Oxytocin, a Novel Treatment for Methamphetamine Use Disorder
Abstract
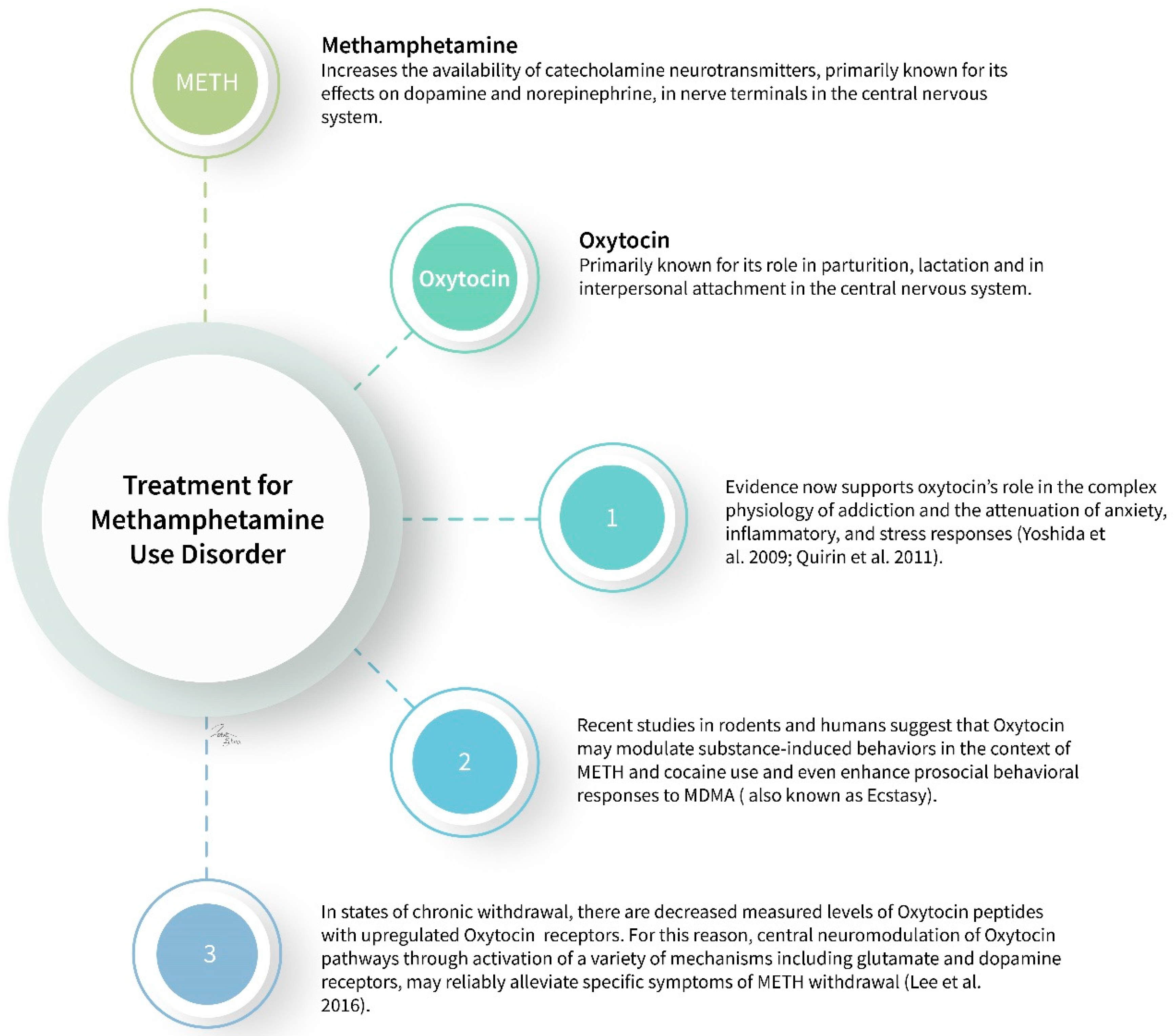
:1. Introduction
2. Methamphetamine
2.1. Epidemiology
2.2. Pathophysiology
2.2.1. Current Treatment of Methamphetamine Use Disorder
2.2.2. Oxytocin
2.3. Mechanism of Action
3. Pre-Clinical Studies
3.1. Subiah et al.
3.2. Qi et al.
3.3. Carson and Cornish et al.
3.4. Carson and Hunt et al.
3.5. Baracz and Parker et al.
4. Stauffer et al. and Possible Clinical Efficacy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DARK Classics in Chemical Neuroscience: Methamphetamine|ACS Chemical Neuroscience [Internet]. Available online: https://pubs.acs.org/doi/10.1021/acschemneuro.8b00123 (accessed on 20 November 2021).
- Che, X.; Cai, J.; Liu, Y.; Xu, T.; Yang, J.; Wu, C. Oxytocin signaling in the treatment of drug addiction: Therapeutic opportunities and challenges. Pharmacol. Ther. 2021, 223, 107820. [Google Scholar] [CrossRef] [PubMed]
- Davidson, C. Developing treatments for stimulant abuse: A brief overview. East Asian Arch. Psychiatry 2016, 26, 52–60. [Google Scholar] [PubMed]
- Ciccarone, D. Stimulant Abuse: Pharmacology, Cocaine, Methamphetamine, Treatment, Attempts at Pharmacotherapy. Prim. Care 2011, 38, 41–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oxytocin in the Socioemotional Brain: Implications for Psychiatric Disorders [Internet]. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4734884/ (accessed on 20 November 2021).
- Sundar, M.; Patel, D.; Young, Z.; Leong, K.-C. Oxytocin and Addiction: Potential Glutamatergic Mechanisms. Int. J. Mol. Sci. 2021, 22, 2405. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.R.; Rohn, M.C.H.; Tanda, G.; Leggio, L. Targeting the Oxytocin System to Treat Addictive Disorders: Rationale and Progress to Date. CNS Drugs 2016, 30, 109–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowen, M.T.; Neumann, I.D. Rebalancing the Addicted Brain: Oxytocin Interference with the Neural Substrates of Addiction. Trends Neurosci. 2017, 40, 691–708. [Google Scholar] [CrossRef] [PubMed]
- Rawson, R.A.; Anglin, M.D.; Ling, W. Will the methamphetamine problem go away? J. Addict. Dis. 2002, 21, 5–19. [Google Scholar] [CrossRef]
- Maxwell, J.C.; Brecht, M.-L. Methamphetamine: Here we go again? Addict. Behav. 2011, 36, 1168–1173. [Google Scholar] [CrossRef] [Green Version]
- Paulus, M.P.; Stewart, J.L. Neurobiology, Clinical Presentation, and Treatment of Methamphetamine Use Disorder: A Review. JAMA Psychiatry 2020, 77, 959–966. [Google Scholar] [CrossRef]
- Key Substance Use and Mental Health Indicators in the United States: Results from the 2019 National Survey on Drug Use and Health [Internet]. Available online: https://www.samhsa.gov/data/sites/default/files/reports/rpt29393/2019NSDUHFFRPDFWHTML/2019NSDUHFFR090120.htm (accessed on 3 October 2021).
- Barr, A.M.; Panenka, W.J.; MacEwan, G.W.; Thornton, A.E.; Lang, D.J.; Honer, W.G.; Lecomte, T. The need for speed: An update on methamphetamine addiction. J. Psychiatry Neurosci. JPN 2006, 31, 301–313. [Google Scholar]
- Brown, J.M.; Hanson, G.R.; Fleckenstein, A.E. Regulation of the vesicular monoamine transporter-2: A novel mechanism for cocaine and other psychostimulants. J. Pharmacol. Exp. Ther. 2001, 296, 762–767. [Google Scholar]
- Khoshbouei, H.; Wang, H.; Lechleiter, J.D.; Javitch, J.A.; Galli, A. Amphetamine-induced dopamine efflux. A voltage-sensitive and intracellular Na+-dependent mechanism. J. Biol. Chem. 2003, 278, 12070–12077. [Google Scholar] [CrossRef] [Green Version]
- Johnson, R.A.; Eshleman, A.J.; Meyers, T.; Neve, K.A.; Janowsky, A. [3H]substrate- and cell-specific effects of uptake inhibitors on human dopamine and serotonin transporter-mediated efflux. Synapse 1998, 30, 97–106. [Google Scholar] [CrossRef]
- Brown, J.M.; Hanson, G.R.; Fleckenstein, A.E. Methamphetamine rapidly decreases vesicular dopamine uptake. J. Neurochem. 2000, 74, 2221–2223. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, O.; Hattori, H.; Asano, M.; Oya, M.; Katsumata, Y. Inhibition of monoamine oxidase by d-methamphetamine. Biochem. Pharmacol. 1980, 29, 2071–2073. [Google Scholar] [CrossRef]
- Prakash, M.D.; Tangalakis, K.; Antonipillai, J.; Stojanovska, L.; Nurgali, K.; Apostolopoulos, V. Methamphetamine: Effects on the brain, gut and immune system. Pharmacol. Res. 2017, 120, 60–67. [Google Scholar] [CrossRef] [Green Version]
- Cadet, J.L.; Bisagno, V.; Milroy, C.M. Neuropathology of substance use disorders. Acta Neuropathol. 2014, 127, 91–107. [Google Scholar] [CrossRef]
- Scott, J.C.; Woods, S.P.; Matt, G.E.; Meyer, R.A.; Heaton, R.K.; Atkinson, J.H.; Grant, I. Neurocognitive effects of methamphetamine: A critical review and meta-analysis. Neuropsychol. Rev. 2007, 17, 275–297. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, R.C.; Cunningham, J.K.; Sykes, J.; Kish, S.J. Increased risk of Parkinson’s disease in individuals hospitalized with conditions related to the use of methamphetamine or other amphetamine-type drugs. Drug Alcohol Depend. 2012, 120, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Rusyniak, D.E. Neurologic manifestations of chronic methamphetamine abuse. Psychiatr. Clin. N. Am. 2013, 36, 261–275. [Google Scholar] [CrossRef] [Green Version]
- Effects of Exposure to Amphetamine Derivatives on Passive Avoidance Performance and the Central Levels of Monoamines and Their Metabolites in Mice: Correlations between Behavior and Neurochemistry—PubMed [Internet]. Available online: https://pubmed.ncbi.nlm.nih.gov/21993877/ (accessed on 28 November 2021).
- Moszczynska, A.; Callan, S.P. Molecular, Behavioral, and Physiological Consequences of Methamphetamine Neurotoxicity: Implications for Treatment. J. Pharmacol. Exp. Ther. 2017, 362, 474–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krasnova, I.N.; Cadet, J.L. Methamphetamine toxicity and messengers of death. Brain Res. Rev. 2009, 60, 379–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potashkin, J.A.; Meredith, G.E. The role of oxidative stress in the dysregulation of gene expression and protein metabolism in neurodegenerative disease. Antioxid. Redox Signal. 2006, 8, 144–151. [Google Scholar] [CrossRef]
- Tata, D.A.; Yamamoto, B.K. Chronic stress enhances methamphetamine-induced extracellular glutamate and excitotoxicity in the rat striatum. Synapse 2008, 62, 325–336. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Northcutt, A.L.; Cochran, T.A.; Zhang, X.; Fabisiak, T.J.; Haas, M.E.; Amat, J.; Li, H.; Rice, K.C.; Maier, S.F.; et al. Methamphetamine Activates Toll-Like Receptor 4 to Induce Central Immune Signaling within the Ventral Tegmental Area and Contributes to Extracellular Dopamine Increase in the Nucleus Accumbens Shell. ACS Chem. Neurosci. 2019, 10, 3622–3634. [Google Scholar] [CrossRef]
- Pålsson-McDermott, E.M.; O’Neill, L.A.J. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology 2004, 113, 153–162. [Google Scholar] [CrossRef]
- Wilson, J.M.; Kalasinsky, K.S.; Levey, A.I.; Bergeron, C.; Reiber, G.; Anthony, R.M.; Schmunk, G.A.; Shannak, K.; Haycock, J.W.; Kish, S.J. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat. Med. 1996, 2, 699–703. [Google Scholar] [CrossRef]
- Moszczynska, A.; Fitzmaurice, P.; Ang, L.; Kalasinsky, K.S.; Schmunk, G.A.; Peretti, F.J.; Aiken, S.S.; Wickham, D.J.; Kish, S.J. Why is parkinsonism not a feature of human methamphetamine users? Brain J. Neurol. 2004, 127, 363–370. [Google Scholar] [CrossRef] [Green Version]
- Chang, L.; Alicata, D.; Ernst, T.; Volkow, N. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addict. Abingdon. Engl. 2007, 102 (Suppl. S1), 16–32. [Google Scholar] [CrossRef]
- Jan, R.K.; Kydd, R.R.; Russell, B.R. Functional and structural brain changes associated with methamphetamine abuse. Brain Sci. 2012, 2, 434–482. [Google Scholar] [CrossRef]
- Siefried, K.J.; Acheson, L.S.; Lintzeris, N.; Ezard, N. Pharmacological Treatment of Methamphetamine/Amphetamine Dependence: A Systematic Review. CNS Drugs 2020, 34, 337–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stuart, A.M.; Baker, A.L.; Denham, A.M.J.; Lee, N.K.; Hall, A.; Oldmeadow, C.; Dunlop, A.; Bowman, J.; McCarter, K. Psychological treatment for methamphetamine use and associated psychiatric symptom outcomes: A systematic review. J. Subst. Abus. Treat. 2020, 109, 61–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKetin, R.; Dean, O.M.; Baker, A.L.; Carter, G.; Turner, A.; Kelly, P.J.; Berk, M. A potential role for N-acetylcysteine in the management of methamphetamine dependence. Drug Alcohol Rev. 2017, 36, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Carson, D.S.; Guastella, A.J.; Taylor, E.R.; McGregor, I.S. A brief history of oxytocin and its role in modulating psychostimulant effects. J. Psychopharmacol. Oxf. Engl. 2013, 27, 231–247. [Google Scholar] [CrossRef]
- Yoshida, M.; Takayanagi, Y.; Inoue, K.; Kimura, T.; Young, L.J.; Onaka, T.; Nishimori, K. Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 2259–2271. [Google Scholar] [CrossRef]
- Quirin, M.; Kuhl, J.; Düsing, R. Oxytocin buffers cortisol responses to stress in individuals with impaired emotion regulation abilities. Psychoneuroendocrinology 2011, 36, 898–904. [Google Scholar] [CrossRef]
- Lee, H.-J.; Macbeth, A.H.; Pagani, J.; Young, W.S. Oxytocin: The Great Facilitator of Life. Prog. Neurobiol. 2009, 88, 127–151. [Google Scholar] [CrossRef] [Green Version]
- Gimpl, G.; Fahrenholz, F. The Oxytocin Receptor System: Structure, Function, and Regulation. Physiol. Rev. 2001, 81, 629–683. [Google Scholar] [CrossRef] [Green Version]
- Knobloch, H.S.; Charlet, A.; Hoffmann, L.C.; Eliava, M.; Khrulev, S.; Cetin, A.H.; Osten, P.; Schwarz, M.K.; Seeburg, P.H.; Stoop, R.; et al. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron 2012, 73, 553–566. [Google Scholar] [CrossRef] [Green Version]
- Eliava, M.; Melchior, M.; Knobloch-Bollmann, H.S.; Wahis, J.; da Silva Gouveia, M.; Tang, Y.; Ciobanu, A.C.; Del Rio, R.T.; Roth, L.C.; Althammer, F.; et al. A New Population of Parvocellular Oxytocin Neurons Controlling Magnocellular Neuron Activity and Inflammatory Pain Processing. Neuron 2016, 89, 1291–1304. [Google Scholar] [CrossRef] [Green Version]
- Cox, B.M.; Bentzley, B.S.; Regen-Tuero, H.; See, R.E.; Reichel, C.M.; Aston-Jones, G. Oxytocin acts in nucleus accumbens to attenuate methamphetamine seeking and demand. Biol. Psychiatry. 2017, 81, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Baracz, S.J.; Rourke, P.I.; Pardey, M.C.; Hunt, G.E.; McGregor, I.S.; Cornish, J.L. Oxytocin directly administered into the nucleus accumbens core or subthalamic nucleus attenuates methamphetamine-induced conditioned place preference. Behav. Brain Res. 2012, 228, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Moaddab, M.; Hyland, B.I.; Brown, C.H. Oxytocin excites nucleus accumbens shell neurons in vivo. Mol. Cell. Neurosci. 2015, 68, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Leong, K.-C.; Cox, S.; King, C.; Becker, H.; Reichel, C.M. Oxytocin and Rodent Models of Addiction. Int. Rev. Neurobiol. 2018, 140, 201–247. [Google Scholar] [PubMed]
- Carson, D.S.; Cornish, J.L.; Guastella, A.J.; Hunt, G.E.; McGregor, I.S. Oxytocin decreases methamphetamine self-administration, methamphetamine hyperactivity, and relapse to methamphetamine-seeking behaviour in rats. Neuropharmacology 2010, 58, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Subiah, C.O.; Mabandla, M.V.; Phulukdaree, A.; Chuturgoon, A.A.; Daniels, W.M.U. The effects of vasopressin and oxytocin on methamphetamine–induced place preference behaviour in rats. Metab. Brain Dis. 2012, 27, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Carson, D.S.; Hunt, G.E.; Guastella, A.J.; Barber, L.; Cornish, J.L.; Arnold, J.C.; Boucher, A.A.; McGregor, I.S. Systemically administered oxytocin decreases methamphetamine activation of the subthalamic nucleus and accumbens core and stimulates oxytocinergic neurons in the hypothalamus: Oxytocin and addiction. Addict. Biol. 2010, 15, 448–463. [Google Scholar] [CrossRef] [PubMed]
- Baracz, S.J.; Parker, L.M.; Suraev, A.S.; Everett, N.A.; Goodchild, A.K.; McGregor, I.S.; Cornish, J.L. Chronic Methamphetamine Self-Administration Dysregulates Oxytocin Plasma Levels and Oxytocin Receptor Fibre Density in the Nucleus Accumbens Core and Subthalamic Nucleus of the Rat. J. Neuroendocrinol. 2016, 28. [Google Scholar] [CrossRef]
- Everett, N.A.; Baracz, S.J.; Cornish, J.L. The effect of chronic oxytocin treatment during abstinence from methamphetamine self-administration on incubation of craving, reinstatement, and anxiety. Neuropsychopharmacology 2020, 45, 597–605. [Google Scholar] [CrossRef]
- Baracz, S.J.; Everett, N.A.; Cornish, J.L. The Involvement of Oxytocin in the Subthalamic Nucleus on Relapse to Methamphetamine-Seeking Behaviour. Fuchs R, editor. PLoS ONE 2015, 10, e0136132. [Google Scholar] [CrossRef]
- Everett, N.; Baracz, S.; Cornish, J. Oxytocin treatment in the prelimbic cortex reduces relapse to methamphetamine-seeking and is associated with reduced activity in the rostral nucleus accumbens core. Pharmacol. Biochem. Behav. 2019, 183, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Stauffer, C.S.; Moschetto, J.M.; McKernan, S.; Meinzer, N.; Chiang, C.; Rapier, R.; Hsiang, E.; Norona, J.; Borsari, B.; Woolley, J.D. Oxytocin-enhanced group therapy for methamphetamine use disorder: Randomized controlled trial. J. Subst. Abus. Treat. 2020, 116, 108059. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Yang, J.-Y.; Wang, F.; Zhao, Y.-N.; Song, M.; Wu, C.-F. Effects of oxytocin on methamphetamine-induced conditioned place preference and the possible role of glutamatergic neurotransmission in the medial prefrontal cortex of mice in reinstatement. Neuropharmacology 2009, 56, 856–865. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edinoff, A.N.; Thompson, E.; Merriman, C.E.; Alvarez, M.R.; Alpaugh, E.S.; Cornett, E.M.; Murnane, K.S.; Kozinn, R.L.; Shah-Bruce, M.; Kaye, A.M.; et al. Oxytocin, a Novel Treatment for Methamphetamine Use Disorder. Neurol. Int. 2022, 14, 186-198. https://doi.org/10.3390/neurolint14010015
Edinoff AN, Thompson E, Merriman CE, Alvarez MR, Alpaugh ES, Cornett EM, Murnane KS, Kozinn RL, Shah-Bruce M, Kaye AM, et al. Oxytocin, a Novel Treatment for Methamphetamine Use Disorder. Neurology International. 2022; 14(1):186-198. https://doi.org/10.3390/neurolint14010015
Chicago/Turabian StyleEdinoff, Amber N., Elliot Thompson, Chandler E. Merriman, Mark R. Alvarez, E. Saunders Alpaugh, Elyse M. Cornett, Kevin S. Murnane, Rachel L. Kozinn, Mila Shah-Bruce, Adam M. Kaye, and et al. 2022. "Oxytocin, a Novel Treatment for Methamphetamine Use Disorder" Neurology International 14, no. 1: 186-198. https://doi.org/10.3390/neurolint14010015
APA StyleEdinoff, A. N., Thompson, E., Merriman, C. E., Alvarez, M. R., Alpaugh, E. S., Cornett, E. M., Murnane, K. S., Kozinn, R. L., Shah-Bruce, M., Kaye, A. M., & Kaye, A. D. (2022). Oxytocin, a Novel Treatment for Methamphetamine Use Disorder. Neurology International, 14(1), 186-198. https://doi.org/10.3390/neurolint14010015









