Modified TPP-MoS2 QD Blend as a Bio-Functional Model for Normalizing Microglial Dysfunction in Alzheimer’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Modified TPP-MoS2 Blend
2.3. Preparation of Aβ Solution
2.4. Cell Culture and Treatment
2.5. Cell Viability Assay and Morphological Studies
2.6. Morphological Assessment of Apoptotic Cells Nuclei with DAPI Staining
2.7. Intracellular Reactive Oxygen Species (ROS) Level
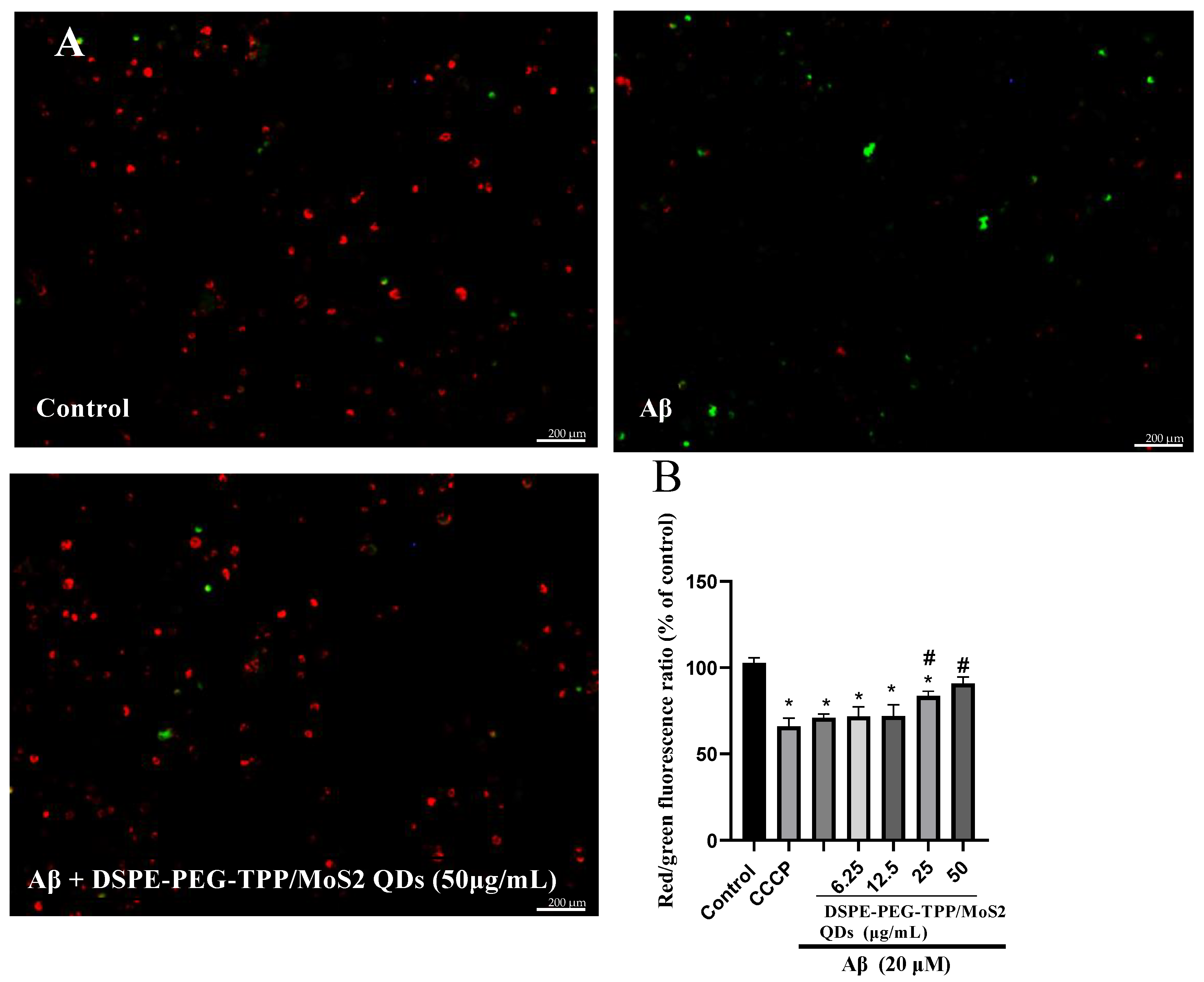
2.8. Mitochondrial Membrane Potential (MPP) Assay
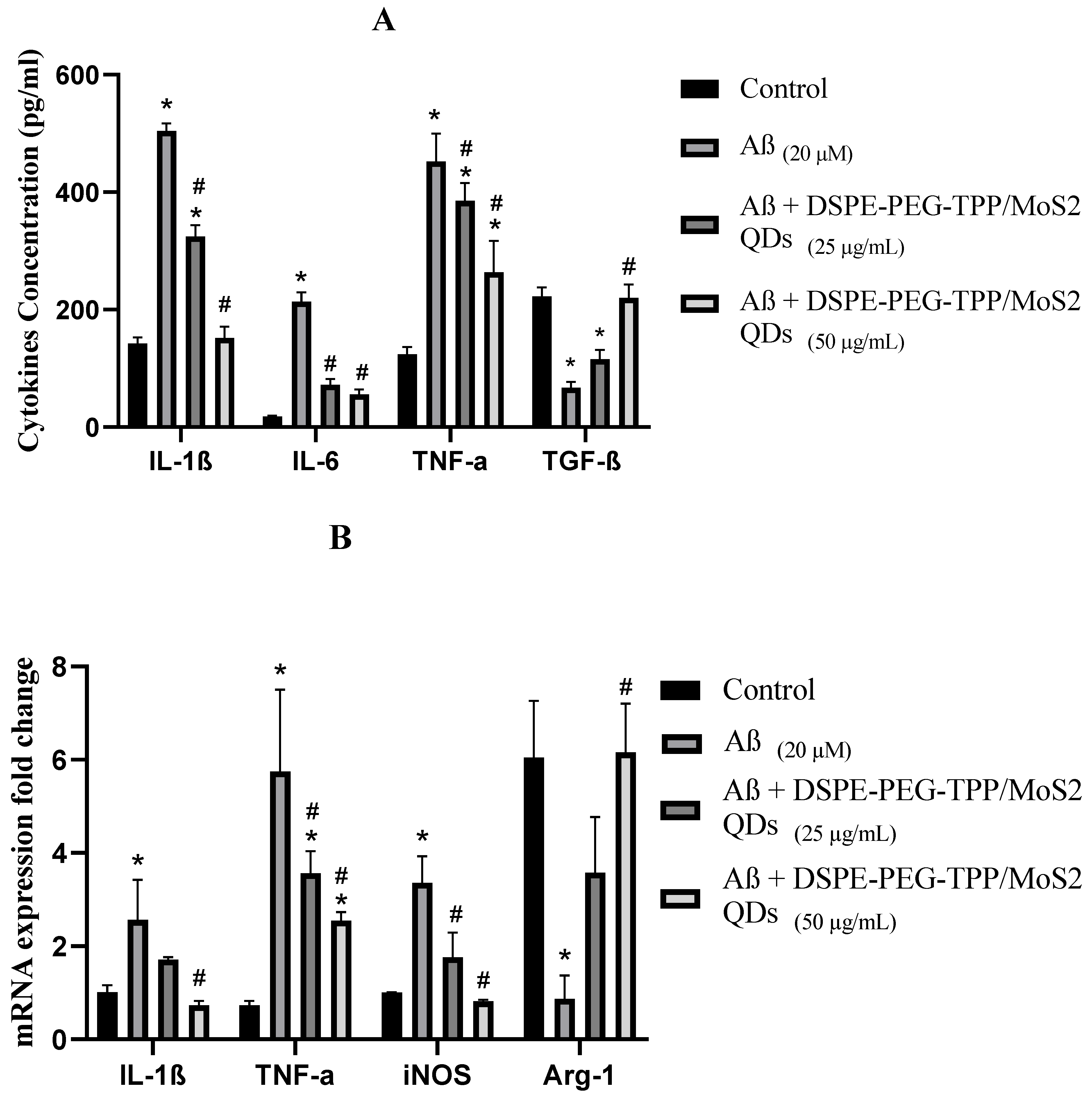
2.9. Cytokine Assay
- (a)
- Enzyme-Linked Immunosorbent Assay (ELISA)
- (b)
- Quantitative Polymerase Chain Reaction
2.10. Statistical Analysis of Data
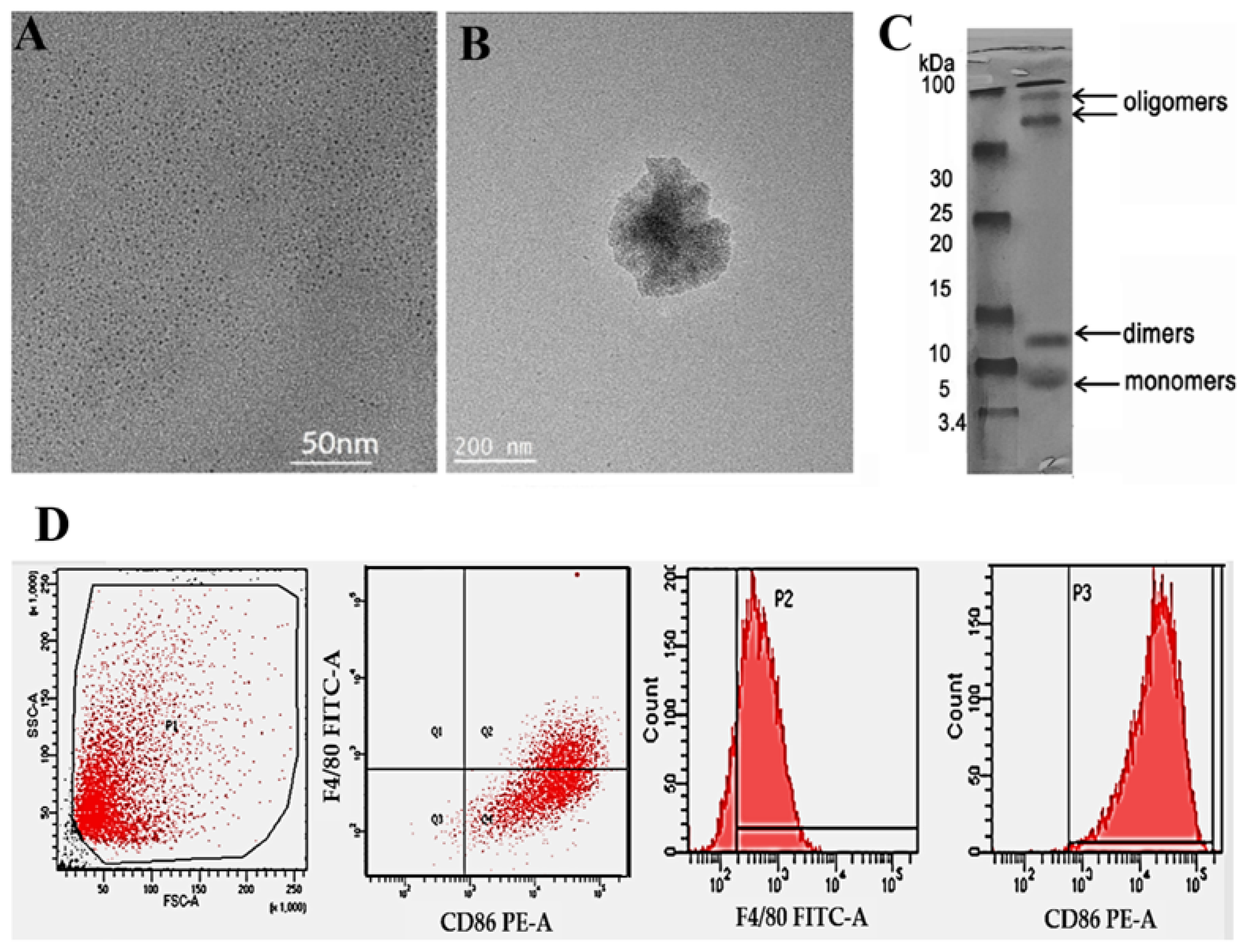
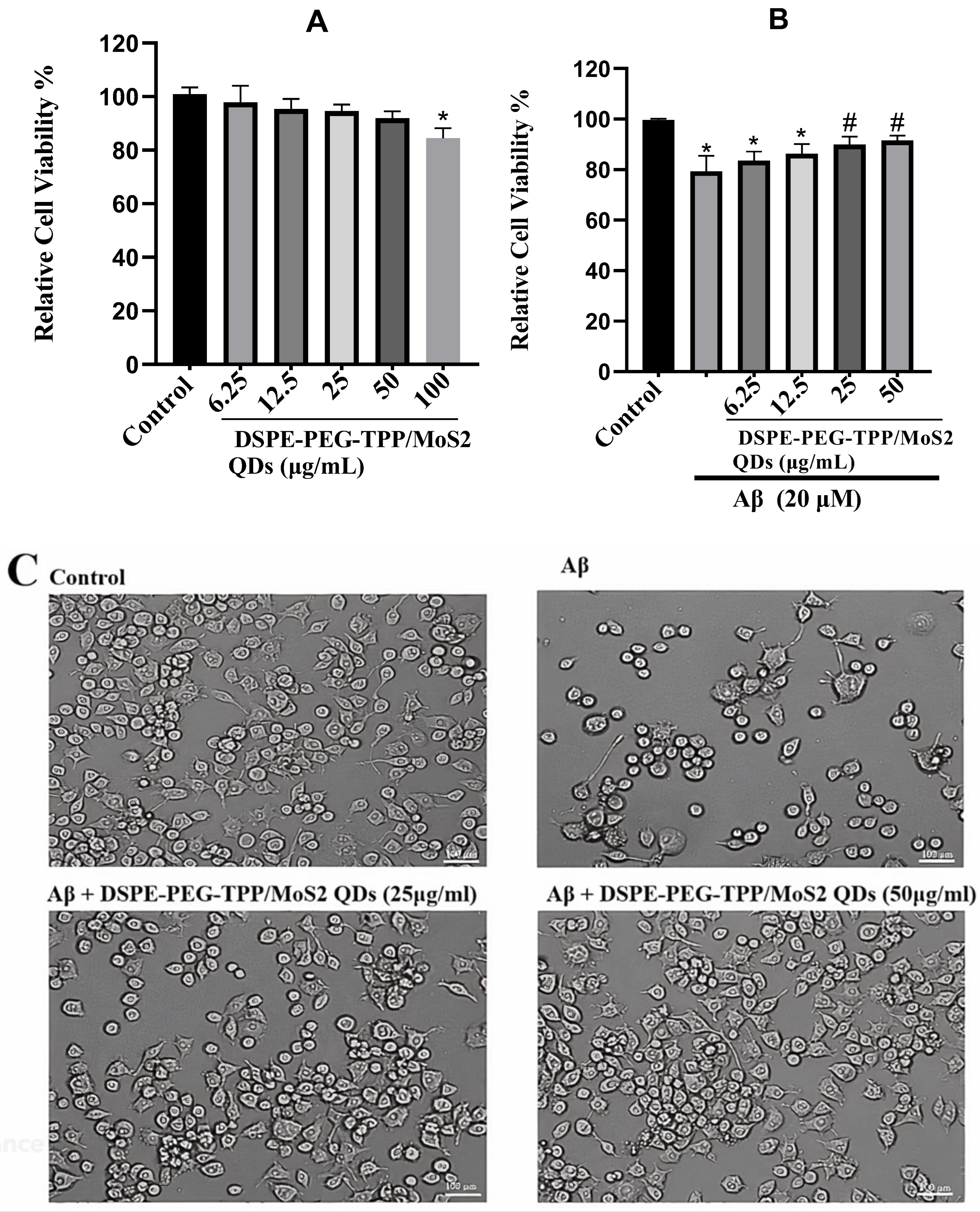
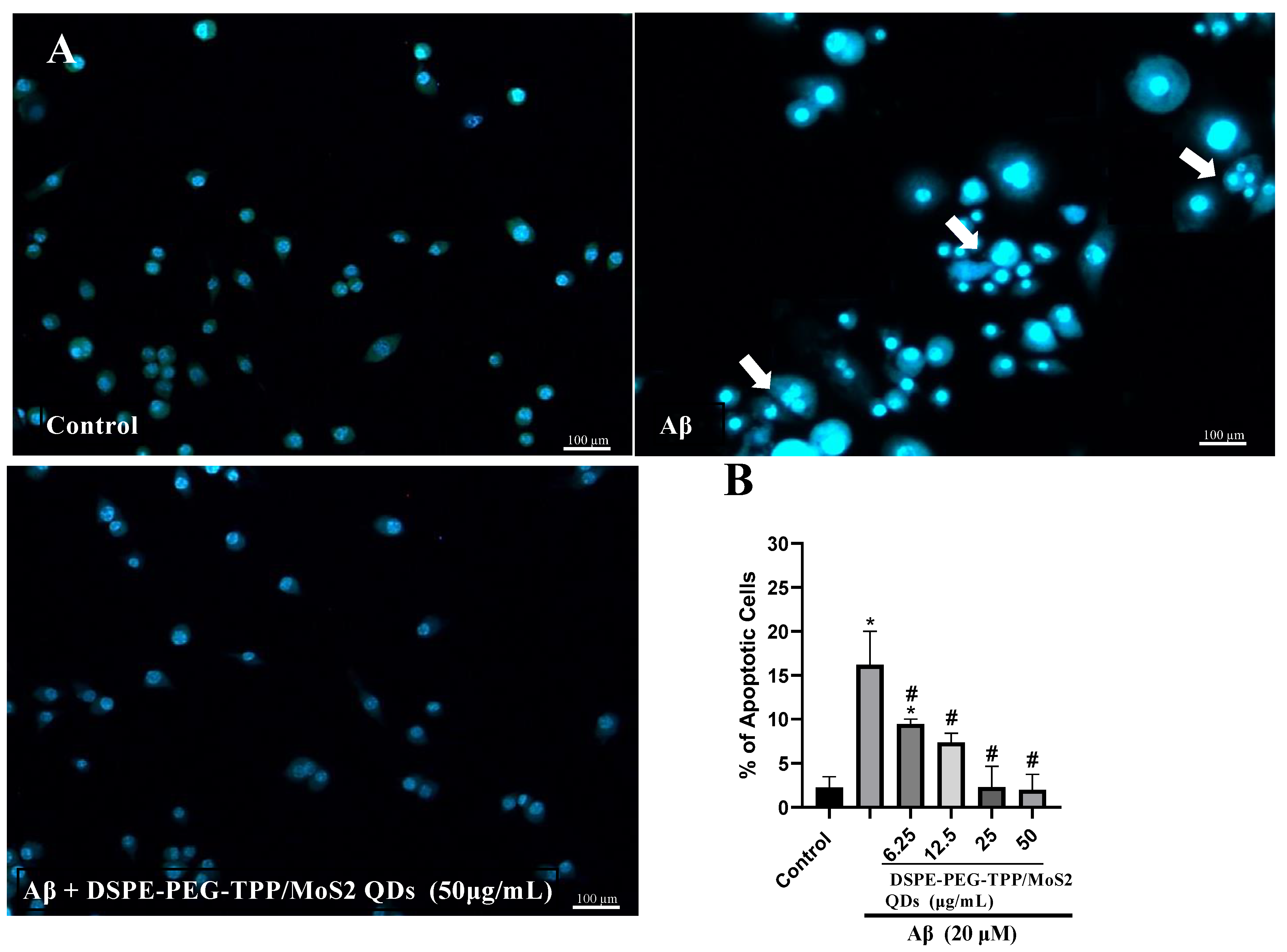
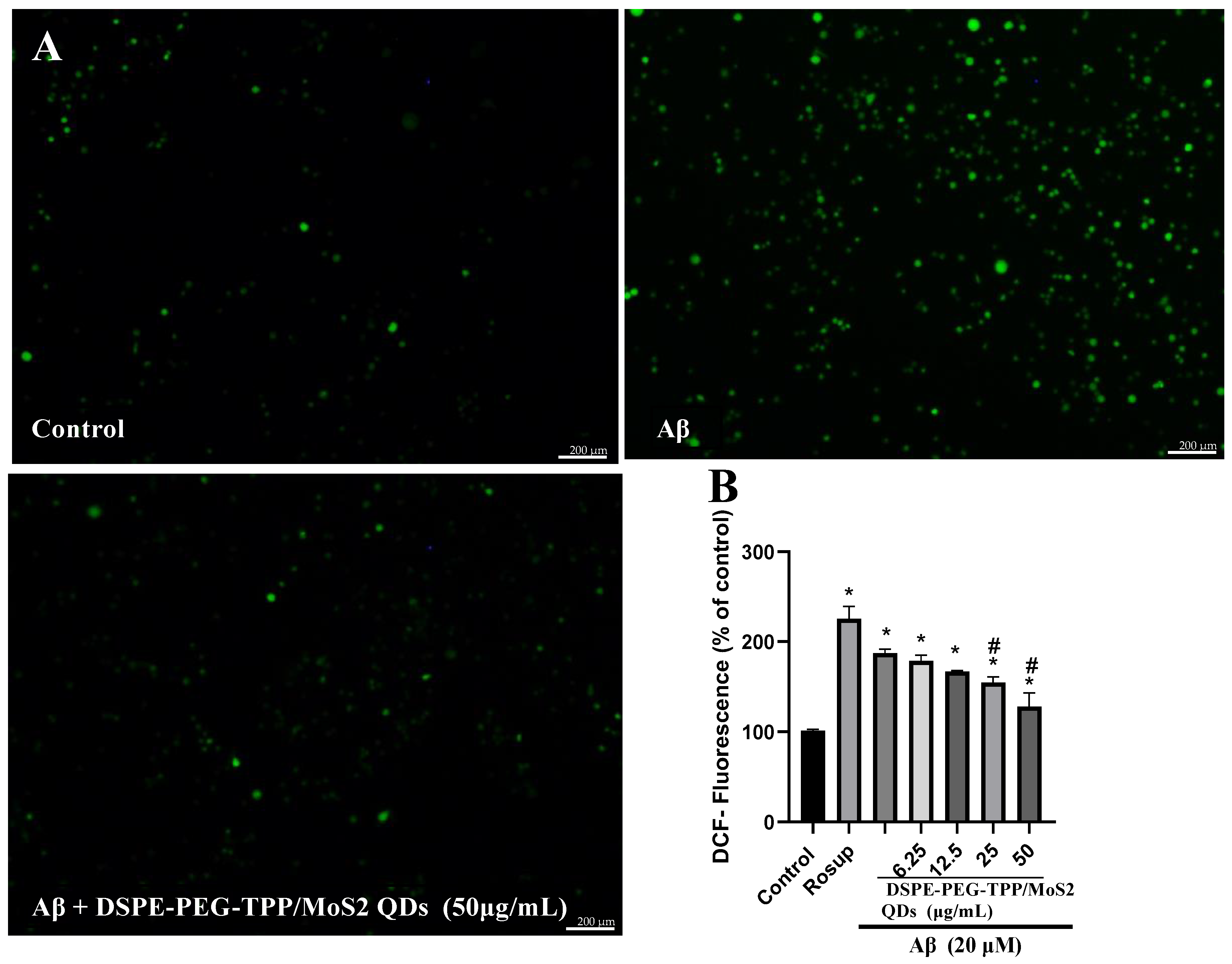
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Breijyeh, Z.; Karaman, R. Comprehensive review on Alzheimer’s disease: Causes and treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef] [PubMed]
- Plascencia-Villa, G.; Perry, G. Neuropathological changes provide insights into key mechanisms related to Alzheimer’s disease and related dementia. Am. J. Pathol. 2022, 192, 1340–1346. [Google Scholar] [CrossRef]
- Sehar, U.; Rawat, P.; Reddy, A.P.; Kopel, J.; Reddy, P.H. Amyloid Beta in Aging and Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 12924. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, I.; Jha, S. Mitochondrial dysfunction and Alzheimer’s disease: Role of microglia. Front. Aging Neurosci. 2020, 12, 252. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Hussain, M.D.; Yan, L.J. Microglia, neuroinflammation, and beta-amyloid protein in Alzheimer’s disease. Int. J. Neurosci. 2014, 124, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Islam, R.; Choudhary, H.; Rajan, R.; Vrionis, F.; Hanafy, K.A. An overview on microglial origin, distribution, and phenotype in Alzheimer’s disease. J. Cell. Physiol. 2022. [Google Scholar] [CrossRef]
- Edler, M.K.; Mhatre-Winters, I.; Richardson, J.R. Microglia in aging and Alzheimer’s disease: A comparative species review. Cells 2021, 10, 1138. [Google Scholar] [CrossRef]
- Wendimu, M.Y.; Hooks, S.B. Microglia phenotypes in aging and neurodegenerative diseases. Cells 2022, 11, 2091. [Google Scholar] [CrossRef]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Salvadores, N.; Moreno-Gonzalez, I.; Gamez, N.; Quiroz, G.; Vegas-Gomez, L.; Escandón, M.; Soto, C. Aβ oligomers trigger necroptosis-mediated neurodegeneration via microglia activation in Alzheimer’s disease. Acta Neuropathol. Commun. 2022, 10, 31. [Google Scholar] [CrossRef]
- Li, Y.; Xia, X.; Wang, Y.; Zheng, J.C. Mitochondrial dysfunction in microglia: A novel perspective for pathogenesis of Alzheimer’s disease. J. Neuroinflam. 2022, 19, 248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wang, Z.; Hu, H.; Zhao, M.; Sun, L. Microglia in Alzheimer’s disease: A target for therapeutic intervention. Front. Cell. Neurosci. 2021, 15, 749587. [Google Scholar] [CrossRef] [PubMed]
- Althafar, Z.M. Targeting microglia in Alzheimer’s disease: From molecular mechanisms to potential therapeutic targets for small molecules. Molecules 2022, 27, 4124. [Google Scholar] [CrossRef]
- Bazaka, K.; Levchenko, I.; Lim JW, M.; Baranov, O.; Corbella, C.; Xu, S.; Keidar, M. MoS2-based nanostructures: Synthesis and applications in medicine. J. Phys. D Appl. Phys. 2019, 52, 183001. [Google Scholar] [CrossRef]
- Guo, Y.; Li, J. MoS2 quantum dots: Synthesis, properties and biological applications. Mater. Sci. Eng. C 2020, 109, 110511. [Google Scholar] [CrossRef]
- Lin, H.; Wang, C.; Wu, J.; Xu, Z.; Huang, Y.; Zhang, C. Colloidal synthesis of MoS 2 quantum dots: Size-dependent tunable photoluminescence and bioimaging. New J. Chem. 2015, 39, 8492–8497. [Google Scholar] [CrossRef]
- Presutti, D.; Agarwal, T.; Zarepour, A.; Celikkin, N.; Hooshmand, S.; Nayak, C.; Maiti, T.K. Transition metal dichalcogenides (TMDC)-based nanozymes for biosensing and therapeutic applications. Materials 2022, 15, 337. [Google Scholar] [CrossRef]
- Xu, J.; Cai, R.; Zhang, Y.; Mu, X. Molybdenum disulfide-based materials with enzyme-like characteristics for biological applications. Colloids Surf. B Biointerfaces 2021, 200, 111575. [Google Scholar] [CrossRef]
- Chen, T.; Zou, H.; Wu, X.; Liu, C.; Situ, B.; Zheng, L.; Yang, G. Nanozymatic antioxidant system based on MoS2 nanosheets. ACS Appl. Mater. Interfaces 2018, 10, 12453–12462. [Google Scholar] [CrossRef]
- Li, Y.; Tang, H.; Zhu, H.; Kakinen, A.; Wang, D.; Andrikopoulos, N.; Ke, P.C. Ultrasmall molybdenum disulfide quantum dots cage alzheimer’s amyloid beta to restore membrane fluidity. ACS Appl. Mater. Interfaces 2021, 13, 29936–29948. [Google Scholar] [CrossRef]
- Han, Q.; Cai, S.; Yang, L.; Wang, X.; Qi, C.; Yang, R.; Wang, C. Molybdenum disulfide nanoparticles as multifunctional inhibitors against Alzheimer’s disease. ACS Appl. Mater. Interfaces 2017, 9, 21116–21123. [Google Scholar] [CrossRef]
- Kwon, H.J.; Cha, M.Y.; Kim, D.; Kim, D.K.; Soh, M.; Shin, K.; Mook-Jung, I. Mitochondria-targeting ceria nanoparticles as antioxidants for Alzheimer’s disease. ACS Nano 2016, 10, 2860–2870. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Li, D.; Zhou, Q.; Hu, X. Mitochondria-targeted TPP-MoS2 with dual enzyme activity provides efficient neuroprotection through M1/M2 microglial polarization in an Alzheimer’s disease model. Biomaterials 2020, 232, 119752. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zeng, Z.; Wang, B.; Guo, S. Trans-caryophyllene inhibits amyloid β (Aβ) oligomer-induced neuroinflammation in BV-2 microglial cells. Int. Immunopharmacol. 2017, 51, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Roushandeh, A.M.; Tomita, K.; Kuwahara, Y.; Jahanian-Najafabadi, A.; Igarashi, K.; Roudkenar, M.H.; Sato, T. Transfer of healthy fibroblast-derived mitochondria to HeLa ρ 0 and SAS ρ 0 cells recovers the proliferation capabilities of these cancer cells under conventional culture medium, but increase their sensitivity to cisplatin-induced apoptotic death. Mol. Biol. Rep. 2020, 47, 4401–4411. [Google Scholar] [CrossRef] [PubMed]
- Guest, F.L.; Rahmoune, H.; Guest, P.C. Early Diagnosis and Targeted Treatment Strategy for Improved Therapeutic Outcomes in Alzheimer’s Disease. In Reviews on New Drug Targets in Age-Related Disorders; Guest, P.C., Ed.; Springer: Cham, Switzerland, 2020; pp. 175–191. [Google Scholar]
- Lee, J.W.; Chun, W.; Lee, H.J.; Kim, S.M.; Min, J.H.; Kim, D.Y.; Lee, S.U. The role of microglia in the development of neurodegenerative diseases. Biomedicines 2021, 9, 1449. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Francis, N.L.; Calvelli, H.R.; Moghe, P.V. Microglia-targeting nanotherapeutics for neurodegenerative diseases. APL Bioeng. 2020, 4, 030902. [Google Scholar] [CrossRef]
- Yadav, V.; Roy, S.; Singh, P.; Khan, Z.; Jaiswal, A. 2D MoS2-based nanomaterials for therapeutic, bioimaging, and biosensing applications. Small 2019, 15, 1803706. [Google Scholar] [CrossRef]
- Wang, J.; Sui, L.; Huang, J.; Miao, L.; Nie, Y.; Wang, K.; Ai, K. MoS2-based nanocomposites for cancer diagnosis and therapy. Bioact. Mater. 2021, 6, 4209–4242. [Google Scholar] [CrossRef]
- Domi, B.; Bhorkar, K.; Rumbo, C.; Sygellou, L.; Yannopoulos, S.N.; Quesada, R.; Tamayo-Ramos, J.A. Fate assessment of commercial 2D MoS2 aqueous dispersions at physicochemical and toxicological level. Nanotechnology 2020, 31, 445101. [Google Scholar] [CrossRef]
- Song, J.; Choi, S.M.; Kim, B.C. Adiponectin regulates the polarization and function of microglia via PPAR-γ signaling under amyloid β toxicity. Front. Cell. Neurosci. 2017, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Liaw, K.; Sharma, R.; Zhang, Z.; Kannan, S.; Kannan, R.M. Targeting mitochondrial dysfunction and oxidative stress in activated microglia using dendrimer-based therapeutics. Theranostics 2018, 8, 5529. [Google Scholar] [CrossRef] [PubMed]
- Karthivashan, G.; Ganesan, P.; Park, S.-Y.; Kim, J.-S.; Choi, D.-K. Therapeutic strategies and nano-drug delivery applications in management of aging Alzheimer’s disease. Drug Delivery 2018, 25, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Chen, D.; Wang, Y.; Li, H.; Zhang, Y.; Chen, H.; Huo, M. Nanozymes-recent development and biomedical applications. J. Nanobiotechnol. 2022, 20, 92. [Google Scholar] [CrossRef]
- Sun, L.J.; Qu, L.; Yang, R.; Yin, L.; Zeng, H.J. Cysteamine functionalized MoS2 quantum dots inhibit amyloid aggregation. Int. J. Biol. Macromol. 2019, 128, 870–876. [Google Scholar] [CrossRef]
- Mangrulkar, S.V.; Wankhede, N.L.; Kale, M.B.; Upaganlawar, A.B.; Taksande, B.G.; Umekar, M.J.; Behl, T. Mitochondrial Dysfunction as a Signaling Target for Therapeutic Intervention in Major Neurodegenerative Disease. Neurotox. Res. 2023, 1–22. [Google Scholar] [CrossRef]
- Huang, Y.C.; Hsu, S.M.; Shie, F.S.; Shiao, Y.J.; Chao, L.J.; Chen, H.W.; Tsay, H.J. Reduced mitochondria membrane potential and lysosomal acidification are associated with decreased oligomeric Aβ degradation induced by hyperglycemia: A study of mixed glia cultures. PLoS ONE 2022, 17, e0260966. [Google Scholar] [CrossRef]
- Ke, S.; Yang, P.; Wang, Y.; Ye, S.; Kou, S. Flower-Like Molybdenum Disulfide Nanostructures for Promoting Mitochondrial Homeostasis and Attenuating Inflammatory Endothelial Dysfunction. ACS Appl. Nano Mater. 2021, 4, 11709–11722. [Google Scholar] [CrossRef]
- Sun, G.; Yang, S.; Cai, H.; Shu, Y.; Han, Q.; Wang, B.; Yin, Z. Molybdenum disulfide nanoflowers mediated anti-inflammation macrophage modulation for spinal cord injury treatment. J. Colloid Interface Sci. 2019, 549, 50–62. [Google Scholar] [CrossRef]
- Tom, S.; Rane, A.; Katewa, A.S.; Chamoli, M.; Matsumoto, R.R.; Andersen, J.K.; Chinta, S.J. Gedunin inhibits oligomeric Aβ1–42-induced microglia activation via modulation of Nrf2-NF-κB signaling. Mol. Neurobiol. 2019, 56, 7851–7862. [Google Scholar] [CrossRef]
| Forward | Reverse | |
|---|---|---|
| GAPDH | ACTCCACTCACGGCAAATTC | TCTCCATGGTGGTGAAGACA |
| TNF-α | AAATGGCCTCCCTCTCATCAG | GTCACTCGAATTTTGAGAAGATGATC |
| IL-1β | AGCTTCAGGCAGGCAGTATC | AAGGTCCACGGGAAAGACAC |
| iNOS | GTTCTCAGCCCAACAATACAAGA | GTGGACGGGTCGATGTCAC |
| Arg-1 | CACAGTCTGGCAGTTGGAAG | GGGAGTGTTGATGTCAGTGTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alomari, O.A.; Qusti, S.; Balgoon, M.; Aljoud, F.; Alamry, K.A.; Hussein, M.A. Modified TPP-MoS2 QD Blend as a Bio-Functional Model for Normalizing Microglial Dysfunction in Alzheimer’s Disease. Neurol. Int. 2023, 15, 954-966. https://doi.org/10.3390/neurolint15030061
Alomari OA, Qusti S, Balgoon M, Aljoud F, Alamry KA, Hussein MA. Modified TPP-MoS2 QD Blend as a Bio-Functional Model for Normalizing Microglial Dysfunction in Alzheimer’s Disease. Neurology International. 2023; 15(3):954-966. https://doi.org/10.3390/neurolint15030061
Chicago/Turabian StyleAlomari, Ohoud A., Safaa Qusti, Maha Balgoon, Fadwa Aljoud, Khalid A. Alamry, and Mahmoud A. Hussein. 2023. "Modified TPP-MoS2 QD Blend as a Bio-Functional Model for Normalizing Microglial Dysfunction in Alzheimer’s Disease" Neurology International 15, no. 3: 954-966. https://doi.org/10.3390/neurolint15030061
APA StyleAlomari, O. A., Qusti, S., Balgoon, M., Aljoud, F., Alamry, K. A., & Hussein, M. A. (2023). Modified TPP-MoS2 QD Blend as a Bio-Functional Model for Normalizing Microglial Dysfunction in Alzheimer’s Disease. Neurology International, 15(3), 954-966. https://doi.org/10.3390/neurolint15030061






