Clinical and Radiological Characteristics for Recurrence of Chronic Subdural Hematoma: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Database Search
2.2. Criteria for Online Search
2.3. Inclusion Criteria for Studies
2.4. Exclusion Criteria
2.5. Evaluation of the Quality of the Studies Included
2.6. Analysis of Data
2.6.1. Clinical Risk Factors
2.6.2. Radiological Risk Factors
2.6.3. Statistical Methods Used
3. Results
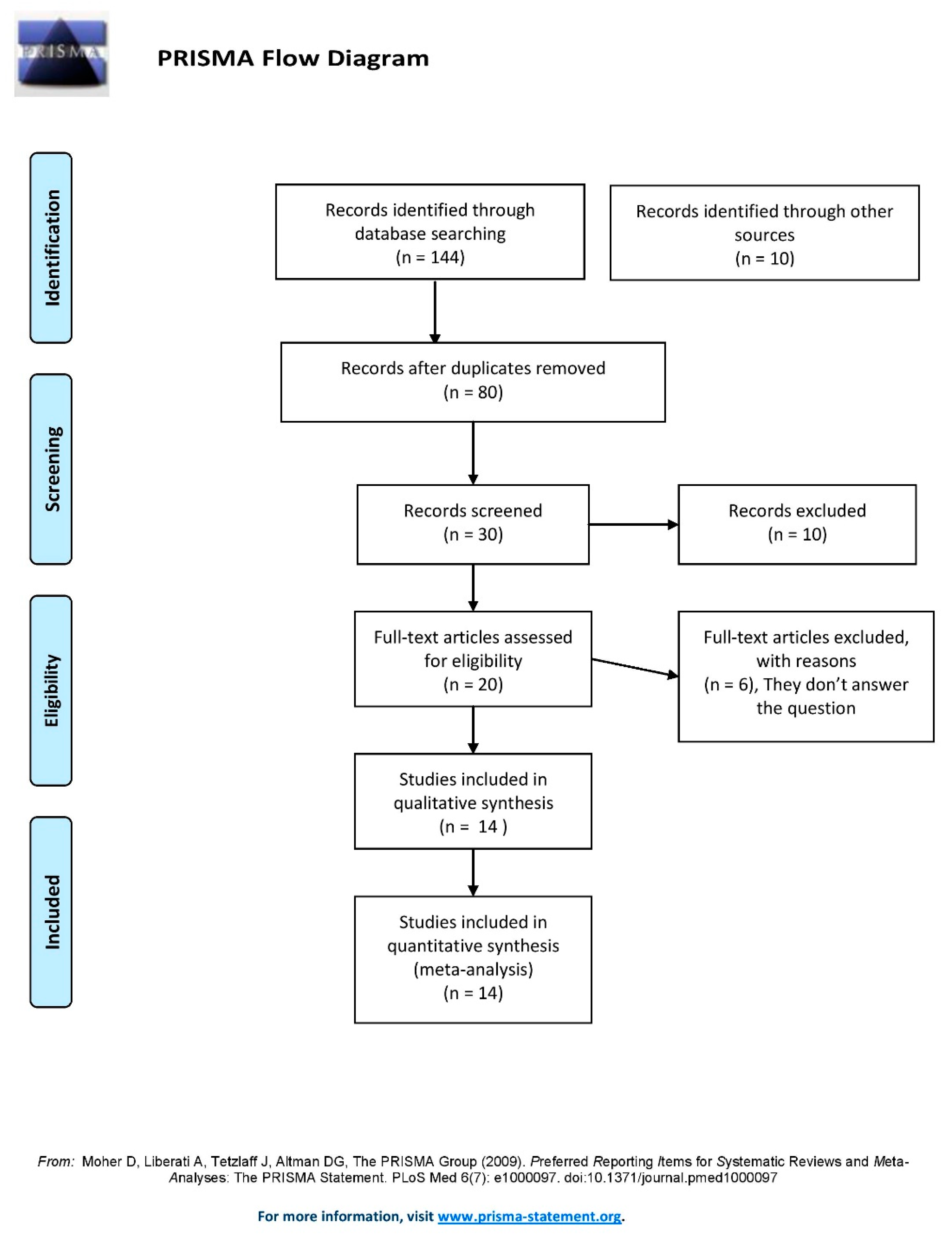
3.1. Study Selection
3.2. Study Characteristics
3.3. Study Quality
3.4. Outcome Measures
3.5. Results Synthesis and Meta-Analysis
3.5.1. Clinical Risk Factors
3.5.2. Radiological Risk Factors
4. Discussion
4.1. Age
4.2. Gender
4.3. Co-Morbidities: Hypertension, Diabetes, and Obesity
4.4. Anticoagulation and Anti-Aggregation Therapy
4.5. Concomitant Stroke, Malignancy, and Chronic Heart, Kidney, and Liver Diseases
4.6. Trauma, Alcohol Consumption, and Smoking Habits
4.7. Midline Shift > 10 mm and Hematoma Width >20 mm on Imaging
4.8. Hematoma Characteristics
4.9. Bilaterality, Severe Brain Atrophy, and Postoperative Pneumocephalus
4.10. Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Andersen-Ranberg, N.C.; Debrabant, B.; Poulsen, F.R.; Bergholt, B.; Hundsholt, T.; Fugleholm, K. The Danish chronic subdural hematoma study-predicting recurrence of chronic subdural hematoma. Acta Neurochir. 2019, 161, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Chon, K.H.; Lee, J.M.; Koh, E.J.; Choi, H.Y. Independent predictors for recurrence of chronic subdural hematoma. Acta Neurochir. 2012, 154, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Hammer, A.; Tregubow, A.; Kerry, G.; Schrey, M.; Hammer, C.; Steiner, H.-H. Predictors for Recurrence of Chronic Subdural Hematoma. Turk. Neurosurg. 2017, 27, 756–762. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Han, M.-H.; Ryu, J.I.; Kim, C.H.; Kim, J.M.; Cheong, J.H.; Yi, H.-J. Predictive factors for recurrence and clinical outcomes in patients with chronic subdural hematoma. J. Neurosurg. 2017, 127, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Moon, J.; Kim, T.; Ahn, S.; Hwang, G.; Bang, J.; Kwon, O.-K.; Oh, C.W. Risk Factor Analysis for the Recurrence of Chronic Subdural Hematoma: A Review of 368 Consecutive Surgical Cases. Korean J. Neurotrauma 2015, 11, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Leroy, H.-A.; Aboukaïs, R.; Reyns, N.; Bourgeois, P.; Labreuche, J.; Duhamel, A.; Lejeune, J.-P. Predictors of functional outcomes and recurrence of chronic subdural hematomas. J. Clin. Neurosci. 2015, 22, 1895–1900. [Google Scholar] [CrossRef]
- Motoie, R.; Karashima, S.; Otsuji, R.; Ren, N.; Nagaoka, S.; Maeda, K.; Ikai, Y.; Uno, J.; Gi, H. Recurrence in 787 Patients with Chronic Subdural Hematoma: Retrospective Cohort Investigation of Associated Factors Including Direct Oral Anticoagulant Use. World Neurosurg. 2018, 118, e87–e91. [Google Scholar] [CrossRef] [PubMed]
- Ohba, S.; Kinoshita, Y.; Nakagawa, T.; Murakami, H. The risk factors for recurrence of chronic subdural hematoma. Neurosurg. Rev. 2013, 36, 145–149, discussion 149–150. [Google Scholar] [CrossRef]
- Oishi, M.; Toyama, M.; Tamatani, S.; Kitazawa, T.; Saito, M. Clinical factors of recurrent chronic subdural hematoma. Neurol. Med.-Chir. 2001, 41, 382–386. [Google Scholar] [CrossRef]
- dos Santos, R.G.; Xander, P.A.W.; Rodrigues, L.H.D.S.; da Costa, G.H.F.; Veiga, J.C.E.; de Aguiar, G.B. Analysis of predisposing factors for chronic subdural hematoma recurrence. Rev. Assoc. Med. Bras. 2019, 65, 834–838. [Google Scholar] [CrossRef]
- Shen, J.; Yuan, L.; Ge, R.; Wang, Q.; Zhou, W.; Jiang, X.C.; Shao, X. Clinical and radiological factors predicting recurrence of chronic subdural hematoma: A retrospective cohort study. Injury 2019, 50, 1634–1640. [Google Scholar] [CrossRef]
- Torihashi, K.; Sadamasa, N.; Yoshida, K.; Narumi, O.; Chin, M.; Yamagata, S. Independent predictors for recurrence of chronic subdural hematoma: A review of 343 consecutive surgical cases. Neurosurgery 2008, 63, 1125–1129, discussion 1129. [Google Scholar] [CrossRef]
- Yamamoto, H.; Hirashima, Y.; Hamada, H.; Hayashi, N.; Origasa, H.; Endo, S. Independent predictors of recurrence of chronic subdural hematoma: Results of multivariate analysis performed using a logistic regression model. J. Neurosurg. 2003, 98, 1217–1221. [Google Scholar] [CrossRef]
- You, W.; Zhu, Y.; Wang, Y.; Liu, W.; Wang, H.; Wen, L.; Yang, X. Prevalence of and risk factors for recurrence of chronic subdural hematoma. Acta Neurochir. 2018, 160, 893–899. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Spallone, A.; Giuffre, R.; Gagliardi, F.M.; Vagnozzi, R. Chronic subdural hematoma in extremely aged patients. Eur. Neurol. 1989, 29, 18–22. [Google Scholar] [CrossRef]
- Mori, K.; Maeda, M. Surgical treatment of chronic subdural hematoma in 500 consecutive cases: Clinical characteristics, surgical outcome, complications, and recurrence rate. Neurol. Med. -Chir. 2001, 41, 371–381. [Google Scholar] [CrossRef]
- Lind, C.R.; Lind, C.J.; Mee, E.W. Reduction in the number of repeated operations for the treatment of subacute and chronic subdural hematomas by placement of subdural drains. J. Neurosurg. 2003, 99, 44–46. [Google Scholar] [CrossRef]
- Abouzari, M.; Rashidi, A.; Rezaii, J.; Esfandiari, K.; Asadollahi, M.; Aleali, H.; Abdollahzadeh, M. The role of postoperative patient posture in the recurrence of traumatic chronic subdural hematoma after burr-hole surgery. Neurosurgery 2007, 61, 794–797, discussion 797. [Google Scholar] [CrossRef]
- Ko, B.-S.; Lee, J.-K.; Seo, B.-R.; Moon, S.-J.; Kim, J.-H.; Kim, S.-H. Clinical analysis of risk factors related to recurrent chronic subdural hematoma. J. Korean Neurosurg. Soc. 2008, 43, 11–15. [Google Scholar] [CrossRef]
- Abdelsadg, M.; Kanodia, A.K.; Abbas, A.; Sheikh, A. Chronic subdural haematoma: Systematic review highlighting risk factors for recurrent bleeds. Neuro. Open J. 2017, 4, 16–24. [Google Scholar] [CrossRef]
- Weigel, R.; Hohenstein, A.; Schlickum, L.; Weiss, C.; Schilling, L. Angiotensin converting enzyme inhibition for arterial hypertension reduces the risk of recurrence in patients with chronic subdural hematoma possibly by an antiangiogenic mechanism. Neurosurgery 2007, 61, 788–792. [Google Scholar] [CrossRef]
| Study | Representativeness of Sample | Size Sample | Source of Information | Demonstration That Outcome Was Not Present at Study Start | Confusion Variable Control | Assessment of Outcome | Enough Follow-Up Period |
|---|---|---|---|---|---|---|---|
| Oishi et al. 2001 | ★ | ★ | ★ | ★ | |||
| Yamamoto et al. 2003 | ★ | ★ | ★ | ★ | ★ | ||
| Torihashi et al. 2008 | ★ | ★ | ★ | ★ | ★ | ★ | |
| Chon et al. 2012 | ★ | ★ | ★ | ★ | ★ | ||
| Ohba et al. 2012 | ★ | ★ | ★ | ★ | ★ | ★ | |
| Leroy et al. 2015 | ★ | ★ | ★ | ★ | ★ | ||
| Kim et al. 2015 | ★ | ★ | ★ | ★ | ★ | ★ | ★ |
| Han et al. 2016 | ★ | ★ | ★ | ★ | ★ | ★ | ★ |
| Hammer et al. 2017 | ★ | ★ | ★ | ★ | |||
| Motoie et al. 2018 | ★ | ★ | ★ | ★ | ★ | ★ | ★ |
| You et al. 2018 | ★ | ★ | ★ | ★ | ★ | ★ | |
| Andersen et al. 2019 | ★ | ★ | ★ | ★ | ★ | ★ | |
| Dos Santos et al. 2019 | ★ | ★ | ★ | ★ | ★ | ||
| Shen et al. 2019 | ★ | ★ | ★ | ★ | ★ | ★ |
| Study | Year | Type | Subgroup | Clinical Risk Factors | Risk Factor in CT | Newcastle–Ottawa Scale Scoring |
|---|---|---|---|---|---|---|
| Oishi et al. | 2001 | Observational retrospective cohort |
|
|
| 4/7 |
| Yamamoto et al. | 2003 | Observational retrospective cohort |
|
|
| 4/7 |
| Torihashi et al. | 2008 | Observational retrospective cohort |
|
| Bilateral hematoma | 6/7 |
| Chon et al. | 2012 | Observational retrospective cohort |
|
|
| 5/7 |
| Ohba et al. | 2012 | Observational retrospective cohort |
|
|
| 6/7 |
| Leroy et al. | 2015 | Observational retrospective cohort |
|
|
| 5/7 |
| Kim et al. | 2015 | Observational retrospective cohort |
|
|
| 7/7 |
| Han et al. | 2016 | Observational retrospective cohort |
|
|
| 7/7 |
| Hammer et al. | 2017 | Observational retrospective cohort |
|
|
| 4/7 |
| Motoie et al. | 2018 | Observational retrospective cohort |
|
| Bilateral hematoma | 7/7 |
| You et al. | 2018 | Observational retrospective cohort |
|
|
| 6/7 |
| Andersen et al. | 2019 | Observational retrospective cohort |
|
|
| 6/7 |
| Dos Santos et al. | 2019 | Observational retrospective cohort |
|
| Bilateral hematoma | 5/7 |
| Shen et al. | 2019 | Observational retrospective cohort |
|
|
| 6/7 |
| S. No. | Risk Factor | Odd’s Ratio (95% Confidence Interval) | p-Value |
|---|---|---|---|
| 1. | Age > 75 years | 1.05 (1.03–1.07) | p < 0.00001 |
| 2. | Alcohol consumption | 1.10 (0.81–1.48) | p = 0.55 |
| 3. | Anticoagulation or anti-aggregation therapy | 1.28 (1.02–1.62) | p = 0.03 |
| 4. | Chronic kidney disease | 1.21 (0.63–2.35) | p = 0.56 |
| 5. | Diabetes mellitus | 1.53 (1.24–1.90) | p < 0.0001 |
| 6. | Gender male | 1.20 (0.96–1.49) | p = 0.10 |
| 7. | Trauma | 0.94 (0.71–1.24) | p = 0.65 |
| 8. | Heart Diseases | 1.23 (0.83–1.84) | p = 0.31 |
| 9. | Hypertension | 1.12 (0.95–1.32) | p = 0.18 |
| 10. | Liver disease | 1.83 (1.23–2.73) | p = 0.003 |
| 11. | Malignancy | 1.10 (0.70–1.72) | p = 0.67 |
| 12. | Obesity | 1.81 (1.09–3.01) | p = 0.02 |
| 13. | Seizure | 2.78 (1.57–4.92) | p = 0.0004 |
| 14. | Smoking | 1.03 (0.74–1.42) | p = 0.87 |
| 15. | Stroke | 1.25 (0.88–1.77) | p = 0.21 |
| 16. | Bilateral hematoma | 1.31 (1.05–1.63) | p = 0.02 |
| 17. | Severe brain atrophy | 2.61 (1.88–3.64) | p < 0.00001 |
| 18. | Internal architecture of the hematoma: homogeneous | 1.42 (1.07–1.88) | p = 0.01 |
| 19. | Hematoma hyperdensity | 1.33 (0.81–2.17) | p = 0.26 |
| 20. | Hematoma hypodensity | 0.86 (0.56–1.34) | p = 0.51 |
| 21. | Hematoma isodensity | 0.78 (0.57–1.07) | p = 0.12 |
| 22. | Internal architecture of the hematoma: laminar | 1.57 (1.03–2.39) | p = 0.04 |
| 23. | Midline shift > 10 mm | 1.75 (1.49–2.07) | p < 0.00001 |
| 24. | Postoperative pneumocephalus | 2.36 (1.41–3.96) | p = 0.001 |
| 25. | Internal architecture of the hematoma: separated | 2.33 (1.69–3.19) | p < 0.00001 |
| 26. | Internal architecture of the hematoma: trabeculate | 0.89 (0.55–1.42) | p = 0.61 |
| 27. | Width of hematoma > 20 mm | 1.22 (1.05–1.41) | p = 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, R.; Deora, H.; Florez-Perdomo, W.A.; Moscote-Salazar, L.R.; Garcia-Ballestas, E.; Rahman, M.M.; Shrivastava, A.; Raj, S.; Chavda, V.; Montemurro, N.; et al. Clinical and Radiological Characteristics for Recurrence of Chronic Subdural Hematoma: A Systematic Review and Meta-Analysis. Neurol. Int. 2022, 14, 683-695. https://doi.org/10.3390/neurolint14030057
Mishra R, Deora H, Florez-Perdomo WA, Moscote-Salazar LR, Garcia-Ballestas E, Rahman MM, Shrivastava A, Raj S, Chavda V, Montemurro N, et al. Clinical and Radiological Characteristics for Recurrence of Chronic Subdural Hematoma: A Systematic Review and Meta-Analysis. Neurology International. 2022; 14(3):683-695. https://doi.org/10.3390/neurolint14030057
Chicago/Turabian StyleMishra, Rakesh, Harsh Deora, William Andres Florez-Perdomo, Luis Rafael Moscote-Salazar, Ezequiel Garcia-Ballestas, Md Moshiur Rahman, Adesh Shrivastava, Sumit Raj, Vishal Chavda, Nicola Montemurro, and et al. 2022. "Clinical and Radiological Characteristics for Recurrence of Chronic Subdural Hematoma: A Systematic Review and Meta-Analysis" Neurology International 14, no. 3: 683-695. https://doi.org/10.3390/neurolint14030057
APA StyleMishra, R., Deora, H., Florez-Perdomo, W. A., Moscote-Salazar, L. R., Garcia-Ballestas, E., Rahman, M. M., Shrivastava, A., Raj, S., Chavda, V., Montemurro, N., & Agrawal, A. (2022). Clinical and Radiological Characteristics for Recurrence of Chronic Subdural Hematoma: A Systematic Review and Meta-Analysis. Neurology International, 14(3), 683-695. https://doi.org/10.3390/neurolint14030057








