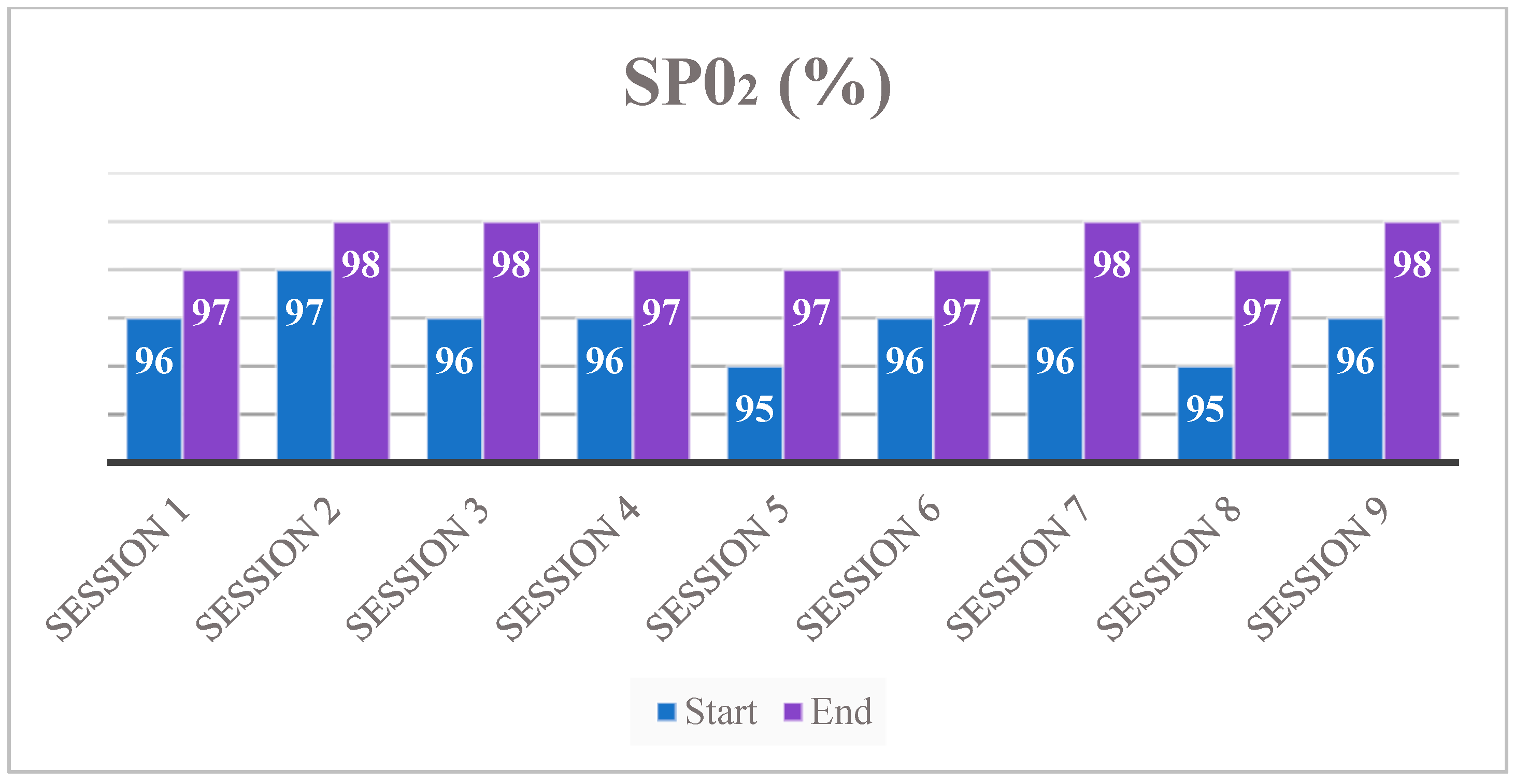
3. Results
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reich, D.S.; Lucchinetti, C.F.; Calabresi, P.A. Multiple Sclerosis. N. Engl. J. Med. 2018, 378, 169–187. [Google Scholar] [CrossRef]
- Cambier, J.; Masson, M.; Masson, C.; Dehen, H. Neurologia; Edra Spa: Milano, Italy, 2012. [Google Scholar]
- Calabresi, P.; Schiess, N. Multiple Sclerosis. Semin. Neurol. 2016, 36, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Ron, M.; Esther, K. Multiple sclerosis: Geoepidemiology, genetics and the environment. Autoimmun. Rev. 2010, 9, 387–394. [Google Scholar]
- Jameson, J.L.; Fauci, A.S.; Kasper, D.L.; Hause, S.L.; Longo, D.L.; Loscalzo, J. Harrison’s Principle of Internal of Medicine; Smat.: Tehran, Iran, 2002. [Google Scholar]
- Randall, T.; Schapiro, M. Managing symptoms of multiple sclerosis. Neurol. Clin. 2007, 23, 135–145. [Google Scholar]
- Doshi, A.; Chataway, J. Multiple sclerosis, a treatable disease. Clin. Med. 2016, 16 (Suppl. 6), s53–s59. [Google Scholar] [CrossRef]
- Henze, T.; Rieckmann, P.; Toyka, K.V. Multiple Sclerosis Therapy Consensus Group of the German Multiple Sclerosis Society. Symptomatic treatment of multiple sclerosis. Eur. Neurol. 2006, 56, 78–105. [Google Scholar] [CrossRef]
- Rietberg, M.B.; Brooks, D.; Uitdehaag, B.M.; Kwakkel, G. Exercise therapy for multiple sclerosis. Cochrane Database Syst. Rev. 2005, 2005, CD003980. [Google Scholar] [CrossRef] [PubMed]
- Paltamaa, J.; Sjögren, T.; Peurala, S.; Heinonen, A. Effects of physiotherapy interventions on balance in multiple sclerosis: A systematic review and meta-analysis of randomized controlled trials. J. Rehabil. Med. 2012, 44, 811–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cramer, H.; Lauche, R.; Azizi, H.; Dobos, G.; Langhorst, J. Yoga for Multiple Sclerosis: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e112414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres-Pareja, M.; Sánchez-Lastra, M.A.; Iglesias, L.; Suárez-Iglesias, D.; Mendoza, N.; Ayán, C. Exercise Interventions for Improving Flexibility in People with Multiple Sclerosis: A Systematic Review and Meta-Analysis. Medicina 2019, 55, 726. [Google Scholar] [CrossRef] [Green Version]
- Campbell, E.; Coulter, E.H.; Paul, L. High intensity interval training for people with multiple sclerosis: A systematic review. Mult. Scler. Relat. Disord. 2018, 24, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Comber, L.; Galvin, R.; Coote, S. Gait deficits in people with multiple sclerosis: A systematic review and meta-analysis. Gait Posture 2017, 51, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Kalron, A.; Dvir, Z.; Achiron, A. Walking while talking—Difficulties incurred during the initial stages of multiple sclerosis disease process. Gait Posture 2010, 32, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Allali, G.; Laidet, M.; Assal, F.; Armand, S.; Lalive, P. Walking while talking in patients with multiple sclerosis: The impact of specific cognitive loads. Neurophysiol. Clin. 2014, 44, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, M.G.; Piperno, R.; Simoncini, L.; Bonato, P.; Tonini, A.; Giannini, S. Gait abnormalities in minimally impaired multiple sclerosis patients. Mult. Scler. J. 1999, 5, 363–368. [Google Scholar] [CrossRef]
- Flegel, M.; Knox, K.; Nickel, D. Step-Length Variability in Minimally Disabled Women with Multiple Sclerosis or Clinically Isolated Syndrome. Int. J. MS Care 2012, 14, 26–30. [Google Scholar] [CrossRef] [Green Version]
- Gianfrancesco, M.A.; Tricheb, E.W.; Fawcettc, J.A.; Labasc, M.; Patterson, T.S.; Lo, A.C. Speed- and cane-related alterations in gait parameters in individuals with multiple sclerosis. Gait Posture 2011, 33, 140–142. [Google Scholar] [CrossRef]
- Givon, U.; Zeilig, G.; Achiron, A. Gait analysis in multiple sclerosis: Characterization of temporal–spatial parameters using GAITRite functional ambulation system. Gait Posture 2009, 29, 138–142. [Google Scholar] [CrossRef]
- Denommé, L.T.; Mandalfino, P.; Cinelli, M.E. Strategies used by individuals with multiple sclerosis and with mild disability to maintain dynamic stability during a steering task. Exp. Brain Res. 2014, 232, 1811–1822. [Google Scholar] [CrossRef]
- Dollar, A.M.; Herr, H. Lower Extremity Exoskeletons and Active Orthoses: Challenges and State-of-the-Art. IEEE Trans. Robot. 2008, 24, 144–158. [Google Scholar] [CrossRef]
- He, Y.; Eguren, D.; Luu, T.P.; Contreras-Vidal, J.L. Risk management and regulations for lower limb medical exoskeletons: A review. Med. Devices 2017, 10, 89–107. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.Y.; Shin, J.-H.; Yang, S.P.; Shin, M.A.; Lee, S.H. Robot-assisted gait training for balance and lower extremity function in patients with infratentorial stroke: A single-blinded randomized controlled trial. J. Neuroeng. Rehabil. 2019, 16, 1–12. [Google Scholar] [CrossRef]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AVEN. Guideline for the Identification of Experimental Thesis to Be Submitted to the Ethics Committee Approval. Available online: https://www.aou.mo.it/ComitatoEticoAVEN (accessed on 8 June 2021).
- Fulk, G.D.; Echternach, J.L.; Nof, L.; O’Sullivan, S. Clinometric properties of the six-minute walk test in individuals undergoing rehabilitation poststroke. Physiother. Theory Pract. 2008, 24, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef]
- Krupp, L.B.; LaRocca, N.G.; Muir-Nash, J.; Steinberg, A.D. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch. Neurol. 1989, 46, 1121–1123. [Google Scholar] [CrossRef] [PubMed]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Likert, R. Technique for the measure of attitudes. Arch. Psychol. 1932, 22, 55. [Google Scholar]
- Allan, J.; Kozlowski, D.M. Time and effort required by persons with spinal cord injury to learn to use a powered exoskeleton for assisted walking. Top Spinal Cord Inj. Rehabil. 2015, 21, 110–121. [Google Scholar]
- Donati, A.R.C.; Shokur, S.; Morya, E.; Campos, D.F.S.; Moioli, R.C.; Gitti, C.M.; Augusto, P.B.; Tripodi, S.; Pires, C.G.; Pereira, G.A.; et al. Long-Term Training with a Brain-Machine Inter-face-Based Gait Protocol Induces Partial Neurological Recovery in Paraplegic Patients. Sci. Rep. 2016, 6, 30383. [Google Scholar] [CrossRef] [Green Version]
- Riley, D.S.; Barber, M.S.; Kienle, G.S.; Aronson, J.K.; von Schoen-Angerer, T.; Tugwell, P.; Kiene, H.; Helfand, M.; Altman, D.G.; Sox, H.; et al. CARE guidelines for case reports: Explanation and elaboration document. J. Clin. Epidemiol. 2017, 89, 218–235. [Google Scholar] [CrossRef]
- Delgado-Mendilívar, J.M.; Cadenas-Díaz, J.C.; Fernández-Torrico, J.M.; Navarro-Mascarell, G.; Izquierdo, G. A study of the quality of life in cases of multiple sclerosis. Rev. Neurol. 2005, 41, 257–262. [Google Scholar]
- Alcobendas-Maestro, M.; Esclarín-Ruz, A.; Casado-López, R.M.; Muñoz-González, A.; Pérez-Mateos, G.; González-Valdizán, E.; Martín, J.L.R. Lokomat robotic-assisted versus overground training within 3 546 to 6 months of incomplete spinal cord lesion: Randomized controlled trial. Neurorehabil. Neural Repair 2012, 6, 1058–1063. [Google Scholar] [CrossRef]
- Niu, X.; Varoqui, D.; Kindig, M.; Mirbagheri, M.M. Prediction of gait recovery in spinal cord injured individuals trained with robotic gait orthosis. J. Neuroeng. Rehabil. 2014, 11, 42. [Google Scholar] [CrossRef] [Green Version]
- Varoqui, D.; Niu, X.; Mirbagheri, M.M. Ankle voluntary movement enhancement following robotic-assisted locomotor training in spinal cord injury. J. Neuroeng. Rehabil. 2014, 11, 46. [Google Scholar] [CrossRef] [Green Version]
- Chang, M.; Kim, Y.; Lee, Y.; Jeon, D. A research on the postural stability of a person wearing the lower limb exoskeletal robot by the HAT model. IEEE Int. Conf. Rehabil. Robot. 2017, 2017, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Lippi, V.; Mergner, T. A Challenge: Support of Standing Balance in Assistive Robotic Devices. Appl. Sci. 2020, 10, 5240. [Google Scholar] [CrossRef]
- Khalili, M.; Borisoff, J.F.; Van der Loos, H.M. Developing safe fall strategies for lower limb exoskeletons. IEEE Int. Conf. Rehabil. Robot. 2017, 2017, 314–319. [Google Scholar] [CrossRef]
- Khalili, M.; Van Der Loos, H.M.; Borisoff, J.F. Studies on Practical Applications of Safe-Fall Control Strategies for Lower Limb Exoskeletons. IEEE Int. Conf. Rehabil. Robot. 2019, 2019, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Tokur, D.; Grimmer, M.; Seyfarth, A. Review of balance recovery in response to external perturbations during daily activities. Hum. Mov. Sci. 2020, 69, 102546. [Google Scholar] [CrossRef] [PubMed]
- Swinnen, E.; Beckwée, D.; Pinte, D.; Meeusen, R.; Baeyens, J.-P.; Kerckhofs, E. Treadmill Training in Multiple Sclerosis: Can Body Weight Support or Robot Assistance Provide Added Value? A Systematic Review. Mult. Scler. Int. 2012, 2012, 240274. [Google Scholar] [CrossRef]
- Xie, X.; Sun, H.; Zeng, Q.; Lu, P.; Zhao, Y.; Fan, T.; Huang, G. Do Patients with Multiple Sclerosis Derive More Benefit from Robot-Assisted Gait Training Compared with Conventional Walking Therapy on Motor Function? A Meta-analysis. Front. Neurol. 2017, 8, 260. [Google Scholar] [CrossRef] [Green Version]
- Pompa, A.; Morone, G.; Iosa, M.; Pace, L.; Catani, S.; Casillo, P.; Clemenzi, A.; Troisi, E.; Tonini, A.; Paolucci, S.; et al. Does robot-assisted gait training improve ambulation in highly disabled multiple sclerosis people? A pilot randomized control trial. Mult. Scler. J. 2016, 23, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Gervasoni, E.; Cattaneo, D.; Jonsdottir, J. Effect of treadmill training on fatigue in multiple sclerosis: A pilot study. Int. J. Rehabil. Res. 2014, 37, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Morrison, E.H.; Cooper, D.M.; White, L.J.; Larson, J.; Leu, S.-Y.; Zaldivar, F.; Ng, A.V. Ratings of Perceived Exertion During Aerobic Exercise in Multiple Sclerosis. Arch. Phys. Med. Rehabil. 2008, 89, 1570–1574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Straudi, S.; Fanciullacci, C.; Martinuzzi, C.; Pavarelli, C.; Rossi, B.; Chisari, C.; Basaglia, N. The effects of robot-assisted gait training in progressive multiple sclerosis: A randomized controlled trial. Mult. Scler. J. 2015, 22, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, M.; Geroin, C.; Picelli, A.; Munari, D.; Waldner, A.; Tamburin, S.; Marchioretto, F.; Smania, N. Robot-assisted vs. sensory integration training in treating gait and balance dysfunctions in patients with multiple sclerosis: A randomized controlled trial. Front. Hum. Neurosci. 2014, 8, 318. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Upper limbs able to handle a walker | History of severe neurological diseases associated with severe systemic diseases (e.g., infections, circulatory or heart problems, lung problems) |
| Absence of unconsolidated fractures | Presence of pressure sores |
| Good general health | Severe spasticity |
| Height between 160 and 195 cm | Heterotopic ossifications that reduce ROM |
| Weight not exceeding 100 kg | Spinal instability or pelvic or AAII fractures not healed |
| Good bone mineral density | Important retractions |
| Psychiatric or cognitive problems that can interfere with the correct use of the device |
| Session Number | Treatment |
|---|---|
| 0 | Explanation of the study Preliminary assessment Setting of the exoskeleton Dressing Up and Down |
| 1 | IMPROVEMENT in PRESSURE POINTS DRESSING UP and DOWN |
| 2 | Dressing Up and Down Warm-Up |
| 3 | Up and Down Warm-Up Pointing exercises to increase device control |
| 4 | Up and Down Warm-Up Step |
| 5 | Up and Down Warm-Up Step |
| 6 | Up and Down Warm-Up Step Walk |
| 7 | Up and Down Warm-Up Step Walk |
| 8 | Up and Down Warm-Up Step Walk |
| 9 | Up and Down Warm-Up Step Walk |
| Start Session | End Session | |
|---|---|---|
| Session 1 | 130/70 mmHg | 135/80 mmHg |
| Session 2 | 135/70 mmHg | 135/70 mmHg |
| Session 3 | 125/80 mmHg | 120/80 mmHg |
| Session 4 | 130/70 mmHg | 120/70 mmHg |
| Session 5 | 135/70 mmHg | 140/80 mmHg |
| Session 6 | 130/70 mmHg | 130/70 mmHg |
| Session 7 | 135/60 mmHg | 135/70 mmHg |
| Session 8 | 110/70 mmHg | 120/70 mmHg |
| Session 9 | 135/80 mmHg | 140/80 mmHg |
| M.L. | Before | After | Differences |
|---|---|---|---|
| Physical function (PF) | 15 | 20 | 5 |
| Role phyisical (RP) | 0 | 0 | - |
| Role emotional (RE) | 33.3 | 66.8 | 33.6 |
| Emotional well-being (EWB) | 35 | 35 | - |
| Social functioning (SF) | 60 | 76 | 16 |
| Pain (BP) | 62.2 | 37.6 | −24.9 |
| General health (GH) | 55 | 65 | 10 |
| Energy/fatigue (VT) | 25 | 25 | - |
| Health change (HC) | 0 | 0 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sesenna, G.; Calzolari, C.; Gruppi, M.P.; Ciardi, G. Walking with UAN.GO Exoskeleton: Training and Compliance in a Multiple Sclerosis Patient. Neurol. Int. 2021, 13, 428-438. https://doi.org/10.3390/neurolint13030042
Sesenna G, Calzolari C, Gruppi MP, Ciardi G. Walking with UAN.GO Exoskeleton: Training and Compliance in a Multiple Sclerosis Patient. Neurology International. 2021; 13(3):428-438. https://doi.org/10.3390/neurolint13030042
Chicago/Turabian StyleSesenna, Gianluca, Cecilia Calzolari, Maria Paola Gruppi, and Gianluca Ciardi. 2021. "Walking with UAN.GO Exoskeleton: Training and Compliance in a Multiple Sclerosis Patient" Neurology International 13, no. 3: 428-438. https://doi.org/10.3390/neurolint13030042
APA StyleSesenna, G., Calzolari, C., Gruppi, M. P., & Ciardi, G. (2021). Walking with UAN.GO Exoskeleton: Training and Compliance in a Multiple Sclerosis Patient. Neurology International, 13(3), 428-438. https://doi.org/10.3390/neurolint13030042






