Chronic Administration of Melatonin: Physiological and Clinical Considerations
Abstract
1. Introduction
2. Literature Search
3. Endogenous Melatonin Physiology
4. Pharmacokinetics of Melatonin
5. Uses of Exogenous Melatonin
6. Available Formulations of Melatonin
7. Effectiveness of Exogenous Melatonin
8. Short-Term Side Effects of Taking Melatonin
9. Melatonin: Usage and Considerations
9.1. Anti-Aging and Oxidation Protection
9.2. Melatonin as a Potential Marker in Alzheimer’s Disease
9.3. Therapeutic for Mild Cognitive Impairment
9.4. Delay of Puberty
9.5. Effect on Seizures
9.6. Increase in Bone Fractures
9.7. Impairment of Balance or Cognition
9.8. Worsening of Restless Leg Syndrome
9.9. Worsening of Asthma
9.10. Worsening of Depression and Bipolar Disorder
10. Summary
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kimland, E.E.; Bardage, C.; Collin, J.; Järleborg, A.; Ljung, R.; Iliadou, A.N. Pediatric use of prescribed melatonin in Sweden 2006–2017: A register based study. Eur. Child Adolesc. Psychiatry 2021, 30, 1339–1350. [Google Scholar] [CrossRef] [PubMed]
- Trends in Use of Melatonin Supplements among US Adults, 1999–2018|Complementary and Alternative Medicine|JAMA|JAMA Network. Available online: https://jamanetwork.com/journals/jama/fullarticle/2788539 (accessed on 9 March 2023).
- Tordjman, S.; Chokron, S.; Delorme, R.; Charrier, A.; Bellissant, E.; Jaafari, N.; Fougerou, C. Melatonin: Pharmacology, Functions and Therapeutic Benefits. Curr. Neuropharmacol. 2017, 15, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Neurobiology, Pathophysiology, and Treatment of Melatonin Deficiency and Dysfunction. Sci. World J. 2012, 2012, 640389. [Google Scholar] [CrossRef]
- Gandhi, A.V.; Mosser, E.; Oikonomou, G.; Prober, D.A. Melatonin is required for the circadian regulation of sleep. Neuron 2015, 85, 1193–1199. [Google Scholar] [CrossRef]
- Carrillo-Vico, A.; Lardone, P.J.; Álvarez-Sánchez, N.; Rodríguez-Rodríguez, A.; Guerrero, J.M. Melatonin: Buffering the Immune System. Int. J. Mol. Sci. 2013, 14, 8638–8683. [Google Scholar] [CrossRef]
- 6 Hydroxymelatonin—An Overview|ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/neuroscience/6-hydroxymelatonin (accessed on 9 March 2023).
- Metabolism of Melatonin by Human Cytochromes P450|Drug Metabolism & Disposition. Available online: https://dmd.aspetjournals.org/content/33/4/489 (accessed on 9 March 2023).
- Melatonin: What You Need to Know. NCCIH. Available online: https://www.nccih.nih.gov/health/melatonin-what-you-need-to-know (accessed on 9 March 2023).
- Melatonin Natural Health Products and Supplements: Presence of Serotonin and Significant Variability of Melatonin Content—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/27855744/ (accessed on 9 March 2023).
- Grigg-Damberger, M.M.; Ianakieva, D. Poor Quality Control of Over-the-Counter Melatonin: What They Say Is often Not What You Get. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2017, 13, 163–165. [Google Scholar] [CrossRef] [PubMed]
- Jan, J.E.; Wasdell, M.B. Melatonin—An orphan drug. Dev. Med. Child Neurol. 2008, 50, 558. [Google Scholar] [CrossRef]
- Buscemi, N.; VanderMeer, B.; Hooton, N.; Pandya, R.; Tjosvold, L.; Hartling, L.; Baker, G.B.; Klassen, T.; Vohra, S. The efficacy and safety of exogenous melatonin for primary sleep disorders. A meta-analysis. J. Gen. Intern. Med. 2005, 20, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, D.A.; Frye, R.E. Melatonin in autism spectrum disorders. Curr. Clin. Pharmacol. 2014, 9, 326–334. [Google Scholar] [CrossRef]
- Melatonin in Autism Spectrum Disorders: A Systematic Review and Meta-Analysis—ROSSIGNOL—2011—Developmental Medicine & Child Neurology—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1111/j.1469-8749.2011.03980.x (accessed on 9 March 2023).
- Zambrelli, E.; Lividini, A.; Spadavecchia, S.; Turner, K.; Canevini, M.P. Effects of Supplementation with Antioxidant Agents on Sleep in Autism Spectrum Disorder: A Review. Front. Psychiatry 2021, 12, 689277. Available online: https://www.frontiersin.org/articles/10.3389/fpsyt.2021.689277 (accessed on 9 March 2023). [CrossRef]
- Attia, A.M.; Montaser, B.A.; Abdallah, N.K. Role of Melatonin in Constitutional Delayed Puberty in Boys. Menoufia Med. J. 2020, 33, 283. Available online: https://www.mmj.eg.net/article.asp?issn=1110-2098;year=2020;volume=33;issue=1;spage=283;epage=287;aulast=Attia (accessed on 9 March 2023).
- Treatment Options for Sundowning in Patients with Dementia|Mental Health Clinician. Available online: https://meridian.allenpress.com/mhc/article/4/4/189/37067/Treatment-options-for-sundowning-in-patients-with (accessed on 9 March 2023).
- The Use of Melatonin in Alzheimer’s Disease—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/12019347/ (accessed on 9 March 2023).
- Lee, J.G.; Woo, Y.S.; Park, S.W.; Seog, D.H.; Seo, M.K.; Bahk, W.M. The Neuroprotective Effects of Melatonin: Possible Role in the Pathophysiology of Neuropsychiatric Disease. Brain Sci. 2019, 9, 285. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, D.P. Melatonin: Clinical Perspectives in Neurodegeneration. Front. Endocrinol. 2019, 10, 480. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, D.P.; Vigo, D.E.; Olivar, N.; Vidal, M.F.; Furio, A.M.; Brusco, L.I. Therapeutic application of melatonin in mild cognitive impairment. Am. J. Neurodegener. Dis. 2012, 1, 280–291. [Google Scholar]
- Medeiros, C.A.M.; Carvalhedo de Bruin, P.F.; Lopes, L.A.; Magalhães, M.C.; de Lourdes Seabra, M.; de Bruin, V.M.S. Effect of exogenous melatonin on sleep and motor dysfunction in Parkinson’s disease. A randomized, double blind, placebo-controlled study. J. Neurol. 2007, 254, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Neural Correlates of Sleep Recovery Following Melatonin Treatment for Pediatric Concussion: A Randomized Control Trial|medRxiv. Available online: https://www.medrxiv.org/content/10.1101/2020.08.02.20166918v1.full (accessed on 9 March 2023).
- Zwart, T.C.; Smits, M.G.; Egberts, T.C.; Rademaker, C.M.A.; van Geijlswijk, I.M. Long-Term Melatonin Therapy for Adolescents and Young Adults with Chronic Sleep Onset Insomnia and Late Melatonin Onset: Evaluation of Sleep Quality, Chronotype, and Lifestyle Factors Compared to Age-Related Randomly Selected Population Cohorts. Healthcare 2018, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Hoebert, M.; Van Der Heijden, K.B.; van Geijlswijk, I.M.; Smits, M.G. Long-term follow-up of melatonin treatment in children with ADHD and chronic sleep onset insomnia. J. Pineal Res. 2009, 47, 1–7. [Google Scholar] [CrossRef]
- Carr, R.; Wasdell, M.B.; Hamilton, D.; Weiss, M.D.; Freeman, R.D.; Tai, J.; Rietveld, W.J.; Jan, J.E. Long-term effectiveness outcome of melatonin therapy in children with treatment-resistant circadian rhythm sleep disorders. J. Pineal Res. 2007, 43, 351–359. [Google Scholar] [CrossRef]
- Wasdell, M.B.; Jan, J.E.; Bomben, M.M.; Freeman, R.D.; Rietveld, W.J.; Tai, J.; Hamilton, D.; Weiss, M.D. A randomized, placebo-controlled trial of controlled release melatonin treatment of delayed sleep phase syndrome and impaired sleep maintenance in children with neurodevelopmental disabilities. J. Pineal Res. 2008, 44, 57–64. [Google Scholar] [CrossRef]
- Long-Term Efficacy and Safety of Pediatric Prolonged-Release Melatonin for Insomnia in Children with Autism Spectrum Disorder. J. Child Adolesc. Psychopharmacol. 2018, 28, 699–710. Available online: https://pubmed.ncbi.nlm.nih.gov/30132686/ (accessed on 9 March 2023). [CrossRef]
- Gringras, P.; Nir, T.; Breddy, J.; Frydman-Marom, A.; Findling, R.L. Efficacy and Safety of Pediatric Prolonged-Release Melatonin for Insomnia in Children with Autism Spectrum Disorder. J. Am. Acad. Child Adolesc. Psychiatry 2017, 56, 948–957.e4. [Google Scholar] [CrossRef] [PubMed]
- Van Geijlswijk, I.M.; Mol, R.H.; Egberts, T.C.G.; Smits, M.G. Evaluation of sleep, puberty and mental health in children with long-term melatonin treatment for chronic idiopathic childhood sleep onset insomnia. Psychopharmacology 2011, 216, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Seabra, M.L.; Bignotto, M.; Pinto, L.R.; Tufik, S. Randomized, double-blind clinical trial, controlled with placebo, of the toxicology of chronic melatonin treatment. J. Pineal Res. 2000, 29, 193–200. [Google Scholar] [CrossRef]
- Andersen, L.P.H.; Gögenur, I.; Rosenberg, J.; Reiter, R.J. The Safety of Melatonin in Humans. Clin. Drug Investig. 2016, 36, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Van der Heijden, K.B.; Smits, M.G.; VAN Someren, E.J.; Ridderinkhof, K.R.; Gunning, W.B. Effect of melatonin on sleep, behavior, and cognition in ADHD and chronic sleep-onset insomnia. J. Am. Acad. Child Adolesc. Psychiatry 2007, 46, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, P.H.; Spillmann, M.; Bärtschi, C.; Ehlert, U.; Von Känel, R. Oral melatonin reduces blood coagulation activity: A placebo-controlled study in healthy young men. J. Pineal Res. 2008, 44, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Ashy, N.; Shroff, K.V. Evaluation of the potential drug interaction of melatonin and warfarin: A case series. Life Sci. J. 2016, 13, 46–51. [Google Scholar] [CrossRef]
- Otmani, S.; Demazières, A.; Staner, C.; Jacob, N.; Nir, T.; Zisapel, N.; Staner, L. Effects of prolonged-release melatonin, zolpidem, and their combination on psychomotor functions, memory recall, and driving skills in healthy middle aged and elderly volunteers. Hum. Psychopharmacol. 2008, 23, 693–705. [Google Scholar] [CrossRef]
- Tarocco, A.; Caroccia, N.; Morciano, G.; Wieckowski, M.R.; Ancora, G.; Garani, G.; Pinton, P. Melatonin as a master regulator of cell death and inflammation: Molecular mechanisms and clinical implications for newborn care. Cell Death Dis. 2019, 10, 317. [Google Scholar] [CrossRef]
- Martín Giménez, V.M.; de las Heras, N.; Lahera, V.; Tresguerres, J.A.F.; Reiter, R.J.; Manucha, W. Melatonin as an Anti-Aging Therapy for Age-Related Cardiovascular and Neurodegenerative Diseases. Front. Aging Neurosci. 2022, 14, 491. Available online: https://www.frontiersin.org/articles/10.3389/fnagi.2022.888292 (accessed on 9 March 2023). [CrossRef]
- How Biomarkers Help Diagnose Dementia|National Institute on Aging. Available online: https://www.nia.nih.gov/health/how-biomarkers-help-diagnose-dementia (accessed on 9 March 2023).
- Melatonin Levels in the Alzheimer’s Disease Continuum: A Systematic Review. Available online: https://www.researchgate.net/publication/349541215_Melatonin_levels_in_the_Alzheimer%27s_disease_continuum_a_systematic_review (accessed on 9 March 2023).
- Mild Cognitive Impairment (MCI)|Symptoms & Treatments|alz.org. Available online: https://www.alz.org/alzheimers-dementia/what-is-dementia/related_conditions/mild-cognitive-impairment (accessed on 9 March 2023).
- Neurocognitive Effects of Melatonin Treatment in Healthy Adults and Individuals with Alzheimer’s Disease and Insomnia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/33957167/ (accessed on 9 March 2023).
- Boafo, A.; Greenham, S.; Alenezi, S.; Robillard, R.; Pajer, K.; Tavakoli, P.; De Koninck, J. Could long-term administration of melatonin to prepubertal children affect timing of puberty? A clinician’s perspective. Nat. Sci. Sleep 2019, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, S.H. Pro-convulsant effects of oral melatonin in neurologically disabled children. Lancet 1998, 351, 1254. [Google Scholar] [CrossRef] [PubMed]
- Melatonin Effect on Seizures in Children with Severe Neurologic Deficit Disorders—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/11580772/ (accessed on 9 March 2023).
- Maghbooli, M.; Alyan NajafAbadi, S.; MalekMahmoudi, G.; Molseghi, M.H. Effect of add-on melatonin on seizure outcomes and quality of sleep in epilepsy with idiopathic generalized tonic-clonic seizures alone in adult patients: Cross-sectional, randomized, double-blind, placebo-controlled clinical trial. Brain Behav. 2023, 13, e2860. [Google Scholar] [CrossRef] [PubMed]
- Frisher, M.; Gibbons, N.; Bashford, J.; Chapman, S.; Weich, S. Melatonin, hypnotics and their association with fracture: A matched cohort study. Age Ageing 2016, 45, 801–806. [Google Scholar] [CrossRef]
- Lui, M.F.G.; Chow, H.K.D.; Wong, W.M.K.; Tsang, W.N.W. Melatonin Affects Postural Control in Community-Dwelling Older Adults While Dual Tasking: A Randomized Observation Study. J. Aging Phys. Act. 2019, 27, 102–107. [Google Scholar] [CrossRef]
- Gooneratne, N.S.; Edwards, A.Y.Z.; Zhou, C.; Cuellar, N.; Grandner, M.A.; Barrett, J.S. Melatonin pharmacokinetics following two different oral surge-sustained release doses in older adults. J. Pineal Res. 2012, 52, 437–445. [Google Scholar] [CrossRef]
- Whittom, S.; Dumont, M.; Petit, D.; Desautels, A.; Adam, B.; Lavigne, G.; Montplaisir, J. Effects of melatonin and bright light administration on motor and sensory symptoms of RLS. Sleep Med. 2010, 11, 351–355. [Google Scholar] [CrossRef]
- Aurora, R.N.; Kristo, D.A.; Bista, S.R.; Rowley, J.A.; Zak, R.; Casey, K.; Lamm, C.I.; Tracy, S.; Rosenberg, R.S. The treatment of restless legs syndrome and periodic limb movement disorder in adult—An update for 2012: Practice parameters with an evidence-based systematic review and meta-analyses: An American Academy of Sleep Medicine Clinical Practice Guideline. Sleep 2012, 35, 1039–1062. [Google Scholar] [CrossRef]
- Campos, F.L.; da Silva-Júnior, F.P.; de Bruin, V.M.S.; de Bruin, P.F.C. Melatonin improves sleep in asthma: A randomized, double-blind, placebo-controlled study. Am. J. Respir. Crit. Care Med. 2004, 170, 947–951. [Google Scholar] [CrossRef]
- Carman, J.S.; Post, R.M.; Buswell, R.; Goodwin, F.K. Negative effects of melatonin on depression. Am. J. Psychiatry 1976, 133, 1181–1186. [Google Scholar] [CrossRef]
- Hansen, M.; Danielsen, A.; Hageman, I.; Rosenberg, J.; Gögenur, I. The therapeutic or prophylactic effect of exogenous melatonin against depression and depressive symptoms: A systematic review and meta-analysis. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2014, 24, 1719–1728. [Google Scholar] [CrossRef] [PubMed]
| Author (Year) (Citation) | Type of Study | Results and Findings | Conclusions |
|---|---|---|---|
| Kimland (2021) [1] | Study of the prevalence and incidence of melatonin prescription and long-term use in children and adolescents aged 0–17 in Sweden during 2006–2017. | In 2017, nearly 2% of the pediatric population in Sweden was dispensed at least 1 prescription of melatonin, representing a 15-fold increase for girls and a 20-fold increase for boys; nearly 80% had concomitant prescriptions of psychotropic medications. | The increase in melatonin use in children, often concomitant with psychotropic medications, suggests the need for further studies of the safety of long-term melatonin use. |
| Li (2022) [2] | Research letter regarding the use of melatonin in the U.S. from 1999–2018. | Among U.S. adults, melatonin use increased from 0.4% to 2.1% between 1999 and 2018; use of melatonin in doses of greater than 5 mg per day also increased during the same period. | |
| Tordiman (2017) [3] | Review of the effects of melatonin pharmacologically, physiologically, and pathologically. | ||
| Hardeland (2012) [4] | Review of melatonin levels during aging and in various diseases. | Melatonin levels decrease during aging; reduced melatonin levels are also found in dementia, mood disorders, severe pain, cancer, and type 2 diabetes mellitus. | |
| Gandhi (2015) [5] | Zebrafish (a diurnal vertebrate) lacking melatonin were studied to determine the effect on sleep. | Endogenous melatonin plays a significant role in promoting the initiation and maintenance of sleep in zebrafish. | Melatonin is required for the circadian regulation of sleep in a diurnal vertebrate (zebrafish). |
| Carrillo-Vico (2013) [6] | Review of the effects of melatonin in the immune system. | Melatonin acts as an immune buffer. Acts as a stimulant under Basal or immunosuppressive conditions. Acts as an anti-inflammatory in exacerbated immune responses. | |
| Kohlmeier (2015) [7] | Description of the metabolism of melatonin. | ||
| Ma (2004) [8] | Study aimed to unveil the finer details of melatonin metabolism. | Melatonin was found to be metabolized by CYP1A2 principally, and for the first time found that CYP1B1 plays a part in metabolism. | Presence of CYP1B1 is extrahepatic meaning that melatonin is metabolized both in the large intestine and the brain. |
| NCCIH (2022) [9] | General overview on melatonin. Seemingly meant for patients. | ||
| Erland (2017) [10] | Review of frequency of melatonin use in U.S. children and adults and the variability of the actual melatonin content of available supplements. | The authors recommend that melatonin be regarded as a medicine in the U.S., available only by prescription and that the content and purity of melatonin supplements be more closely monitored. | |
| Grigg-Damberger (2017) [11] | 30 commercial melatonin supplements were quantified for melatonin and serotonin. | Melatonin content was found to range from −83% to +478% of the labeled content; lot-to-lot variability of the same product varied by as much as 465%; serotonin was found in 26% of supplements tested. | Label claim and actual melatonin content of available melatonin products vary widely, and contamination with serotonin is common. |
| Jan (2008) [12] | Letter to the Editor regarding the status of melatonin as a “supplement” in the U.S. | The writers recommend that melatonin should be regarded as a medication in the U.S., made available only by prescription, and regulated the same way as other pharmaceutical products. | |
| Buscemi (2005) [13] | Meta-analysis of the efficacy and safety of exogenous melatonin for primary sleep disorders. | Use of melatonin reduced average sleep onset latency in all patients by an average of 11.7 min and by 38.8 min in patients with delayed sleep phase syndrome. | Melatonin reduces sleep onset latency by a small amount in most patients. |
| Rossignol (2014) [14] | Review of the use of melatonin in individuals with autistic spectrum disorder (ASD). | Melatonin use in ASD can safely decrease sleep onset latency, increase total sleep duration, and improve daytime behaviors. | |
| Rossignol (2011) [15] | Review and meta-analysis of the use of melatonin in autistic spectrum disorders (ASD). | Melatonin administration in ASD is associated with improved sleep parameters, better daytime behavior, and minimal side effects. | |
| Zambreli (2021) [16] | Review of the effects of antioxidant agents, including melatonin, on sleep in autistic spectrum disorder (ASD). | Treatment with immediate-release melatonin at doses of 2–10 mg/day was effective in shortening sleep-onset latency (SOL), reducing the number of awakenings per night and bedtime resistance, and increasing total sleep time; 2–10 mg/day of prolonged-release melatonin had similar effects on sleep and decreased disruptive behaviors and parenting stress. | Immediate-release and controlled-release melatonin are safe and effective in improving sleep in children with ASD; controlled-release melatonin decreases disruptive behavior and parenting stress. |
| Attia (2020) [17] | Case-control study of 25 boys aged 14–18 years old with constitutional delay of puberty (CDP) and 25 age-matched individuals to study melatonin levels. | Melatonin is significantly higher in boys with CDP compared to age-matched boys, and melatonin levels are negatively correlated with testosterone and FSH. | Melatonin is associated with the delayed onset of puberty onset and may be a cause of the delay. More research is needed to assess its role in hormonal development and puberty onset. |
| Blaise (2014) [18] | Review of pharmacological and nonpharmacological management of sundowning in patients with AD. | The number of studies supporting treatment of sundowning in AD patients is sparce and in need of further study. | |
| Cardinali (2002) [19] | Study on 45 AD patients suffering from sleep disturbances, administered 6 mg melatonin for 4 months. | Improved sleep and decreased sundowning. | Melatonin seems to be effective in ameliorating sleep disturbances and sundowning. |
| Lee (2019) [20] | Review of the neuroprotective effects of melatonin in cerebral ischemia, Alzheimer’s disease (AD), and depression | Exogenous melatonin prolongs sleep time in patients with AD and dementia; anti-depressant effects of melatonin have not been established; the role of melatonin in the treatment of cerebral ischemia requires further study | |
| Cardinali (2019) [21] | Review of the use of melatonin in the management of Alzheimer’s disease (AD) and Parkinson’s disease (PD). | Melatonin improved the quality of sleep, decreased “sundowning”, and improved cognitive performance in AD patients, and it improved the quality of sleep in PD patients with no effect on motor symptoms. | Melatonin improves the quality of sleep in AD and PD patients and is useful for the treatment of insomnia in these patients; its effect on cognitive performance in AD requires further study. |
| Cardinali (2012) [22] | Retrospective study of 96 patient with mild cognitive impairment (MCI) treated with 3–9 mg of immediate-release melatonin at bedtime for up to 3 years. | MCI patients treated with melatonin scored better on multiple neuropsychological assessments and were treated with benzodiazepines less frequently. | Melatonin can be a useful medication as an adjunct to standard medications in the treatment of MCI. |
| Medeiros (2007) [23] | A randomized, double-blind, placebo-controlled study of 18 patients with Parkinson’s disease given 3 mg of melatonin 1 h before bedtime for 4 weeks. | Melatonin significantly improved subjective quality of sleep, but not objective quality as evaluated by polysomnography; melatonin did not improve motor dysfunction. | Melatonin improves subjective quality of sleep in PD patients. |
| Iyer (2020) [24] | A randomized, double-blind, placebo-controlled trial of 3 mg or 10 mg in 62 children with persistent post-concussion syndrome (PPCS) over 28 days. | Melatonin treatment did not result in overall recovery from PCCS; however, it did improve subjective and objective sleep parameters. | Melatonin improves sleep parameters in PPCS patients; the long-term benefits of melatonin treatment on overall PPCS recovery needs further study. |
| Zwart (2018) [25] | A follow-up study of 69 children aged 6–12 years with chronic sleep onset insomnia (CSOI); the overall average treatment duration was 7.1 years; sleep timing, sleep quality, adverse events, and reasons for cessation of therapy were assessed. | Long-term melatonin therapy appears to be safe after an average of 7.1 years; adverse effects were few and mild; there was no effect on long-term sleep quality; there were subjective concerns about delay in onset of puberty in 31.3% of those studied (compared to 17% in the general population). | Long-term melatonin therapy appears to be safe; there is subjective concern about possible delay in onset of puberty (this requires further study). |
| Hoebart (2009) [26] | Parents of 105 children with attention-deficit/hyperactivity disorder (ADHD) and chronic sleep onset insomnia (CSOI) who had been treated with melatonin over a mean 3.7-year period responded to a structured questionnaire with a 93% response rate. | No serious adverse effects or treatment-related co-morbidities were reported; long-term melatonin treatment was judged to be effective against sleep problems in 88% of cases; there were improvements in behavior and mood in 71% and 61%, respectively. | Melatonin is an effective and safe therapy for the long-term treatment of CSOI in children with ADHD. |
| Carr (2007) [27] | Placebo-controlled, double-blind, cross-over trial of controlled-release melatonin in 44 children with neurodevelopmental disabilities and treatment-resistant circadian rhythm sleep disorders for up to 3.8 years. | Subjective improvement in sleep, behavior and learning were reported by caregivers; adverse reaction to melatonin therapy and development of tolerance were not evident. | Controlled-release melatonin is safe and effective for children with neuro-developmental disabilities and sleep maintenance difficulties. |
| Wasdell (2008) [28] | Randomized, placebo-controlled crossover trial of 51 children aged 2–18 years with neurodevelopmental disabilities (NDD), including autistic spectrum disorder (ASD) and with delayed sleep phase syndrome (DSPS) and impaired sleep maintenance (ISM); children were treated with 5 mg of controlled-release melatonin, increased as needed for optimal beneficial effects. | Those receiving melatonin had improvement in total nighttime sleep and sleep latency of approximately 30 min in 47 of 61 children; there was also a reduction in family stress; there was no evidence of significant side effects. | Controlled-release melatonin is safe and effective in improving both sleep latency and sleep duration in children with NDD, including ASD and with DSPS and ISM. |
| Maras (2018) [29] | A double-blind, randomized placebo-controlled study of 19 children aged 2–17.5 years with autistic spectrum disorder (ASD) and neurogenetic disorders (NGD) given 2–10 mg of prolonged-release melatonin for 52 weeks. | In those given melatonin, sleep latency, total sleep time, and sleep quality were improved; 5.3% of subjects reported fatigue, and 3.2% reported mood swings. | Prolonged-release melatonin is safe and efficacious for long-term treatment (up to 52 weeks) of insomnia in children with ASD and NGDs. |
| Gringras (2017) [30] | Randomized, double-blind, placebo-controlled study of 125 children and adolescents with autistic spectrum disorder (ASD) and neurogenetic disorders (NGD) treated with 2–5 mg of prolonged-release melatonin at bedtime for 13 weeks. | In those patients given melatonin, sleep latency decreased by 39.6 min, and length of sleep increased by 57.5 min compared to placebo; somnolence, headache, and fatigue were reported as side effects; no tolerance was observed. | Prolonged-release melatonin decreases sleep latency and increases the length of sleep in children and adolescents with ASD and NGD with only mild side effects. |
| Van Geijlswijk (2011) [31] | A follow-up research study of children aged 6–12 years with chronic idiopathic childhood sleep onset insomnia (CSOI) treated for an average of 3.1 years with 0.3–10 mg of melatonin. | There was no significant difference in puberty development, social development, and mental health scores as compared with the general population; adverse effects occurred infrequently and led to cessation of melatonin use in 1.6%. | Melatonin treatment in children with CSOI can be sustained over an average of 3 years without significant adverse effects. |
| Seabra (2000) [32] | Randomized, double-blind, placebo-controlled study of the toxicology of chronic melatonin treatment in 40 male adults aged 22–55 years administered 10 mg of melatonin for 28 days. | Analysis of multiple parameters (polysomnographic recording, somnolence scale, sleep diary, and clinical laboratory examinations) showed no difference between placebo and melatonin groups. | Melatonin appears to be safe when administered in a dose of 10 mg daily for 28 days. |
| Andersen (2016) [33] | Review of the safety of exogenous melatonin in humans. | The use of melatonin in non-pregnant adults is safe; adverse effects are mild; long-term safety in children and adolescents should be studied further. | |
| Van der Heijden (2007) [34] | Randomized, double-blind, placebo-controlled study of 105 children aged 6–12 years with attention-deficit/hyperactivity disorder (ADHD) and chronic sleep-onset insomnia (SOI) given 3 or 6 mg of melatonin for 4 weeks. | In melatonin-treated children, sleep onset advanced by an average of 26 min, and total time asleep increased by 19.8 min as compared to placebo; there were no significant adverse events and no significant effect on behavior, cognition, or quality of life. | Melatonin induces clinically relevant advances of sleep onset and increased total sleep time in children with ADHD and SOI with no apparent negative effects. |
| Wirtz (2008) [35] | Randomized, single-blinded, placebo-controlled study of 46 healthy men aged 20–34 years old treated with 3 mg oral melatonin or placebo pill to assess effect on plasma levels of procoagulants. | Subjects in the melatonin administration group had significantly lower levels of factor VIII and fibrinogen compared to the placebo group. | The use of melatonin is associated with a significant decrease in certain procoagulant factors (factor VIII, fibrinogen) in a likely dose-response relationship; melatonin should be studied as a potential therapeutic agent for patients at risk of ASCVD. |
| Ashy (2022) [36] | Review Summarizing the consequences of COVID-19 infection on activity and function of coagulation. The effect of melatonin in this process. | Melatonin may help protect against virally induced coagulopathies in COVID-19 patients. | |
| Otmani (2008) [37] | Randomized, double-blind, placebo-controlled, and 4-way crossover study of 16 patients aged 55 years and older treated with 2 mg prolonged-release melatonin, 10 mg zolpidem, and the 2 combined; psychomotor functions, memory recall, and driving skills were evaluated at 1 and 4 h post-dosing. | Prolonged-release melatonin alone did not impair psychomotor functions, memory recall, or driving skills; zolpidem did impair all three parameters, impairments that were exacerbated with co-administration of melatonin. | Prolonged-release melatonin alone does not significantly impair psychomotor functions, memory recall, or driving skills; melatonin should be used cautiously in combination with zolpidem. |
| Tarocco (2019) [38] | Review of the effects of melatonin and its potential role and clinical implications for newborn care and pathologic conditions. | Repeated studies demonstrate that melatonin use is associated with a decrease in oxidative stress; melatonin is not solely made in the pineal gland but also in other tissue types and organ systems like the retina, GI tract, and immune system. | Melatonin may have some important anti-oxidative effects, such as reducing inflammatory biomarkers, providing neuroprotection, which may have a role in improving clinical conditions in neonates. |
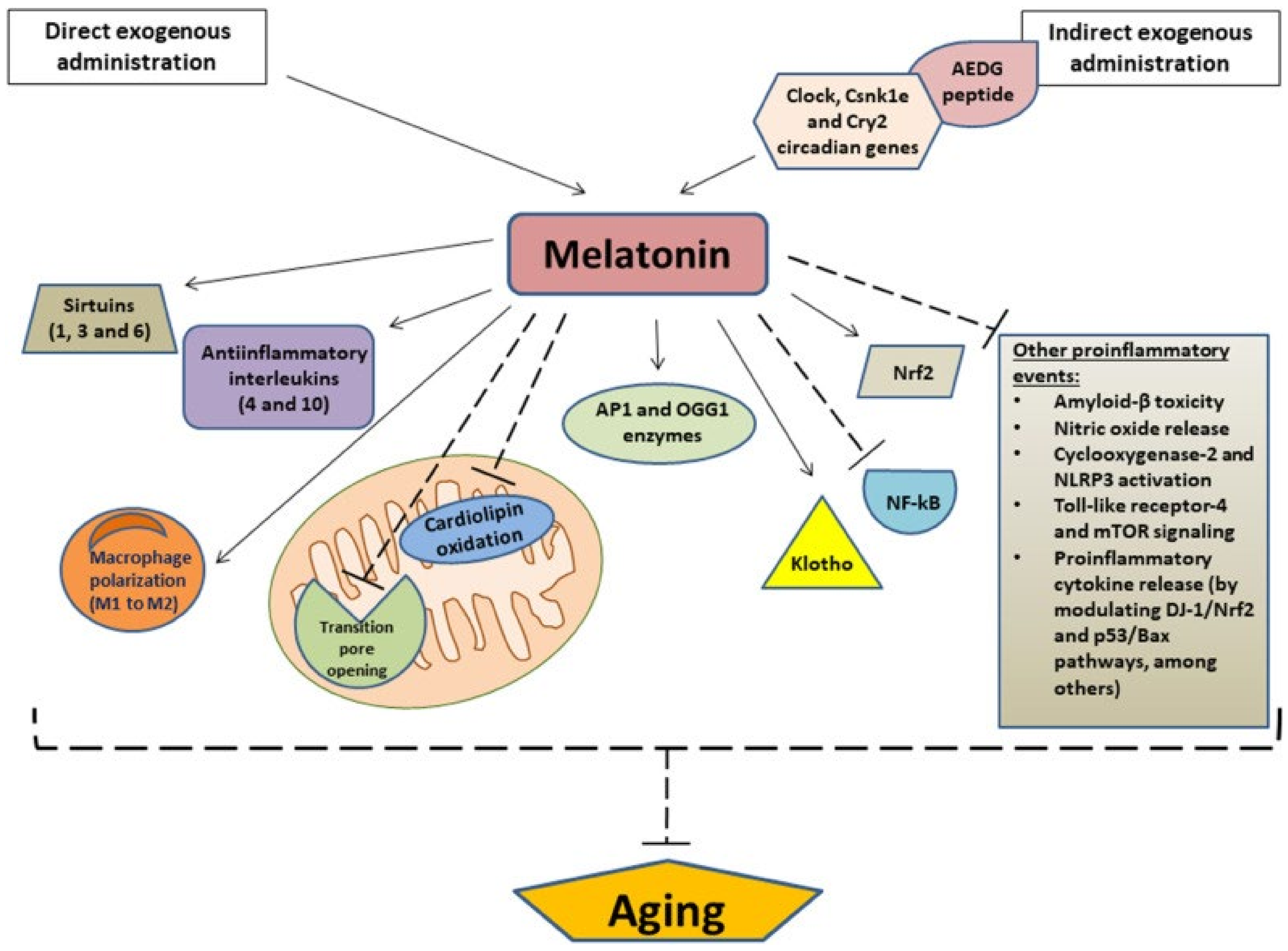
| Gimenez (2022) [39] | Review of the effects of melatonin on the aging process and its associated diseases, such as cardiovascular and neurodegenerative diseases. | Melatonin can decrease mitochondrial dysfunction and cellular aging by modulating the sirtuin1 pathway, limiting the oxidation of cardiolipin, upregulating Nrf2 and downregulating NF-kB, and suppressing proinflammatory markers such as NO, COX-2, NLRP3, and B-amyloids. | The pharmacokinetic features of melatonin that make it anti-oxidative and anti-inflammatory suggests that melatonin may be considered for its therapeutic use as an anti-aging agent. |
| National Institute of Aging (2023) [40] | “How Biomarkers Help Diagnose Dementia” is an article published by the NIH that details the biomarkers and biomarker tests available and studied for dementia research purposes. | Alzheimer’s disease is diagnosed and monitored by tests including CT, MRI, and PET scans looking for the presence of amyloid beta plaques, tau fibers, and fluorodeoxyglucose. | Several types of brain scans and biomarkers exist to help diagnose Alzheimer’s disease and other related dementias; the discovery of new biomarkers is allowing researchers to make advancements in the field of dementia. |
| Nous (2021) [41] | A systematic review of 20 studies analyzing blood and CSF melatonin levels in patients with Alzheimer’s disease (AD) compared to healthy controls. | A significant reduction in CSF levels, nocturnal blood, and nocturnal saliva levels of melatonin were found in patients with AD compared to controls; pineal gland and ventricular CSF melatonin levels have a strong correlation, while the relationship between blood and CSF melatonin levels needs further investigation. | There is indeed altered melatonin production, particularly decreased melatonin blood and CSF levels, as we age, and this reduction may become more significant in patients with AD; altered melatonin production in AD may help to explain the biological basis for circadian rhythm disturbances and sundowning effect commonly seen in patients with AD. |
| Alz.org (2023) [42] | “Mild Cognitive Impairment (MCI)” is an article published by the Alzheimer’s Association that describes the wide spectrum of cognitive changes and medical workup for MCI. | MCI is a clinical diagnosis, and when individuals with MCI have a PET scan or CSF test that detects amyloid beta protein, these individuals are considered to have MCI secondary to Alzheimer’s disease; aducanumab and lecanemab are newly approved by U.S. FDA for the treatment of early Alzheimer’s disease. | MCI causes cognitive changes that may or may not be an early sign of Alzheimer’s disease. A medical workup of MCI is critical to attempt to determine the root cause of MCI, e.g., a new medication or an irreversible neurodegenerative disease like Alzheimer’s disease. |
| Sumsuzzman (2021) [43] | A systemic review and meta-analysis of 22 studies investigating the impact of melatonin on cognition from randomized-control trials of oral melatonin treatment for Alzheimer’s disease, insomnia, and/or healthy subjects. | Melatonin use among patients with mild-stage Alzheimer’s disease significantly improved cognition (measured by MMSE score); chronic nighttime melatonin use among healthy subjects improved memory without any negative cognitive effects and may potentially improve cognition. | Long-term melatonin use is associated with positive cognitive outcomes and may be of particular benefit in patients with mild-stage Alzheimer’s disease. |
| Boafo (2019) [44] | Review of effect of long-term use of melatonin in pre-pubertal children on the timing of puberty. | The effect of long-term use of melatonin of pubertal timing is understudied. | |
| Sheldon (1998) [45] | Research letter reporting on a study of 6 children aged 9 months to 18 years with multiple neurological deficits and chronic severe sleep complaints treated with 5 mg of melatonin. | Melatonin improved sleep-onset latency, sleep continuity, and total sleep time in five of six patients; however, the study was suspended because four of six patients had increased seizure activity. | Melatonin may increase seizure activity in children with multiple neurological deficits and should be used with caution. |
| Peled (2012) [46] | Review of melatonin utility in 6 children aged 2–15 years old with severe, intractable seizures. | Melatonin improved seizure activity in five of six children with intractable seizures. | Melatonin may be an effective adjunct to standard anti-epileptic therapy. |
| Maghbooli (2022) [47] | A cross-sectional, randomized, double-blind, placebo-controlled clinical trial of add-on melatonin therapy for 60 patients with epilepsy with idiopathic generalized tonic-clonic seizures alone (EGTCS). | The addition of melatonin to valproic acid treatment led to a significant decrease in mean severity score of epilepsy and improved sleep quality; the number of attacks and EEG results did not significantly change with melatonin vs. placebo. | Melatonin may be a useful adjunctive therapeutic agent for patients with EGTCS, given its ability to reduce severity of epilepsy and enhance sleep quality. |
| Frisher (2016) [48] | A retrospective cohort study of 1,377 patients ages 45 and older with at least 3 melatonin prescriptions over a 2-year period; records were reviewed for fracture risk. | Prescribed melatonin was associated with a significantly increased risk of fracture after adjusting for potential confounders. | Melatonin use may increase the risk of fractures in older adults. |
| Lui (2018) [49] | A randomized, double-blind, placebo-controlled observation study to determine the effect of melatonin on postural control and cognitive performance in 34 adults aged 60–71; testing while dual-tasking (concurrent physical and cognitive tasks) was performed before and after a 3 mg dose of immediate-release melatonin. | There was a significant decrease in postural control after taking melatonin but no change in cognitive performance. | A single dose of melatonin may disturb postural control in older adults; precautions may be necessary to decrease the risk of falls in older adults after taking melatonin. |
| Gooneratne (2012) [50] | A randomized, double-blind, placebo-controlled study of pharmacokinetics of low (<0.5 mg) and higher dose (>2 mg) combined immediate- and controlled-release melatonin in 27 adults over 65 years of age. | In those given higher dose melatonin, melatonin levels remained elevated for an average of 10 h. | Melatonin levels in older adults given higher dose (>2 mg) combined immediate and controlled release melatonin may remain elevated beyond the typical sleep period. |
| Whittom (2010) [51] | 8 adults aged 38–63 years with restless legs syndrome (RLS) received exogenous melatonin or bright light exposure; the effect on motor and sensory manifestations was measured. | Melatonin administration resulted in an increase in motor manifestations of RLS; bright light exposure resulted in a small but significant decrease in sensory symptoms. | Melatonin causes an increase in motor manifestations of RLS. |
| Aurora (2012) [52] | An evidence-based systematic review and meta-analysis of treatment of restless legs syndrome and periodic limb movement disorder (PLMD) in adults. | 3 mg of melatonin decreased leg movements, sleep arousal, and subjective well-being in PLMD. | Melatonin is useful in the treatment of periodic leg movement disorder (PLMD) in adults. |
| Campos (2004) [53] | A randomized, double-blind, placebo-controlled study of the use of melatonin in 22 adults with mild to moderate asthma. | Melatonin improved subjective sleep quality with no effect on asthma symptoms, use of relief medication, or daily peak expiratory flow. | Melatonin can safely be used in adults with mild to moderate asthma. |
| Carman (1976) [54] | Double blind cross-over study of 6 moderately to severely depressed patients and 2 patients with Huntington’s chorea were treated with PO or IV melatonin at doses of 150–1600 mg daily. | All patients studied showed an increase in psychological symptoms (depression, psychosis, anger, and anxiety) during treatment with melatonin. | Melatonin may worsen depression, psychosis, anger, and anxiety in moderately to severely depressed patients at higher doses than are usually used for the treatment of sleep disorders. |
| Hansen (2014) [55] | Review, qualitative synthesis, and meta-analysis of the therapeutic or prophylactic effect of exogenous melatonin on depression and depressive symptoms. | There is no clear evidence that melatonin worsens or improves depression or depressive symptoms. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Givler, D.; Givler, A.; Luther, P.M.; Wenger, D.M.; Ahmadzadeh, S.; Shekoohi, S.; Edinoff, A.N.; Dorius, B.K.; Jean Baptiste, C.; Cornett, E.M.; et al. Chronic Administration of Melatonin: Physiological and Clinical Considerations. Neurol. Int. 2023, 15, 518-533. https://doi.org/10.3390/neurolint15010031
Givler D, Givler A, Luther PM, Wenger DM, Ahmadzadeh S, Shekoohi S, Edinoff AN, Dorius BK, Jean Baptiste C, Cornett EM, et al. Chronic Administration of Melatonin: Physiological and Clinical Considerations. Neurology International. 2023; 15(1):518-533. https://doi.org/10.3390/neurolint15010031
Chicago/Turabian StyleGivler, Donald, Amy Givler, Patrick M. Luther, Danielle M. Wenger, Shahab Ahmadzadeh, Sahar Shekoohi, Amber N. Edinoff, Bradley K. Dorius, Carlo Jean Baptiste, Elyse M. Cornett, and et al. 2023. "Chronic Administration of Melatonin: Physiological and Clinical Considerations" Neurology International 15, no. 1: 518-533. https://doi.org/10.3390/neurolint15010031
APA StyleGivler, D., Givler, A., Luther, P. M., Wenger, D. M., Ahmadzadeh, S., Shekoohi, S., Edinoff, A. N., Dorius, B. K., Jean Baptiste, C., Cornett, E. M., Kaye, A. M., & Kaye, A. D. (2023). Chronic Administration of Melatonin: Physiological and Clinical Considerations. Neurology International, 15(1), 518-533. https://doi.org/10.3390/neurolint15010031










