Altered Cardiac Autonomic Regulation in Individuals with Myasthenia Gravis—A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Information Sources and Search Strategies
2.2. Eligibility Criteria
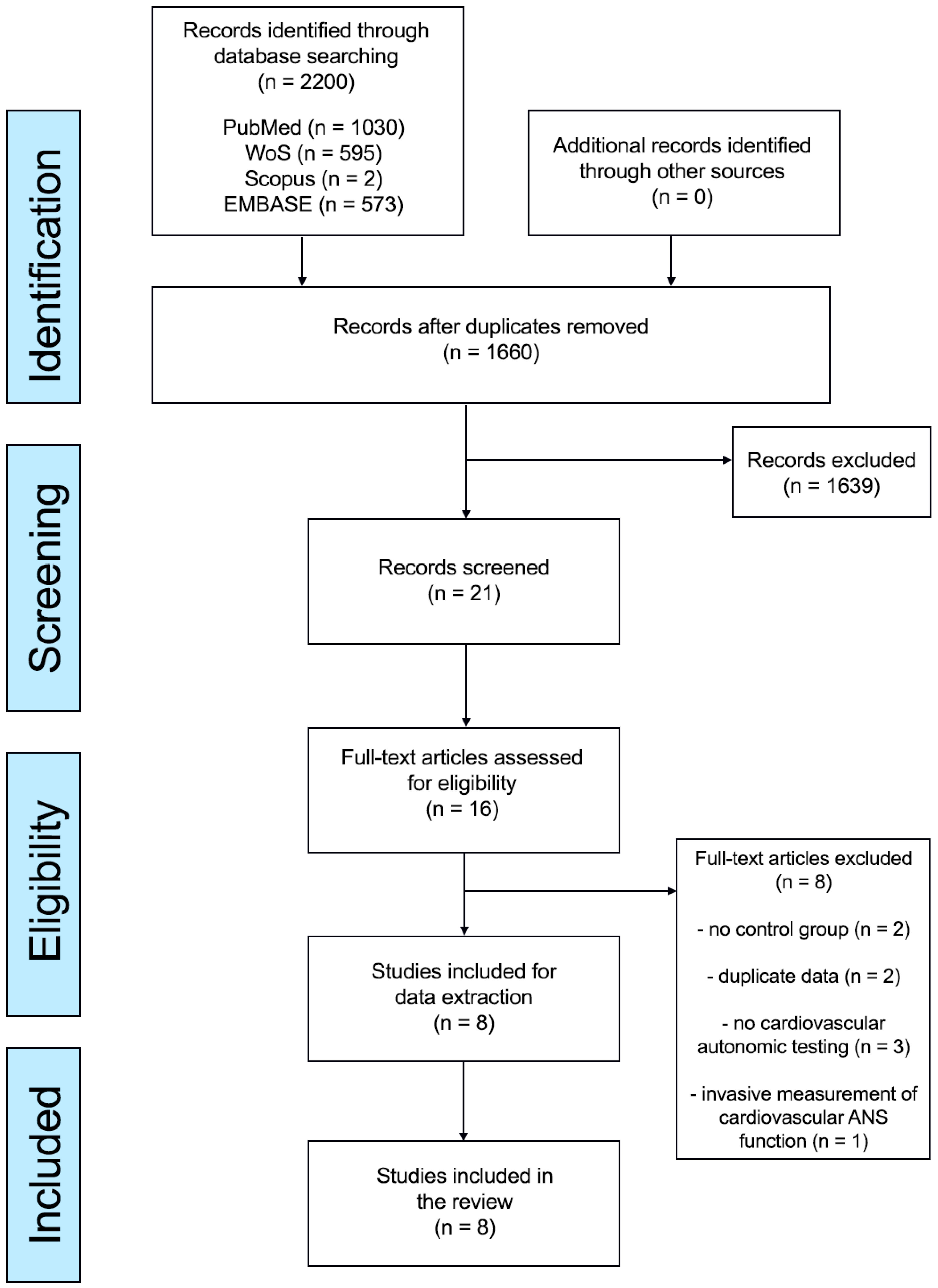
2.3. Study Selection
2.4. Outcomes
2.5. Assessment of Risk of Bias
2.6. Data Synthesis
3. Results
3.1. Characteristics of Included Studies and Participant Details
3.2. Meta-Analysis Results
3.2.1. Time Domain HRV Parameters: SDNN; SDANN, rMSSD, pNN50, SDNN Index
3.2.2. Frequency Domain HRV Parameters at Rest: LFnu, HFnu, LF, HF, LF/HF, LF/HF of HRV, PSD
3.2.3. Frequency Domain HRV Parameters in Response to Tilt: LFtilt, HFtilt, LF/HFtilt, LF/HFtilt of HRV, PSDtilt
3.3. Baroreflex Sensitivity (BRS)
3.4. Valsalva Ratio
3.5. Mean R-R Interval
3.6. E/I Ratio
3.7. Heart Rate and Blood Pressure Parameters at Rest and in Response to Tilt
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sanders, D.B.; Wolfe, G.I.; Benatar, M.; Evoli, A.; Gilhus, N.E.; Illa, I.; Kuntz, N.; Massey, J.M.; Melms, A.; Murai, H.; et al. International consensus guidance for management of myasthenia gravis: Executive summary. Neurology 2016, 87, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Gilhus, N.E.; Tzartos, S.; Evoli, A.; Palace, J.; Burns, T.M.; Verschuuren, J.J.G.M. Myasthenia gravis. Nat. Rev. Dis. Primers 2019, 5, 30. [Google Scholar] [CrossRef]
- Binks, S.; Vincent, A.; Palace, J. Myasthenia gravis: A clinical-immunological update. J. Neurol. 2016, 263, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Khemani, P.; Mehdirad, A.A. Cardiovascular Disorders Mediated by Autonomic Nervous System Dysfunction. Cardiol. Rev. 2020, 28, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Gibson, T.C. The heart in myasthenia gravis. Am. Heart J. 1975, 90, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Owe, J.F.; Davidsen, E.S.; Eide, G.E.; Gerdts, E.; Gilhus, N.E. Left ventricular long-axis function in myasthenia gravis. J. Neurol. 2008, 255, 1777–1784. [Google Scholar] [CrossRef]
- Johannessen, K.-A.; Mygland, A.; Gilhus, N.E.; Aarli, J.; Vik-Mo, H. Left ventricular function in myasthenia gravis. Am. J. Cardiol. 1992, 69, 129–132. [Google Scholar] [CrossRef]
- Nikolić, A.; Perić, S.; Nišić, T.; Popović, S.; Ilić, M.; Stojanović, V.R.; Lavrnić, D. The presence of dysautonomia in different subgroups of myasthenia gravis patients. J. Neurol. 2014, 261, 2119–2127. [Google Scholar] [CrossRef]
- Peric, S.; Rakocevic-Stojanovic, V.; Nisic, T.; Pavlovic, S.; Basta, I.; Popovic, S.; Damjanovic, S.; Lavrnic, D. Cardiac autonomic control in patients with myasthenia gravis and thymoma. J. Neurol. Sci. 2011, 307, 30–33. [Google Scholar] [CrossRef]
- Kocabas, Z.U.; Kizilay, F.; Basarici, I.; Uysal, H. Evaluation of cardiac autonomic functions in myasthenia gravis. Neurol. Res. 2018, 40, 405–412. [Google Scholar] [CrossRef]
- Zawadka-Kunikowska, M.; Rzepiński, Ł.; Tafil-Klawe, M.; Klawe, J.J.; Zalewski, P.; Słomko, J. Association of Cardiac Autonomic Responses with Clinical Outcomes of Myasthenia Gravis: Short-Term Analysis of the Heart-Rate and Blood Pressure Variability. J. Clin. Med. 2022, 11, 3697. [Google Scholar] [CrossRef]
- Shukla, G.; Gupta, S.; Goyal, V.; Singh, S.; Srivastava, A.; Behari, M. Abnormal sympathetic hyper-reactivity in patients with myasthenia gravis: A prospective study. Clin. Neurol. Neurosurg. 2013, 115, 179–186. [Google Scholar] [CrossRef]
- Puneeth, C.S.; Chandra, S.R.; Yadav, R. Heart rate and blood pressure variability in patients with myasthenia gravis. Ann. Indian Acad. Neurol. 2013, 16, 329–332. [Google Scholar]
- Elsais, A.; Kerty, E.; Russell, K.; Toska, K. Does cardiovascular autonomic dysfunction contribute to fatigue in myasthenia gravis? Physiol. Res. 2022, 71, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Nalbantoglu, M.; Akalin, M.A.; Gunduz, A.; Kiziltan, M. Electrophysiological investigation for autonomic dysfunction in patients with myasthenia gravis: A prospective study. Clin. Neurosci. 2021, 74, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-Y.; Cheng, G.-Y.; Shan, Z.-G.; Baritussio, A.; Lorenzoni, G.; Tyminska, A.; Ozieranski, K.; Iliceto, S.; Marcolongo, R.; Gregori, D.; et al. Efficacy of immunosuppressive therapy in myocarditis: A 30-year systematic review and meta analysis. Autoimmun. Rev. 2021, 20, 102710. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, R.N.; Aaron, S.; Sivadasan, A.; Devasahayam, S.; Sebastin, A.; Alexander, M. The Spectrum of Autonomic Dysfunction in Myasthenic Crisis. Ann. Indian Acad. Neurol. 2018, 21, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Rzepiński, Ł.; Zawadka-Kunikowska, M.; Newton, J.L.; Zalewski, P. Cardiac Autonomic Dysfunction in Myasthenia Gravis and Relapsing-Remitting Multiple Sclerosis—A Pilot Study. J. Clin. Med. 2021, 10, 2173. [Google Scholar] [CrossRef] [PubMed]
- Zawadka-Kunikowska, M.; Rzepiński, Ł.; Cieślicka, M.; Klawe, J.J.; Tafil-Klawe, M. Alterations in short-term blood pressure variability related to disease severity and autonomic symptoms in myasthenia gravis patients. Neurol. Sci. 2023, 28. [Google Scholar] [CrossRef]
- Cheshire, W.P.; Freeman, R.; Gibbons, C.H.; Cortelli, P.; Wenning, G.K.; Hilz, M.J.; Spies, J.M.; Lipp, A.; Sandroni, P.; Wada, N.; et al. Electrodiagnostic assessment of the autonomic nervous system: A consensus statement endorsed by the American Autonomic Society, American Academy of Neurology, and the International Federation of Clinical Neurophysiology. Clin. Neurophysiol. 2021, 132, 666–682. [Google Scholar] [CrossRef]
- Low, P.A. Composite autonomic scoring scale for laboratory quantification of generalized autonomic failure. Mayo Clin. Proc. 1993, 68, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Sarkis-Onofre, R.; Catalá-López, F.; Aromataris, E.; Lockwood, C. How to properly use the PRISMA Statement. Syst. Rev. 2021, 10, 117. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 15 June 2023).
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. Trim and Fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef]
- Jammoul, M.; Naddour, J.; Madi, A.; Reslan, M.A.; Hatoum, F.; Zeineddine, J.; Abou-Kheir, W.; Lawand, N. Investigating the possible mechanisms of autonomic dysfunction post-COVID-19. Auton. Neurosci. 2023, 245, 103071. [Google Scholar] [CrossRef]
- Spudich, S.; Nath, A. Nervous system consequences of COVID-19. Science 2022, 375, 267–269. [Google Scholar] [CrossRef]
- Adlan, A.M.; Paton, J.F.; Lip, G.Y.; Kitas, G.D.; Fisher, J.P. Increased sympathetic nerve activity and reduced cardiac baroreflex sensitivity in rheumatoid arthritis. J. Physiol. 2017, 595, 967–981. [Google Scholar] [CrossRef]
- Pongratz, G.; Straub, R.H. The sympathetic nervous response in inflammation. Arthritis Res. Ther. 2014, 16, 504. [Google Scholar] [CrossRef] [PubMed]
- Vernino, S.; Adamski, J.; Kryzer, T.J.; Fealey, R.D.; Lennon, V.A. Neuronal nicotinic ACh receptor antibody in subacute autonomic neuropathy and cancer-related syndromes. Neurology 1998, 50, 1806–1813. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Guatimosim, S.; Prado, V.F.; Gros, R.; Prado, M.A.M. Cholinergic activity as a new target in diseases of the heart ashbeel. Mol. Med. 2015, 20, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Barboza, C.A.; Fukushima, A.R.; Carrozzi, N.; Machi, J.F.; Dourado, P.M.M.; Mostarda, C.T.; Irigoyen, M.C.; Nathanson, L.; Morris, M.; Caperuto, E.C.; et al. Cholinergic Stimulation by Pyridostigmine Bromide Before Myocardial Infarction Prevent Cardiac and Autonomic Dysfunction. Sci. Rep. 2019, 9, 2481. [Google Scholar] [CrossRef]
- Gardim, C.B.; Veiga, A.C.; Aguilar, B.A.; Philbois, S.V.; Souza, H.C.D. Effects of chronic cholinergic stimulation associated with aerobic physical training on cardiac morphofunctional and autonomic parameters in spontaneously hypertensive rats. Sci. Rep. 2021, 11, 17141. [Google Scholar] [CrossRef]
- Robinson, T.G.; Carr, S.J. Cardiovascular autonomic dysfunction in uremia. Kidney Int. 2002, 62, 1921–1932. [Google Scholar] [CrossRef]
- Elezaby, A.; Dexheimer, R.; Sallam, K. Cardiovascular effects of immunosuppression agents. Front. Cardiovasc. Med. 2022, 9, 981838. [Google Scholar] [CrossRef]
- Dias, R.M.; Hoshi, R.A.; Vanderlei, L.C.M.; Monteiro, C.B.d.M.; Alvarez, M.P.B.; Crocetta, T.B.; Grossklauss, L.F.; Fernani, D.C.G.L.; Dantas, M.T.A.P.; Martins, F.P.A.; et al. Influence of Different Types of Corticosteroids on Heart Rate Variability of Individuals with Duchenne Muscular Dystrophy—A Pilot Cross Sectional Study. Life 2021, 11, 752. [Google Scholar] [CrossRef]
- Kucera, P.; Goldenberg, Z.; Kurca, E. Sympathetic skin response: Review of the method and its clinical use. Bratisl Lek List. 2004, 105, 108–116. [Google Scholar]
- Emmer, A.; Mangalo, S.; Kornhuber, M.E. Augmentation of the sympathetic skin response after electrical train stimuli. Front. Neurol. 2012, 3, 152. [Google Scholar] [CrossRef]
- Hubli, M.; Krassioukov, A.V. How reliable are sympathetic skin responses in subjects with spinal cord injury? Clin. Auton. Res. 2015, 25, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Gronda, E.; Dusi, V.; D’elia, E.; Iacoviello, M.; Benvenuto, E.; Vanoli, E. Sympathetic activation in heart failure. Eur. Heart J. Suppl. 2022, 24, E4–E11. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.-H.; Ihm, S.-H.; Lee, D.-H.; Choi, Y.; Chung, W.-B.; Jung, H.O.; Hong, K.-S.; Youn, H.-J. Prehypertension is a comorbid state with autonomic and metabolic dysfunction. J. Clin. Hypertens. 2018, 20, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Carnethon, M.R.; Jacobs, D.R.; Sidney, S.; Liu, K. Influence of autonomic nervous system dysfunction on the development of type 2 diabetes: The CARDIA study. Diabetes Care 2003, 26, 3035–3041. [Google Scholar] [CrossRef]
- Harris, L.; Graham, S.; MacLachlan, S.; Exuzides, A.; Jacob, S. A retrospective longitudinal cohort study of the clinical burden in myasthenia gravis. BMC Neurol. 2022, 22, 172. [Google Scholar] [CrossRef]
- Lehnerer, S.; Jacobi, J.; Schilling, R.; Grittner, U.; Marbin, D.; Gerischer, L.; Stascheit, F.; Krause, M.; Hoffmann, S.; Meisel, A. Burden of disease in myasthenia gravis: Taking the patient’s perspective. J. Neurol. 2022, 269, 3050–3063. [Google Scholar] [CrossRef]
- La Rovere, M.T.; Pinna, G.D.; Hohnloser, S.H.; Marcus, F.I.; Mortara, A.; Nohara, R.; Bigger, J.T., Jr.; Camm, A.J.; Schwartz, P.J. Autonomic Tone and Reflexes after Myocardial Infarcton. Baroreflex sensitivity and heart rate variability in the identification of patients at risk for life-threatening arrhythmias: Implications for clinical trials. Circulation 2001, 103, 2072–2077. [Google Scholar] [CrossRef]
- Rice, J.B.; White, A.G.; Scarpati, L.M.; Wan, G.; Nelson, W.W. Long-term systemic corticosteroid exposure: A systematic literature review. Clin. Ther. 2017, 39, 2216–2229. [Google Scholar] [CrossRef]
- Ruiter, A.M.; Strijbos, E.; de Meel, R.H.; Lipka, A.F.; Raadsheer, W.F.; Tannemaat, M.R.; Verschuuren, J.J. Accuracy of patient-reported data for an online patient registry of autoimmune myasthenia gravis and Lambert-Eaton myasthenic syndrome. Neuromuscul. Disord. 2021, 31, 622–632. [Google Scholar] [CrossRef]
- Chu, H.-T.; Tseng, C.-C.; Liang, C.-S.; Yeh, T.-C.; Hu, L.-Y.; Yang, A.C.; Tsai, S.-J.; Shen, C.-C. Risk of Depressive Disorders Following Myasthenia Gravis: A Nationwide Population-Based Retrospective Cohort Study. Front. Psychiatry 2019, 10, 481. [Google Scholar] [CrossRef]
- Marto, J.P.; Strambo, D.; Livio, F.; Michel, P. Drugs Associated with Ischemic Stroke: A Review for Clinicians. Stroke 2021, 52, e646–e659. [Google Scholar] [CrossRef] [PubMed]
- Westerberg, E.; Punga, A.R. Mortality rates and causes of death in Swedish Myasthenia Gravis patients. Neuromuscul. Disord. 2020, 30, 815–824. [Google Scholar] [CrossRef] [PubMed]
| Study | Country | Total Population (MG, HC) | Mean Age of Population (SD) | Mean Age of MG (SD) | Mean Age of HC (SD) | Total % Female (MG, HC) | Disease Duration (SD) | Disease Severity, n | % Abs AchR+ | % Thymoma | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nalbantoglu et al., 2021 [15] | Turkey | 59 (29, 30) | 45.95 (14.85) | 47.86 (15.08) | 44.1 (14.64) | 57.58 (55.2; 60) | NR | NR | 51.7 | 10.34 | 6 |
| Kocabas et al., 2018 [10] | Turkey | 60 (30, 30) | 44.8 (11.92) | 45.9 (12.08) | 43.77 (11.1) | 48.45 (50; 46.7) | 8.6 (6.3) | Remission: n = 12 MGFA I: n = 4 MGFA IIa: n = 9 MGFA IIb: n = 2 MGFA IIIb: n = 0 MGFA IIIa: 2 MGFA IVa: n = 1 | 73,9 | 26.66 | 7 |
| Shukla et al., 2013 [12] | India | 302 (61, 241) | NR | NR | NR | NR | NR | NR | NR | 6 | 5 |
| Elsais, 2022 [14] | Norway | 34 (17, 17) | 45 (13.8) | 45 (14) | 45 (14.0) | 58.8 (58.8; 58.8) | 13 (11) | Remission: n = 10 MGFA I: n = 7 MGFA IIa: n = 0 MGFA IIb: n = 0 MGFA IIIb: n = 0 MGFA IIIa: n = 0 MGFA IVa: n = 0 | NR | 11.76 | 6 |
| Puneeth et al., 2013 [13] | India | 60 (30, 30) | 36.05 (13.3) | 36.2 (13.6) | 35.9 (13.3) | 60 (60; 60) | NR | Remission: n = 4 Osserman I: n = 2 Osserman IIa, n = 24 | 66.7 | 6.66 | 6 |
| Peric et al., 2011 [9] | Serbia | 42 (21, 21) | 52.9 (10.8) | 53.2 (9.9) | 52.7 (11.9) | 47.6 (47.6; 47.6) | 7,4 (6) | Remission: n = 7 MGFA I: n =1 MGFA IIa: n = 2 MGFA IIb: n = 9 MGFA IIIa: n = 1 MGFA IIIb: n = 1 MGFA IVa: n = 0 | NR | 100 | 6 |
| Nicolic et al., 2014 [8] | Serbia | 130 (75, 55) | 46.80 (13.88) | 46.57 (14.42) | 47.12 (13.25) | 75.1 (75.3; 74.9) | 6.65 (6.32) | Remission: n = 13 MGFA I: n = 24 MGFA IIa: n = 0 MGFA IIb: n = 26 MGFA IIIa: n = 7 MGFA IIIb: n = 5 MGFA IVa: n = 0 | 69.3 | 27 | 6 |
| Zawadka-Kunikowska et al., 2022 [11] | Poland | 68 (38, 30) | 41.1 (9.9) | 42.8 (11.07) | 39 (8) | 81.75 (86.8; 76.7) | 4.4 (2.7) | Remission: n = 0 MGFA I: n = 8 MGFA IIa: n = 19 MGFA IIb: n = 0 MGFA IIIa:11 MGFA IVa: n = 0 | 60.5 | 2.63 | 7 |
| Study | Diagnostic Tool for Cardiac Autonomic Assessment | Cardiovascular Autonomic Parameters | Main Results |
|---|---|---|---|
| Nalbantoglu et al., 2021 [15] | RRIV Valsalva maneuver | RRIV Valsalva ratio | MG patients (both ocular/generalized) exhibit a subclinical parasympathetic abnormality, which is particularly prominent in the AchR antibody-negative group. |
| Kocabas et al., 2018 [10] | BRS Head-up tilt test HRV; frequency domain Handgrip test DBT Valsalva maneuver Active standing: HR, sBP, dBP response | Record time: rest, tilt; sBP, dBP, HR; LF, HF, PSD, LF/HF, LF/HF of HRV BRSrest, BRStilt, E/I ratio Valsalva ratio 30:15 ratio HR and BP response to HG Ewing’s battery: percentage of abnormal results | The balance between sympathetic and vagal activity has been disturbed in favor of sympathetic tone and parasympathetic insufficiency has become more prominent. |
| Shukla et al., 2013 [12] | Head-up tilt: tilt 70° Active standing: 3 min Handgrip test: 3 min Valsalva maneuver R-R interval variation | Record time: rest; RRIV Valsalva ratio HR and sBP, dBP response to HG HR and sBP, dBP response on Valsalva maneuver HR and sBP, dBP response on HUTT HR and sBP, dBP on active standing | Sympathetic hyperreactivity among individuals with MG. |
| Elsais, 2022 [14] | Head-up tilt Handgrip test | Record time: rest, during, after; HR, mBP | MG patients experiencing fatigue show higher resting heart rates compared to suitably matched HCs. This distinction is more pronounced in patients who are not using acetylcholinesterase inhibitors. |
| Puneeth et al., 2013 [13] | DBT Handgrip test Valsalva maneuver HRV; frequency and time domains Active standing | Record time: rest; SDNN, rMSSD; LFnu, HFnu, LF/HF E-I difference Valsalva ratio BP response to HG Orthostatic fall in systolic BP | Reduction in the values of HR-based tests, along with a BP-based test (isometric handgrip test), was observed in the study group in comparison to the controls. This reduction indicates a deficiency in parasympathetic activity and a minimal level of sympathetic deficiency. |
| Peric et al., 2011 [9] | Handgrip test DBT Valsalva maneuver Active standing Orthostatic challenge HRV; frequency and time domains BRS | Record time: rest; LFnu, HFnu, LF/HF of HRV SDNN, SDANN, SDNN index, rMSSD, pNN50 Mean R-R interval BRSrest Ewing’s battery: percentage of abnormal results | Predominantly, there is parasympathetic cardiac impairment in individuals with MG and thymoma. |
| Nicolic et al., 2014 [8] | Handgrip test Active standing Orthostatic hypotension test Valsalva maneuver BRS HRV; frequency and time domains DBT | Record time: rest HR, sBP, dBP LFnu, HFnu, LF, HF, LF/HF ratio of HRV, PSD of HRV, mean R-R interval SDANN, SDNN, SDNN index, rMSSD, pNN50 Ewing’s battery: percentage of abnormal results | The most prominent autonomic failure was noted among MG patients with thymoma association. Mild parasympathetic abnormalities were observed in AChR-positive thymoma-negative MG patients. MuSK-positive MG patients showed a mild degree of AD. |
| Zawadka-Kunikowska et al., 2022 [11] | Head-up tilt HRV; frequency domain BPV BRS DBT | Record time: rest, tilt, delta mBP, sBP, dBP, HR LFnu, HFnu, LF, HF, LF/HF, LF/HF of HRV, PSD of HRV LFnu, HFnu, LF, HF, LF/HF, LF/HF of HRV, PSD of sBPV BRSrest, E/I ratio | CAD with predominant parasympathetic dysfunction. |
| Number of Data Sets | Sample MG | Sample C | Effect Size (95%CI) | p | I2 | p of I2 | Publication Bias | |
|---|---|---|---|---|---|---|---|---|
| HRsupine | 5 | 221 | 373 | 0.40 (−0.47; −1.26) | 0.37 | 94.8 | <0.001 | N/A |
| sBPsupine | 4 | 204 | 356 | 0.39 (0.09; −0.68) | 0.01 | 56.1 | 0.09 | No |
| dBPsupine | 4 | 204 | 356 | −0.08 (−0.42; −0.27) | 0.66 | 68.1 | 0.013 | No |
| mBPsupine | 2 | 55 | 47 | 0.25 (−0.14, 0.66) | 0.20 | 0.0 | 0.412 | N/A |
| LFnusupine | 4 | 164 | 136 | 1.34 (−0.88, 3.55) | 0.24 | 98.5 | <0.001 | No |
| HFnusupine | 4 | 164 | 134 | −2.81 (−7.72, 2.10) | 0.26 | 99.7 | <0.001 | No |
| LFsupine | 2 | 68 | 60 | 0.39 (−0.39, 1.17) | 0.33 | 79.2 | 0.028 | N/A |
| HFsupine | 2 | 68 | 60 | −0.40 (−1.16, 0.36) | 0.30 | 77.9 | 0.033 | N/A |
| LF/HFsupine | 3 | 68 | 60 | 1.80 (−1.04, 4.65) | 0.21 | 98.4 | <0.001 | No |
| LF/HF-RRIsupine | 4 | 164 | 136 | 0.44 (0.21, 068) | <0.001 | 0 | 0.79 | No |
| RRIsupine | 2 | 96 | 76 | 0.16( −0.14, –0.47 | 0.66 | 61.7 | 0.11 | N/A |
| PSD-RRIsupine | 2 | 68 | 60 | −0.11 (−0.46, 0.24) | 0.55 | 0 | 0.47 | N/A |
| SDNN | 3 | 126 | 106 | −1.20 (−3.13, 0.73) | 0.22 | 97.2 | <0.001 | No |
| SDANN | 2 | 96 | 76 | 0.19 (−0.26, 064) | 0.41 | 44.5 | 0.179 | N/A |
| rMSSD | 3 | 126 | 106 | −1.94 (−3.57, −0.32) | 0.02 | 95.1 | <0.001 | No |
| pNN50 | 2 | 96 | 76 | −1.2 (−3.134, 0.735) | 0.004 | 82.8 | 0.016 | N/A |
| SDNN index | 2 | 96 | 76 | −0.83 (−1.37, −0.28) | 0.003 | 55.5 | 0.13 | N/A |
| BRSsupine | 4 | 136 | 164 | −0.56 (−0.80, −0.33) | <0.001 | 0.3 | 0.49 | No |
| HRtilt | 4 | 146 | 318 | 0.19 (−0.57–0.95) | 0.63 | 90.5 | <0.001 | No |
| sBPtilt | 3 | 129 | 301 | 0.34 (−0.27–0.94) | 0.28 | 83.8 | 0.04 | No |
| dBPtilt | 3 | 129 | 301 | 0.04 (−0.61–0.69) | 0.90 | 85.7 | 0.004 | No |
| mBPtilt | 2 | 55 | 47 | −0.04 (−0.92–0.83) | 0.92 | 77.3 | 0.036 | N/A |
| LFtilt | 2 | 68 | 60 | 0.13 (−1.05, 1.32) | 0.83 | 90.9 | <0.001 | N/A |
| HFtilt | 2 | 68 | 60 | −0.75 (−1.11, −0.39) | 0.00 | 0 | 0.98 | N/A |
| LF/HF-RRItilt | 2 | 68 | 60 | 0.86 (0.50, 1.23) | <0.001 | 0. | 0.96 | N/A |
| LF/HFtilt | 2 | 68 | 60 | 0.40 (0.05, 0.75) | 0.02 | 0 | 0.57 | N/A |
| PSD-RRItilt | 2 | 68 | 60 | −0.24 (−0.65, 0.17) | 0.26 | 25.8 | 0.246 | N/A |
| Valsalva ratio | 3 | 120 | 301 | −0.22 (−0.69–0.25) | 0.36 | 72.2 | 0.045 | No |
| E/I ratio | 2 | 68 | 60 | −0.45 (−0.80, −0.09) | 0.01 | 74.7 | 0.047 | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zawadka-Kunikowska, M.; Rzepiński, Ł.; Tafil-Klawe, M.; Veronese, N.; Barbagallo, M.; Habek, M.; Gilhus, N.E. Altered Cardiac Autonomic Regulation in Individuals with Myasthenia Gravis—A Systematic Review and Meta-Analysis. Neurol. Int. 2023, 15, 1140-1154. https://doi.org/10.3390/neurolint15030071
Zawadka-Kunikowska M, Rzepiński Ł, Tafil-Klawe M, Veronese N, Barbagallo M, Habek M, Gilhus NE. Altered Cardiac Autonomic Regulation in Individuals with Myasthenia Gravis—A Systematic Review and Meta-Analysis. Neurology International. 2023; 15(3):1140-1154. https://doi.org/10.3390/neurolint15030071
Chicago/Turabian StyleZawadka-Kunikowska, Monika, Łukasz Rzepiński, Małgorzata Tafil-Klawe, Nicola Veronese, Mario Barbagallo, Mario Habek, and Nils E. Gilhus. 2023. "Altered Cardiac Autonomic Regulation in Individuals with Myasthenia Gravis—A Systematic Review and Meta-Analysis" Neurology International 15, no. 3: 1140-1154. https://doi.org/10.3390/neurolint15030071
APA StyleZawadka-Kunikowska, M., Rzepiński, Ł., Tafil-Klawe, M., Veronese, N., Barbagallo, M., Habek, M., & Gilhus, N. E. (2023). Altered Cardiac Autonomic Regulation in Individuals with Myasthenia Gravis—A Systematic Review and Meta-Analysis. Neurology International, 15(3), 1140-1154. https://doi.org/10.3390/neurolint15030071







