EEG Correlation Coefficient Change with Motor Task Activation Can Be a Predictor of Functional Recovery after Hemiparetic Stroke
Abstract
1. Introduction
2. Subjects and Methods
2.1. Study Subjects
2.2. Evaluation of Motor Function
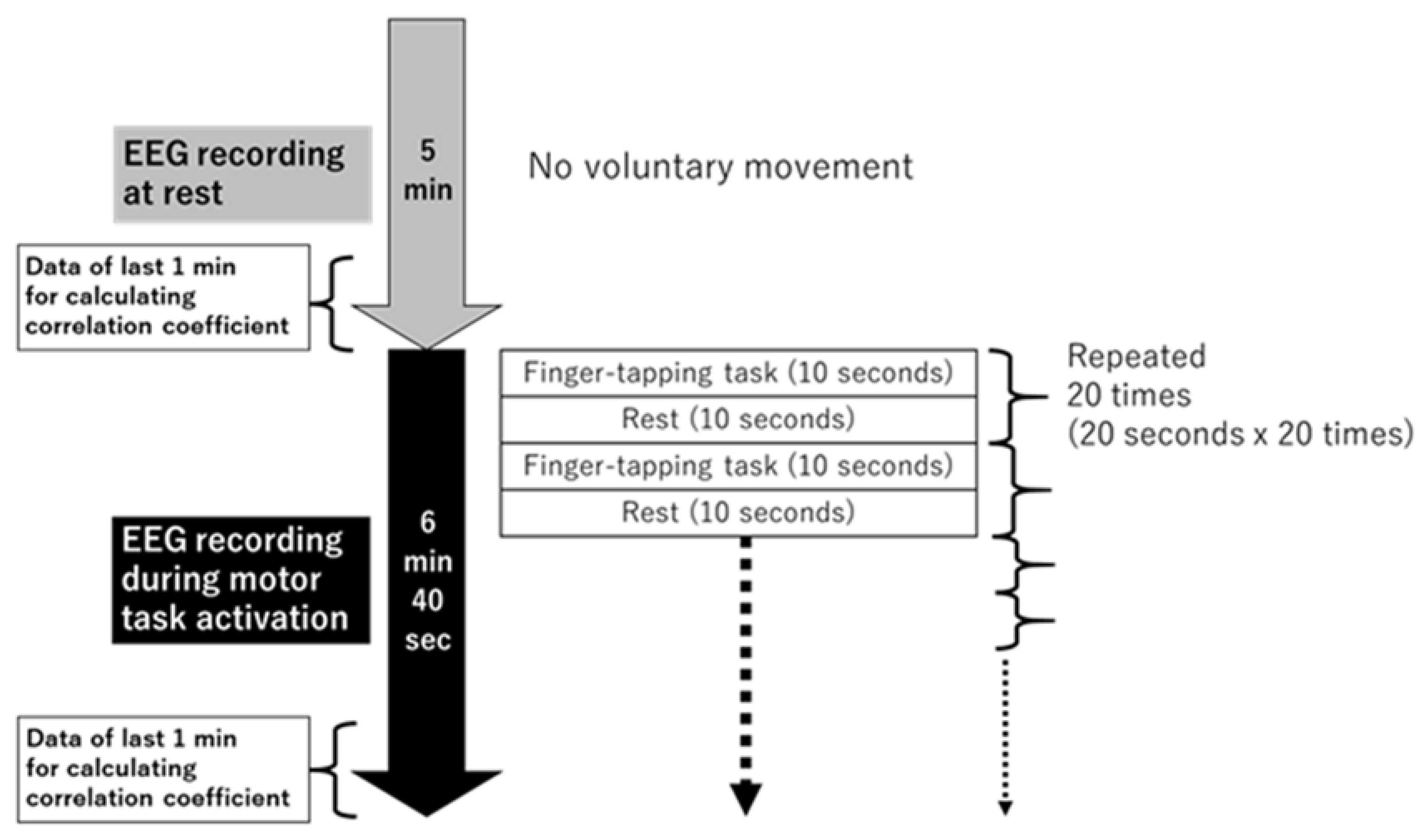
2.3. EEG Recording at Rest and during Motor Task, and Data Preprocessing
2.4. Correlation Coefficient Analysis Based on EEG Data
2.5. Statistical Analysis
3. Results
3.1. Change in Correlation Coefficient with Motor Task Activation in Each Cortico-Cortical Area
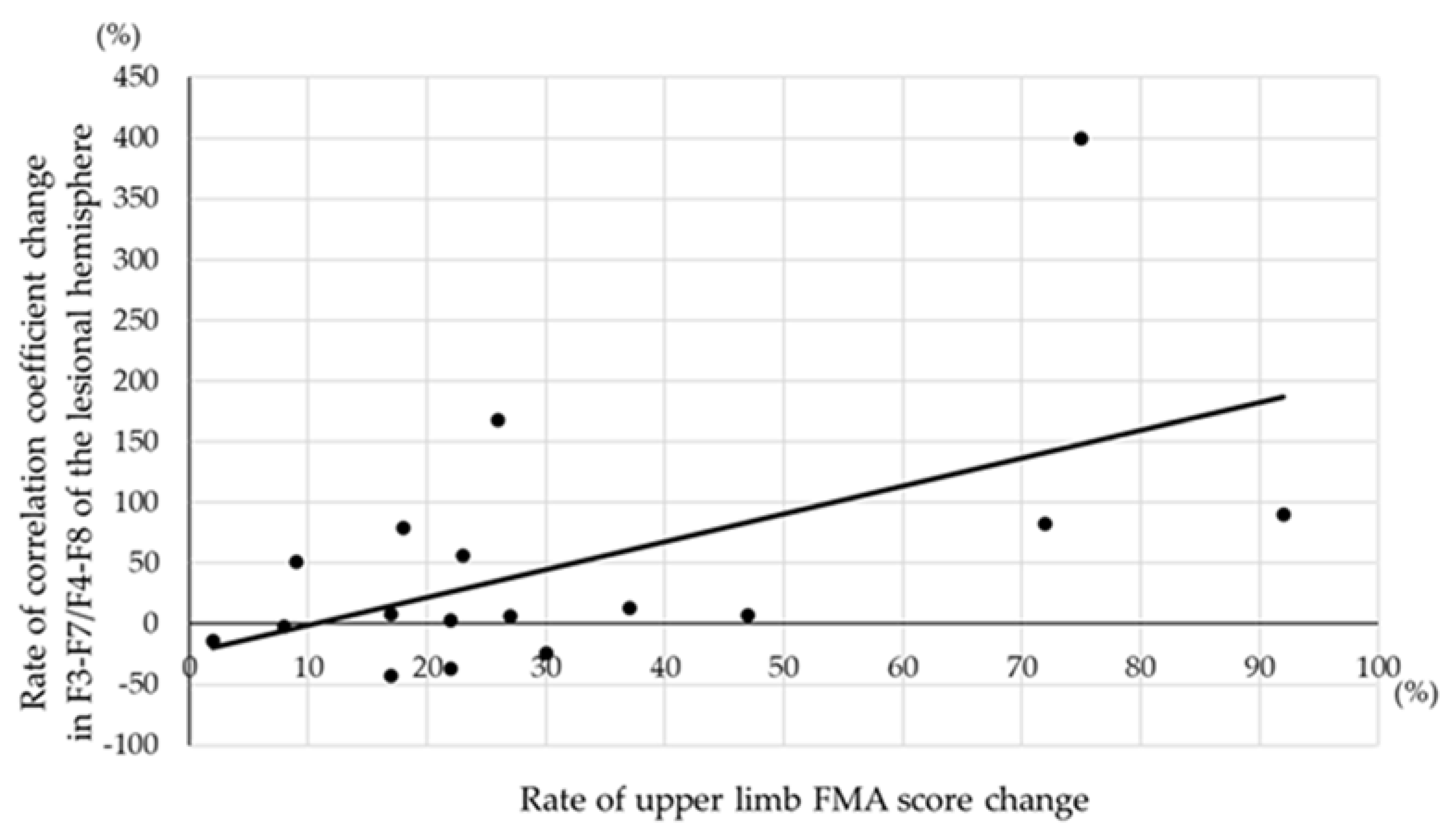
3.2. Correlation between Change in Correlation Coefficient and FMA Score Change
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hata, J.; Ninomiya, T.; Hirakawa, Y.; Nagata, M.; Mukai, N.; Gotoh, S.; Fukuhara, M.; Ikeda, F.; Shikata, K.; Yoshida, D.; et al. Secular trends in cardiovascular disease and its risk factors in Japanese: Half-century data from the Hisayama Study (1961–2009). Circulation 2013, 128, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Waddell, K.J.; Birkenmeier, R.; Bland, M.D.; Lang, C.E. An exploratory analysis of the self-reported goals of individuals with chronic upper-extremity paresis following stroke. Disabil. Rehabil. 2016, 38, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Choi-Kwon, S.; Choi, J.M.; Kwon, S.U.; Kang, D.W.; Kim, J.S. Factors that affect the quality of life at 3 years post-stroke. J. Clin. Neurol. 2006, 2, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Etoom, M.; Hawamdeh, M.; Hawamdeh, Z.; Alwardat, M.; Giordani, L.; Bacciu, S.; Scarpini, C.; Foti, C. Constraint-induced movement therapy as a rehabilitation intervention for upper extremity in stroke patients: Systematic review and meta-analysis. Int. J. Rehabil. Res. 2016, 39, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Dionisio, A.; Duarte, I.C.; Patricio, M.; Castelo-Branco, M. The use of repetitive transcranial magnetic stimulation for stroke rehabilitation: A systematic review. J. Stroke Cerebrovasc. Dis. 2018, 27, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Bertani, R.; Melegari, C.; Cola, M.C.D.; Bramanti, A.; Bramanti, P.; Calabrò, R.S. Effects of robot-assisted upper limb rehabilitation in stroke patients: A systematic review with meta-analysis. Neurol. Sci. 2017, 38, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.K.; Prasanna, S.S. Virtual reality and noninvasive brain stimulation in stroke: How effective is their combination for upper limb motor improvement? A meta-analysis. PM&R 2018, 10, 1261–1270. [Google Scholar] [CrossRef]
- Veerbeek, J.M.; Kwakkel, G.; Wegen, E.E.H.; Ket, J.C.F.; Heymans, M.W. Early prediction of outcome of activities of daily living after stroke: A systematic review. Stroke 2011, 42, 1482–1488. [Google Scholar] [CrossRef]
- Weng, S.C.; Hsu, C.Y.; Shen, C.C.; Huang, J.A.; Chen, P.L.; Lin, S.Y. Combined functional assessment for predicting clinical outcomes in stroke patients after post-acute care: A retrospective multi-center cohort in central Taiwan. Front. Aging Neurosci. 2022, 14, 834273. [Google Scholar] [CrossRef] [PubMed]
- Wendling, F.; Ansari-Asl, K.; Bartolomei, F.; Senhadji, L. From EEG signals to brain connectivity: A model-based evaluation of interdependence measures. J. Neurosci. Methods 2009, 183, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Jalili, M.; Knyazeva, M.G. Constructing brain functional networks from EEG: Partial and unpartial correlations. J. Integr. Neurosci. 2011, 10, 213–232. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Dufor, O.; Merlet, I.; Berrou, C.; Wending, F. EEG source connectivity analysis: From dense array recordings to brain networks. PLoS ONE 2014, 9, e105041. [Google Scholar] [CrossRef] [PubMed]
- Naghdi, S.; Ansari, N.N.; Mansouri, K.; Hasson, S. A neurophysiological and clinical study of Brunnstrom recovery stages in the upper limb following stroke. Brain Inj. 2010, 24, 1372–1378. [Google Scholar] [CrossRef] [PubMed]
- Miyai, I.; Sonoda, S.; Nagai, S.; Takayama, Y.; Inoue, Y.; Kakehi, A.; Kurihara, M.; Ishikawa, M. Results of new policies for inpatient rehabilitation coverage in Japan. Neurorehabil. Neural. Repair 2011, 25, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Platz, T.; Pinkowski, C.; Wijck, F.V.; Kim, I.; Bella, P.D.; Johnson, G. Reliability and validity of arm function assessment with standardized guidelines for the Fugl-Meyer Test, action research arm test and box and black test: A multicenter study. Clin. Rehabil. 2005, 19, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Malaia, R.; Bates, E.; Seitzman, B.; Coppess, K. Altered brain network dynamics in youths with autism spectrum disorder. Exp. Brain Res. 2016, 234, 3425–3431. [Google Scholar] [CrossRef] [PubMed]
- Anusha, A.S.; Ramakrishnan, A.G. Bain functional connectivity as biomarker for propofol-induced alterations of consciousness. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Virtual, 1–5 November 2021; pp. 1928–1931. [Google Scholar] [CrossRef]
- Hassan, M.; Merlet, I.; Mheich, A.; Kabbara, A.; Biraben, A.; Nica, A.; Wendling, F. Identification of interictal epileptic networks from dense-EEG. Brain Topogr. 2017, 30, 60–76. [Google Scholar] [CrossRef]
- Zheng, F.; Sato, S.; Mamada, K.; Ozaki, N.; Kubo, J.; Kakuda, W. Changes of cortico-cortical neural connections with motor functional recovery after stroke. J. Stroke Cerebrovasc. Dis. 2022, 31, 106689. [Google Scholar] [CrossRef] [PubMed]
- Marshall, R.S.; Perera, G.M.; Lazar, R.M.; Krakauer, J.W.; Constantine, R.C.; DeLaPaz, R.L. Evolution of cortical activation during recovery from corticospinal tract infarction. Stroke 2000, 31, 656–661. [Google Scholar] [CrossRef]
- Nelles, G.; Spiekramann, G.; Jueptner, M.; Leonhardt, G.; Müller, S.; Gerhard, H.; Diener, H.C. Evolution of functional reorganization in hemiplegic stroke: A serial positron emission tomographic activation study. Ann. Neurol. 1999, 46, 901–909. [Google Scholar] [CrossRef]
- Loubinoux, I.; Carel, C.; Pariente, J.; Dechaumont, S.; Aibucher, J.; Marque, P.; Manelfe, C.; Chollet, F. Correlation between cerebral reorganization and motor recovery after subcortical infarcts. Neuroimage 2003, 20, 2166–2180. [Google Scholar] [CrossRef] [PubMed]
- Favre, I.; Zeffiro, T.A.; Detante, O.; Krainik, A.; Hommel, M.; Jaillard, A. Upper limb recovery after stroke is associated with ipsilesional primary motor cortical activity: A meta-analysis. Stroke 2014, 45, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Park, E.; Lee, A.; Im, C.H.; Kim, Y.H. Changes in network connectivity during motor imagery and execution. PLoS ONE 2018, 13, e0190715. [Google Scholar] [CrossRef]
- Sharma, N.; Baron, J.; Rowe, J.B. Motor-imagery after stroke: Relating outcome to motor network connectivity. Ann. Neurol. 2009, 66, 604–616. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Baron, J.C. Motor recovery after subcortical stroke depends on modulation of extant motor networks. Front. Neurol. 2015, 6, 230. [Google Scholar] [CrossRef]
| Age at admission to our ward, years old | 73.4 ± 9.6 | |
| Gender | Female: 5 Male: 11 | |
| Diagnosis | Cerebral infarction: 13 Intracerebral hemorrhage: 3 | |
| Period between stroke onset and admission, days | 22.0 ± 9.4 | |
| Side of legion (side of hemiparesis) | Left cerebral hemisphere (right hemiparesis): 8 Right cerebral hemisphere (left hemiparesis): 8 | |
| Brunnstrom Recovery Stage for hand–fingers at admission | Stage II: 3 Stage III: 0 Stage IV: 3 Stage V: 10 | |
| Period between admission and EEG, days | 3.9 ± 2.0 | |
| Length of hospitalization, days | 68.1 ± 26.5 | |
| FMA score at admission, points | Upper limb | 41.2 ± 18.2 |
| Lower limb | 21.8 ± 7.5 | |
| FMA score 4 weeks after admission, points | Upper limb | 51.6 ± 19.5 |
| Lower limb | 27.3 ± 7.3 | |
| Measured Areas | Correlation Coefficient at Rest, % | Correlation Coefficient during Motor Task Activation, % | p-Value | |
|---|---|---|---|---|
| Within lesional hemisphere | C3-F3 or C4-F4 | 18.9 ± 15.0 | 31.1 ± 22.6 | <0.05 |
| C3-F7 or C4-F8 | 17.1 ± 15.0 | 27.6 ± 20.4 | <0.05 | |
| F3-F7 or F4-F8 | 45.2 ± 20.5 | 60.6 ± 17.6 | <0.05 | |
| C3-T3 or C4-T4 | 27.4 ± 16.7 | 35.6 ± 17.5 | 0.061 | |
| F3-T3 or F4-T4 | 14.8 ± 14.5 | 27.9 ± 22.7 | 0.075 | |
| F7-T3 or F8-T4 | 42.9 ± 13.3 | 41.1 ± 17.4 | 0.767 | |
| Within non-lesional hemisphere | C3-F3 or C4-F4 | 25.3 ± 19.8 | 31.4 ± 20.3 | 0.191 |
| C3-F7 or C4-F8 | 20.1 ± 14.3 | 25.5 ± 17.9 | 0.364 | |
| F3-F7 or F4-F8 | 48.4 ± 14.2 | 50.4 ± 19.3 | 0.730 | |
| C3-T3 or C4-T4 | 27.3 ± 17.5 | 32.5 ± 20.6 | 0.393 | |
| F3-T3 or F4-T4 | 17.2 ± 18.6 | 19.6 ± 16.9 | 0.731 | |
| F7-T3 or F8-T4 | 37.9 ± 12.2 | 38.8 ± 21.7 | 0.893 |
| Measured Areas | All Patients (n = 16) | Moderate–Severe Hemiparetic Patients (n = 6) | Mild Hemiparetic Patients (n = 10) | p-Value between Moderate–Severe and Mild Patients | |
|---|---|---|---|---|---|
| Within lesional hemisphere | C3-F3 or C4-F4 | 21.4 (−16.0–45.5) | −17.1 (−55.9–149.2) | 10.8 (−24.7–29.4) | 0.713 |
| C3-F7 or C4-F8 | 13.5 (−23.5–55.4) | 0.0 (−83.9–205.6) | 27.8 (−8.3–90.8) | 0.181 | |
| F3-F7 or F4-F8 | 7.3 (−11.1–81.6) | 82.4 (31.4–245.1) | 0.8 (−16.7–6.2) | 0.181 | |
| C3-T3 or C4-T4 | 4.0 (−13.6–38.7) | 4.0 (−40.0–255.0) | 27.9 (7.3–49.2) | 0.792 | |
| F3-T3 or F4-T4 | 42.9 (4.8–100) | 27.3 (−31.0–116.7) | 52.7 (−2.8–103.6) | 0.864 | |
| F7-T3 or F8-T4 | −4.4 (−27.1–22.7) | −20.8 (−60.2–16.6) | 2.8 (−10.7–30.4) | 0.428 | |
| Within non-lesional hemisphere | C3-F3 or C4-F4 | 1.3 (−27.7–18.0) | −25.0 (−34.1–114.1) | 1.3 (−26.5–10.6) | 0.875 |
| C3-F7 or C4-F8 | 0.0 (−44.0–47.3) | 0.0 (−60.9–0.0) | 40.1 (−14.4–101.3) | 0.108 | |
| F3-F7 or F4-F8 | 5.1 (−33.2–37.8) | 37.8 (−16.1–105.9) | 17.6 (−26.7–47.2) | 0.263 | |
| C3-T3 or C4-T4 | 0.8 (−32.9–25.7) | −12.8 (−45.7–26.7) | 10.5 (−26.0–44.3) | 0.492 | |
| F3-T3 or F4-T4 | −24.2 (−68.1–58.9) | 4.8 (−48.3–68.6) | −30.9 (−75.0–76.8) | 0.298 | |
| F7-T3 or F8-T4 | −18.9 (−43.4–11.1) | −21.8 (−67.4–0.8) | −25.1 (−47.4–−1.0) | 0.792 |
| Upper-Limb FMA Score | Lower-Limb FMA Score | ||||
|---|---|---|---|---|---|
| Coefficient | p-Value | Coefficient | p-Value | ||
| Within lesional hemisphere | C3-F3 or C4-F4 | 0.209 | 0.483 | −0.111 | 0.682 |
| C3-F7 or C4-F8 | −0.004 | 0.987 | −0.332 | 0.208 | |
| F3-F7 or F4-F8 | 0.570 | <0.05 | 0.036 | 0.894 | |
| C3-T3 or C4-T4 | 0.381 | 0.145 | 0.039 | 0.887 | |
| F3-T3 or F4-T4 | −0.068 | 0.804 | −0.251 | 0.349 | |
| F7-T3 or F8-T4 | −0.231 | 0.389 | −0.421 | 0.104 | |
| Within non-lesional hemisphere | C3-F3 or C4-F4 | 0.212 | 0.430 | 0.202 | 0.452 |
| C3-F7 or C4-F8 | −0.238 | 0.374 | −0.141 | 0.602 | |
| F3-F7 or F4-F8 | 0.343 | 0.193 | 0.025 | 0.927 | |
| C3-T3 or C4-T4 | −0.066 | 0.809 | 0.180 | 0.506 | |
| F3-T3 or F4-T4 | −0.098 | 0.718 | −0.067 | 0.804 | |
| F7-T3 or F8-T4 | −0.134 | 0.620 | −0.047 | 0.862 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, F.; Sato, S.; Mamada, K.; Ozaki, N.; Kubo, J.; Kakuda, W. EEG Correlation Coefficient Change with Motor Task Activation Can Be a Predictor of Functional Recovery after Hemiparetic Stroke. Neurol. Int. 2022, 14, 738-747. https://doi.org/10.3390/neurolint14030062
Zheng F, Sato S, Mamada K, Ozaki N, Kubo J, Kakuda W. EEG Correlation Coefficient Change with Motor Task Activation Can Be a Predictor of Functional Recovery after Hemiparetic Stroke. Neurology International. 2022; 14(3):738-747. https://doi.org/10.3390/neurolint14030062
Chicago/Turabian StyleZheng, Fei, Shin Sato, Kenji Mamada, Naoto Ozaki, Jin Kubo, and Wataru Kakuda. 2022. "EEG Correlation Coefficient Change with Motor Task Activation Can Be a Predictor of Functional Recovery after Hemiparetic Stroke" Neurology International 14, no. 3: 738-747. https://doi.org/10.3390/neurolint14030062
APA StyleZheng, F., Sato, S., Mamada, K., Ozaki, N., Kubo, J., & Kakuda, W. (2022). EEG Correlation Coefficient Change with Motor Task Activation Can Be a Predictor of Functional Recovery after Hemiparetic Stroke. Neurology International, 14(3), 738-747. https://doi.org/10.3390/neurolint14030062






