All Roads Lead to the Gut: The Importance of the Microbiota and Diet in Migraine
Abstract
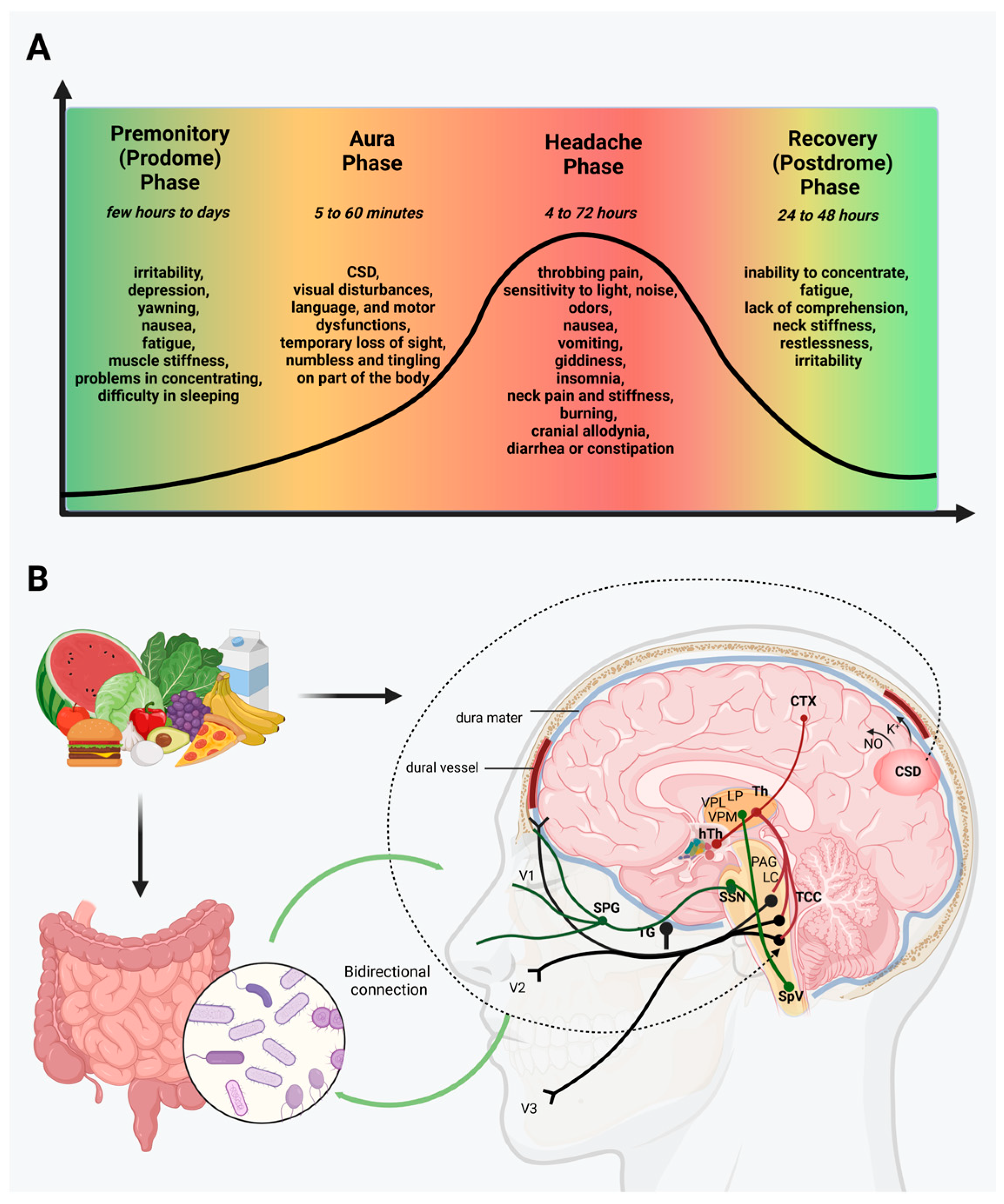
1. Migraine
2. Gut Microbiota and Brain
3. Migraine, the Microbiome, and GI Disorders
4. Migraine and Diets
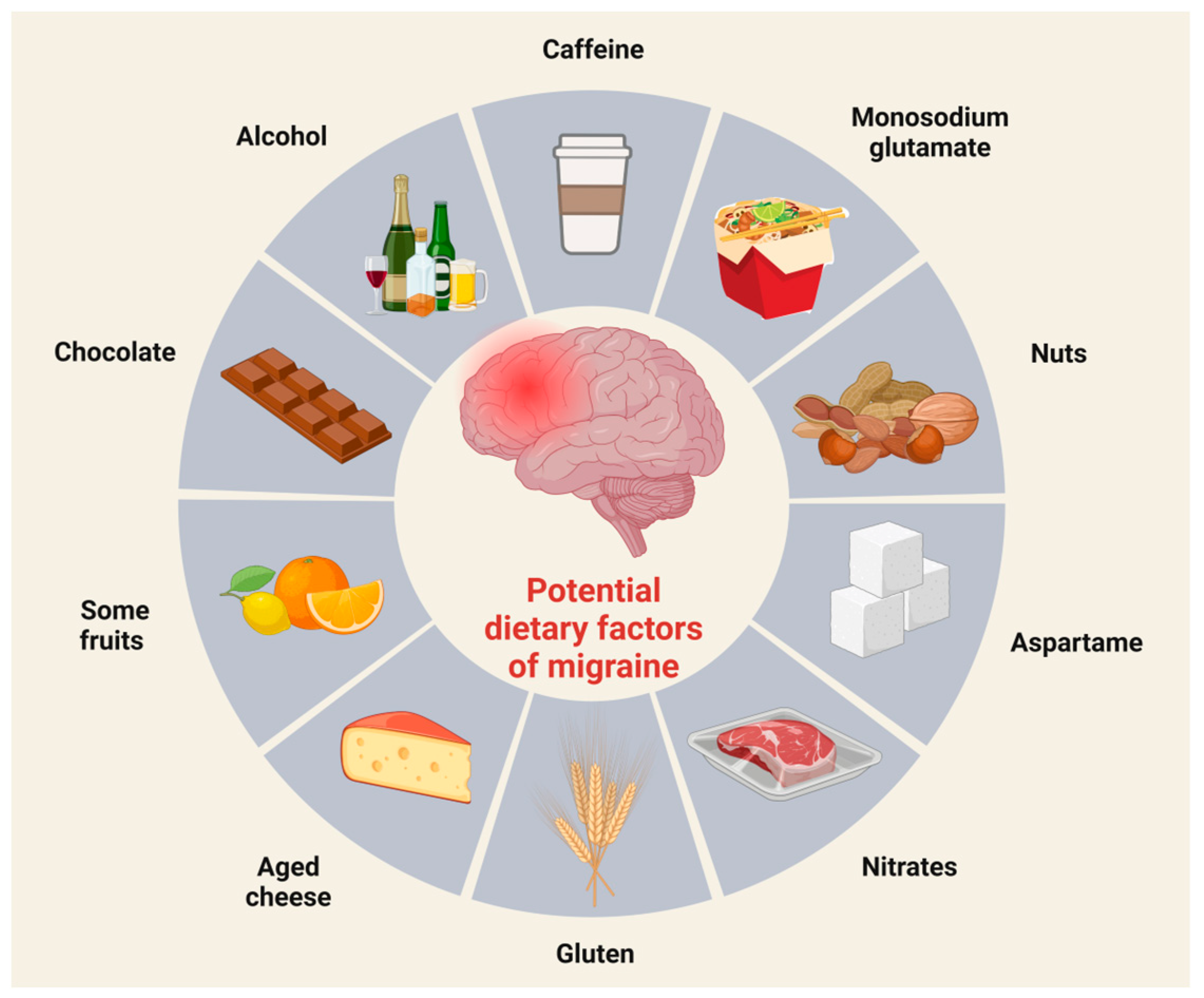
4.1. Nutrition and Dietary Triggers
Potential Dietary Triggers in Migraine
4.2. Diets
4.2.1. Elimination Diets
4.2.2. Migraine and Other Targeted Diets
4.3. Pre- and Probiotics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HT | serotonin |
| Ach | acetylcholine |
| BMI | body mass index |
| CD | celiac disease |
| CGRP | calcitonin gene-related peptide |
| CNS | central nervous system |
| CRH | corticotrophin-releasing hormone |
| CTX | cortex |
| CSD | cortical spreading depression |
| DASH | Dietary Approaches to Stop Hypertension |
| DMV | dorsal motor nucleus of the vagus |
| eNOS | endothelial nitric oxide synthase |
| GABA | gamma-aminobutyric acid |
| GI | gastrointestinal |
| hTh | hypothalamus |
| IBD | inflammatory bowel disease |
| IBS | irritable bowel syndrome |
| IL | interleukin |
| LC | locus coeruleus |
| LP | lateral posterior nucleus |
| LPS | lipopolysaccharides |
| MIND | Mediterranean-DASH Intervention for Neurodegenerative Delay |
| NKA | neurokinin A |
| NO | nitric-oxide |
| NPY | neuropeptide Y |
| PACAP | pituitary adenylate cyclase-activating polypeptide |
| PAG | periaqueductal grey matter |
| PN | personalized or precision nutrition |
| SCFAs | short-chain fatty acids |
| SP | substance P |
| SPG | sphenopalatine ganglion |
| SpV | spinal trigeminal nucleus caudalis |
| SSN | superior salivatory nucleus |
| TCC | trigeminocervical complex |
| TG | trigeminal ganglion |
| Th | thalamus |
| TNC | trigeminal nucleus caudalis |
| TNF-α | tumor necrosis factor-α |
| V1 | ophthalmic nerve |
| V2 | maxillary nerve |
| V3 | mandibular nerve |
| VIP | vasoactive intestinal peptide |
| VPL | ventral posterolateral nucleus |
| VPM | ventral posteromedial nucleus |
References
- Lipton, R.B.; Bigal, M.E.; Steiner, T.J.; Silberstein, S.D.; Olesen, J. Classification of primary headaches. Neurology 2004, 63, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition, (beta version). Cephalalgia 2013, 33, 629–808. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef] [PubMed]
- Al Ghadeer, H.A.; AlSalman, S.A.; Albaqshi, F.M.; Alsuliman, S.R.; Alsowailem, F.A.; Albusror, H.A.; AlAbdi, Z.I.; Alwabari, E.M.; Alturaifi, Z.A.; AlHajji, A.M. Quality of Life and Disability Among Migraine Patients: A Single-Center Study in AlAhsa, Saudi Arabia. Cureus 2021, 13, e19210. [Google Scholar] [CrossRef] [PubMed]
- Goadsby, P.J.; Holland, P.R.; Martins-Oliveira, M.; Hoffmann, J.; Schankin, C.; Akerman, S. Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol. Rev. 2017, 97, 553–622. [Google Scholar] [CrossRef] [PubMed]
- Schulte, L.H.; May, A. The migraine generator revisited: Continuous scanning of the migraine cycle over 30 days and three spontaneous attacks. Brain 2016, 139 Pt 7, 1987–1993. [Google Scholar] [CrossRef]
- Karsan, N.; Bose, P.; Goadsby, P.J. The Migraine Premonitory Phase. Contin. Lifelong Learn. Neurol. 2018, 24, 996–1008. [Google Scholar] [CrossRef]
- Lai, J.; Dilli, E. Migraine Aura: Updates in Pathophysiology and Management. Curr. Neurol. Neurosci. Rep. 2020, 20, 17. [Google Scholar] [CrossRef]
- Chen, P.K.; Wang, S.J. Non-headache symptoms in migraine patients. F1000Research 2018, 7, 188. [Google Scholar] [CrossRef]
- Qubty, W.; Patniyot, I. Migraine Pathophysiology. Pediatr. Neurol. 2020, 107, 1–6. [Google Scholar] [CrossRef]
- Moskowitz, M.A.; Reinhard, J.F., Jr.; Romero, J.; Melamed, E.; Pettibone, D.J. Neurotransmitters and the fifth cranial nerve: Is there a relation to the headache phase of migraine? Lancet 1979, 2, 883–885. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Levy, D.; Kainz, V.; Noseda, R.; Jakubowski, M.; Burstein, R. Activation of central trigeminovascular neurons by cortical spreading depression. Ann. Neurol. 2011, 69, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, C.; Burstein, R. Sensitization of the trigeminovascular pathway: Perspective and implications to migraine pathophysiology. J. Clin. Neurol. 2012, 8, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Spekker, E.; Tanaka, M.; Szabó, Á.; Vécsei, L. Neurogenic Inflammation: The Participant in Migraine and Recent Advancements in Translational Research. Biomedicines 2021, 10, 76. [Google Scholar] [CrossRef] [PubMed]
- Borkum, J.M. Brain Energy Deficit as a Source of Oxidative Stress in Migraine: A Molecular Basis for Migraine Susceptibility. Neurochem. Res. 2021, 46, 1913–1932. [Google Scholar] [CrossRef]
- Edvinsson, L. Role of CGRP in Migraine. Handb. Exp. Pharmacol. 2019, 255, 121–130. [Google Scholar] [CrossRef]
- Robblee, J.; Starling, A.J. SEEDS for success: Lifestyle management in migraine. Cleve Clin. J. Med. 2019, 86, 741–749. [Google Scholar] [CrossRef]
- Gazerani, P. Migraine and Diet. Nutrients 2020, 12, 1658. [Google Scholar] [CrossRef]
- Peroutka, S.J. What turns on a migraine? A systematic review of migraine precipitating factors. Curr. Pain Headache Rep. 2014, 18, 454. [Google Scholar] [CrossRef]
- Nowaczewska, M.; Wiciński, M.; Kaźmierczak, W.; Kaźmierczak, H. To Eat or Not to Eat: A Review of the Relationship between Chocolate and Migraines. Nutrients 2020, 12, 608. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Donelan, J.; Kandere-Grzybowska, K.; Konstantinidou, A. The role of mast cells in migraine pathophysiology. Brain Res. Rev. 2005, 49, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, R. Neurogenic inflammation and its role in migraine. Semin. Immunopathol. 2018, 40, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Holzer, P.; Farzi, A. Neuropeptides and the microbiota-gut-brain axis. Adv. Exp. Med. Biol. 2014, 817, 195–219. [Google Scholar] [CrossRef] [PubMed]
- Kohler, D.R.; Goldspiel, B.R. Ondansetron: A serotonin receptor (5-HT3) antagonist for antineoplastic chemotherapy-induced nausea and vomiting. DICP 1991, 25, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Arzani, M.; Jahromi, S.R.; Ghorbani, Z.; Vahabizad, F.; Martelletti, P.; Ghaemi, A.; Sacco, S.; Togha, M.; School of Advanced Studies of the European Headache Federation (EHF-SAS). Gut-brain Axis and migraine headache: A comprehensive review. J. Headache Pain 2020, 21, 15. [Google Scholar] [CrossRef]
- Cámara-Lemarroy, C.R.; Rodriguez-Gutierrez, R.; Monreal-Robles, R.; Marfil-Rivera, A. Gastrointestinal disorders associated with migraine: A comprehensive review. World J. Gastroenterol. 2016, 22, 8149–8160. [Google Scholar] [CrossRef]
- Hindiyeh, N.A.; Zhang, N.; Farrar, M.; Banerjee, P.; Lombard, L.; Aurora, S.K. The Role of Diet and Nutrition in Migraine Triggers and Treatment: A Systematic Literature Review. Headache 2020, 60, 1300–1316. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, J. Role of intestinal microbiota and metabolites on gut homeostasis and human diseases. BMC Immunol. 2017, 18, 2. [Google Scholar] [CrossRef]
- Romijn, J.A.; Corssmit, E.P.; Havekes, L.M.; Pijl, H. Gut-brain axis. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 518–521. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kasper, L.H. The role of microbiome in central nervous system disorders. Brain Behav. Immun. 2014, 38, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Lai, X.; Li, C.; Ding, D.; Wang, Y.; Zhu, Y. The Role of Gut Microbiota in Various Neurological and Psychiatric Disorders-An Evidence Mapping Based on Quantified Evidence. Mediat. Inflamm. 2023, 2023, 5127157. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Tillisch, K.; Gupta, A. Gut/brain axis and the microbiota. J. Clin. Investig. 2015, 125, 926–938. [Google Scholar] [CrossRef]
- Shreiner, A.B.; Kao, J.Y.; Young, V.B. The gut microbiome in health and in disease. Curr. Opin. Gastroenterol. 2015, 31, 69–75. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef]
- Ustianowska, K.; Ustianowski, Ł.; Machaj, F.; Gorący, A.; Rosik, J.; Szostak, B.; Szostak, J.; Pawlik, A. The Role of the Human Microbiome in the Pathogenesis of Pain. Int. J. Mol. Sci. 2022, 23, 13267. [Google Scholar] [CrossRef]
- Amaral, F.A.; Sachs, D.; Costa, V.V.; Fagundes, C.T.; Cisalpino, D.; Cunha, T.M.; Ferreira, S.H.; Cunha, F.Q.; Silva, T.A.; Nicoli, J.R.; et al. Commensal microbiota is fundamental for the development of inflammatory pain. Proc. Natl. Acad. Sci. USA 2008, 105, 2193–2197. [Google Scholar] [CrossRef]
- Strandwitz, P.; Kim, K.H.; Terekhova, D.; Liu, J.K.; Sharma, A.; Levering, J.; McDonald, D.; Dietrich, D.; Ramadhar, T.R.; Lekbua, A.; et al. GABA-modulating bacteria of the human gut microbiota. Nat. Microbiol. 2019, 4, 396–403. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Sandhu, K.; Peterson, V.; Dinan, T.G. The gut microbiome in neurological disorders. Lancet Neurol. 2020, 19, 179–194. [Google Scholar] [CrossRef]
- Baj, A.; Moro, E.; Bistoletti, M.; Orlandi, V.; Crema, F.; Giaroni, C. Glutamatergic Signaling Along the Microbiota-Gut-Brain Axis. Int. J. Mol. Sci. 2019, 20, 1482. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.; Charles, A. Glutamate and Its Receptors as Therapeutic Targets for Migraine. Neurotherapeutics 2018, 15, 361–370. [Google Scholar] [CrossRef]
- Gonzalez, A.; Hyde, E.; Sangwan, N.; Gilbert, J.A.; Viirre, E.; Knight, R. Migraines Are Correlated with Higher Levels of Nitrate-, Nitrite-, and Nitric Oxide-Reducing Oral Microbes in the American Gut Project Cohort. mSystems 2016, 1, e00105-16. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, Q.; Wang, A.; Lin, Z. Structural and Functional Characterization of the Gut Microbiota in Elderly Women with Migraine. Front. Cell. Infect. Microbiol. 2020, 9, 470. [Google Scholar] [CrossRef] [PubMed]
- Aamodt, A.H.; Stovner, L.J.; Hagen, K.; Zwart, J.A. Comorbidity of headache and gastrointestinal complaints. The Head-HUNT Study. Cephalalgia 2008, 28, 144–151. [Google Scholar] [CrossRef]
- Aurora, S.K.; Shrewsbury, S.B.; Ray, S.; Hindiyeh, N.; Nguyen, L. A link between gastrointestinal disorders and migraine: Insights into the gut-brain connection. Headache 2021, 61, 576–589. [Google Scholar] [CrossRef]
- Schuppan, D.; Junker, Y.; Barisani, D. Celiac disease: From pathogenesis to novel therapies. Gastroenterology 2009, 137, 1912–1933. [Google Scholar] [CrossRef]
- Van Hemert, S.; Breedveld, A.C.; Rovers, J.M.; Vermeiden, J.P.; Witteman, B.J.; Smits, M.G.; de Roos, N.M. Migraine associated with gastrointestinal disorders: Review of the literature and clinical implications. Front. Neurol. 2014, 5, 241. [Google Scholar] [CrossRef]
- Chang, F.Y.; Lu, C.L. Irritable bowel syndrome and migraine: Bystanders or partners? J. Neurogastroenterol. Motil. 2013, 19, 301–311. [Google Scholar] [CrossRef]
- Dimitrova, A.K.; Ungaro, R.C.; Lebwohl, B.; Lewis, S.K.; Tennyson, C.A.; Green, M.W.; Babyatsky, M.W.; Green, P.H. Prevalence of migraine in patients with celiac disease and inflammatory bowel disease. Headache 2013, 53, 344–355. [Google Scholar] [CrossRef]
- Crawford, J.; Liu, S.; Tao, F. Gut microbiota and migraine. Neurobiol. Pain 2022, 11, 100090. [Google Scholar] [CrossRef]
- Li, C.; Yu, S.; Li, H.; Zhou, J.; Liu, J.; Tang, W.; Zhang, L. Clinical features and risk factors for irritable bowel syndrome in Migraine patients. Pak. J. Med. Sci. 2017, 33, 720–725. [Google Scholar] [CrossRef]
- Boyle, R.; Behan, P.O.; Sutton, J.A. A correlation between severity of migraine and delayed gastric emptying measured by an epigastric impedance method. Br. J. Clin. Pharmacol. 1990, 30, 405–409. [Google Scholar] [CrossRef]
- Waelkens, J. Dopamine blockade with domperidone: Bridge between prophylactic and abortive treatment of migraine? A dose-finding study. Cephalalgia 1984, 4, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Acosta, A.; Camilleri, M. Prokinetics in gastroparesis. Gastroenterol. Clin. N. Am. 2015, 44, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Friedman, B.W.; Mulvey, L.; Esses, D.; Solorzano, C.; Paternoster, J.; Lipton, R.B.; Gallagher, E.J. Metoclopramide for acute migraine: A dose-finding randomized clinical trial. Ann. Emerg. Med. 2011, 57, 475–482.e1. [Google Scholar] [CrossRef] [PubMed]
- Shakhatreh, M.; Jehangir, A.; Malik, Z.; Parkman, H.P. Metoclopramide for the treatment of diabetic gastroparesis. Expert. Rev. Gastroenterol. Hepatol. 2019, 13, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Kopishinskaya, S.V.; Gustov, A.V. Gluten migraine. Zhurnal Nevrologii Psikhiatrii Imeni SS Korsakova 2015, 115, 13–17. [Google Scholar] [CrossRef]
- Zis, P.; Julian, T.; Hadjivassiliou, M. Headache Associated with Coeliac Disease: A Systematic Review and Meta-Analysis. Nutrients 2018, 6, 1445. [Google Scholar] [CrossRef]
- Mormile, R. Celiac disease and migraine: Is there a common backstage? Int. J. Color. Dis. 2014, 29, 1571. [Google Scholar] [CrossRef][Green Version]
- Voruganti, V.S. Precision Nutrition: Recent Advances in Obesity. Physiology 2023, 38, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Muniesa, G.; Martinez, J.A.; González-Muniesa, P. Precision Nutrition and Metabolic Syndrome Management. Nutrients 2019, 11, 2411. [Google Scholar] [CrossRef] [PubMed]
- Kokavec, A. Migraine: A disorder of metabolism? Med. Hypotheses 2016, 97, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Rainero, I.; Govone, F.; Gai, A.; Vacca, A.; Rubino, E. Is Migraine Primarily a Metaboloendocrine Disorder? Curr. Pain Headache Rep. 2018, 22, 36. [Google Scholar] [CrossRef]
- Martin, V.T.; Vij, B. Diet and Headache: Part 1. Headache J. Head Face Pain 2016, 56, 1543–1552. [Google Scholar] [CrossRef]
- Cairns, B.E. Influence of pro-algesic foods on chronic pain conditions. Expert. Rev. Neurother. 2016, 16, 415–423. [Google Scholar] [CrossRef]
- Scopp, A.L. MSG and hydrolyzed vegetable protein induced headache: Review and case studies. Headache 1991, 31, 107–110. [Google Scholar] [CrossRef]
- Shimada, A.; Cairns, B.E.; Vad, N.; Ulriksen, K.; Pedersen Lynge, A.M.; Svensson, P.; Baad-Hansen, L. Headache and mechanical sensitization of human pericranial muscles after repeated intake of monosodium glutamate (MSG). J. Headache Pain 2013, 14, 2. [Google Scholar] [CrossRef]
- Juliano, L.M.; Griffiths, R.R. A critical review of caffeine withdrawal: Empirical validation of symptoms and signs, incidence, severity, and associated features. Psychopharmacology 2004, 176, 1–29. [Google Scholar] [CrossRef]
- Nehlig, A. Effects of coffee/caffeine on brain health and disease: What should I tell my patients? Pract. Neurol. 2016, 16, 89–95. [Google Scholar] [CrossRef]
- Shapiro, R.E. Caffeine and headaches. Curr. Pain Headache Rep. 2008, 12, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Boardman, H.F.; Thomas, E.; Millson, D.S.; Croft, P.R. Psychological, sleep, lifestyle, and comorbid associations with headache. Headache 2005, 45, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Xing, Y.; Akan, O.D.; Yang, T. Alcohol-Induced Headache with Neuroinflammation: Recent Progress. Fermentation 2023, 9, 184. [Google Scholar] [CrossRef]
- Panconesi, A. Alcohol and migraine: Trigger factor, consumption, mechanisms. A review. J. Headache Pain 2008, 9, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Mattiuzzi, C.; Cervellin, G. Chocolate and migraine: The history of an ambiguous association. Acta Biomed. 2014, 85, 216–221. [Google Scholar] [PubMed]
- Ellam, S.; Williamson, G. Cocoa and human health. Annu. Rev. Nutr. 2013, 33, 105–128. [Google Scholar] [CrossRef] [PubMed]
- Cady, R.J.; Durham, P.L. Cocoa-enriched diets enhance expression of phosphatases and decrease expression of inflammatory molecules in trigeminal ganglion neurons. Brain Res. 2010, 1323, 18–32. [Google Scholar] [CrossRef]
- Magrone, T.; Russo, M.A.; Jirillo, E. Cocoa and Dark Chocolate Polyphenols: From Biology to Clinical Applications. Front. Immunol. 2017, 8, 677. [Google Scholar] [CrossRef]
- Razeghi Jahromi, S.; Togha, M.; Ghorbani, Z.; Hekmatdoost, A.; Khorsha, F.; Rafiee, P.; Shirani, P.; Nourmohammadi, M.; Ansari, H. The association between dietary tryptophan intake and migraine. Neurol. Sci. 2019, 40, 2349–2355. [Google Scholar] [CrossRef]
- Abbey, M.J.; Patil, V.V.; Vause, C.V.; Durham, P.L. Repression of calcitonin gene-related peptide expression in trigeminal neurons by a Theobroma cacao extract. J. Ethnopharmacol. 2008, 115, 238–248. [Google Scholar] [CrossRef]
- Nattagh-Eshtivani, E.; Sani, M.A.; Dahri, M.; Ghalichi, F.; Ghavami, A.; Arjang, P.; Tarighat-Esfanjani, A. The role of nutrients in the pathogenesis and treatment of migraine headaches: Review. Biomed. Pharmacother. 2018, 102, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Sun-Edelstein, C.; Mauskop, A. Foods and supplements in the management of migraine headaches. Clin. J. Pain 2009, 25, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.T.; Behbehani, M.M. Toward a rational understanding of migraine trigger factors. Med. Clin. N. Am. 2001, 85, 911–941. [Google Scholar] [CrossRef]
- Council of Scientific Affairs. Aspartame: Review of safety issues. JAMA 1985, 254, 400–402. [Google Scholar] [CrossRef]
- Van den Eeden, S.K.; Koepsell, T.D.; Longstreth, W.T., Jr.; van Belle, G.; Daling, J.R.; McKnight, B. Aspartame ingestion and headaches: A randomized crossover trial. Neurology 1994, 44, 1787–1793. [Google Scholar] [CrossRef]
- Loehler, S.M.; Glaros, A. The effect of aspartame on migraine headache. Headache 1988, 28, 10–14. [Google Scholar] [CrossRef]
- Ozon, A.O.; Karadas, O.; Ozge, A. Efficacy of diet restriction on migraines. Arch. Neuropsychiatry 2018, 55, 233–237. [Google Scholar] [CrossRef]
- Aydinla, E.I.; Dikmen, P.Y.; Tiftikci, A.; Saruc, M.; Aksu, M.; Gunsoy, H.G.; Tozun, N. IgG-based elimination diet in migraine plus irritable bowel syndrome. Headache 2013, 53, 514–525. [Google Scholar] [CrossRef]
- Jahromi, S.R.; Ghorbani, Z.; Martelletti, P.; Lampl, C.; Togha, M.; On behalf of the School of Advanced Studies of the European Headache Federation (EHF-SAS). Association of diet and headache. J. Headache Pain 2019, 20, 106–111. [Google Scholar] [CrossRef]
- Gross, E.C.; Klement, R.J.; Schoenen, J.; D’Agostino, D.P.; Fischer, D. Potential Protective Mechanisms of Ketone Bodies in Migraine Prevention. Nutrients 2019, 11, 811. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Coppola, G.; Bracaglia, M.; Di Lenola, D.; Evangelista, M.; Sirianni, G.; Rossi, P.; Di Lorenzo, G.; Serrao, M.; Parisi, V.; et al. Cortical functional correlates of responsiveness to short-lasting preventive intervention with ketogenic diet in migraine: A multimodal evoked potentials study. J. Headache Pain 2016, 17, 58. [Google Scholar] [CrossRef] [PubMed]
- Moskatel, L.S.; Zhang, N. Migraine and Diet: Updates in Understanding. Curr. Neurol. Neurosci. Rep. 2022, 22, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Evcili, G.; Utku, U.; Ogun, M.N.; Ozdemir, G. Early and long period follow-up results of low glycemic index diet for migraine prophylaxis. J. Turk. Soc. Algol. 2018, 30, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Hardy, T.M.; Tollefsbol, T.O. Epigenetic diet: Impact on the epigenome and cancer. Epigenomics 2011, 3, 503–518. [Google Scholar] [CrossRef] [PubMed]
- Fila, M.; Pawłowska, E.; Blasiak, J. Mitochondria in migraine pathophysiology—Does epigenetics play a role? Arch. Med. Sci. 2019, 15, 944–956. [Google Scholar] [CrossRef]
- Thompson, D.F.; Saluja, H.S. Prophylaxis of migraine headaches with riboflavin: A systematic review. J. Clin. Pharm. Ther. 2017, 42, 394–403. [Google Scholar] [CrossRef]
- Daniel, O.; Mauskop, A. Nutraceuticals in acute and prophylactic treatment of migraine. Curr. Treat. Options Neurol. 2016, 18, 14. [Google Scholar] [CrossRef]
- Marashly, E.T.; Bohlega, S.A. Riboflavin has neuroprotective potential: Focus on Parkinson’s disease and migraine. Front. Neurol. 2017, 8, 333. [Google Scholar] [CrossRef]
- Namazi, N.; Heshmati, J.; Tarighat-Esfanjani, A. Supplementation with riboflavin (Vitamin B2) for migraine prophylaxis in adults and children: A review. Int. J. Vitam. Nutr. Res. 2015, 85, 79–87. [Google Scholar] [CrossRef]
- Schoenen, J.; Jacquy, J.; Lenaerts, M. Effectiveness of high-dose riboflavin in migraine prophylaxis. A randomized controlled trial. Neurology 1998, 50, 466–470. [Google Scholar] [CrossRef]
- Karimi, N.; Tavakoli, M.; Charati, J.Y.; Shamsizade, M. Single-dose intravenous sodium valproate (Depakine) versus dexamethasone for the treatment of acute migraine headache: A double-blind randomized clinical trial. Clin. Exp. Emerg. Med. 2017, 4, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ma, T.; Che, X.; Wang, Q.; Yu, S. The efficacy of venlafaxine, flunarizine, and valproic acid in the prophylaxis of vestibular migraine. Front. Neurol. 2017, 8, 524. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, A.; Arimondo, P.B.; Rots, M.G.; Jeronimo, C.; Berdasco, M. The timeline of epigenetic drug discovery: From reality to dreams. Clin. Epigenet. 2019, 11, 17. [Google Scholar] [CrossRef]
- Passaro, D.; Rana, G.; Piscopo, M.; Viggiano, E.; De Luca, B.; Fucci, L. Epigenetic chromatin modifications in the cortical spreading depression. Brain Res. 2010, 1329, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gazerani, P. Current Evidence on the Role of Epigenetic Mechanisms in Migraine: The Way Forward to Precision Medicine. OBM Genet. 2018, 2, 1. [Google Scholar] [CrossRef]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Barnes, L.L.; Bennett, D.A.; Aggarwal, N.T. MIND diet slows cognitive decline with aging. Alzheimers Dement. 2015, 11, 1015–1022. [Google Scholar] [CrossRef]
- Askarpour, M.; Yarizadeh, H.; Sheikhi, A.; Khorsha, F.; Mirzaei, K. Associations between adherence to MIND diet and severity, duration and frequency of migraine headaches among migraine patients. BMC Res. Notes 2020, 13, 341. [Google Scholar] [CrossRef] [PubMed]
- Escott-Stump, S. Nutrition and Diagnosis-Related Care; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011. [Google Scholar]
- Deen, M.; Christensen, C.E.; Hougaard, A.; Hansen, H.D.; Knudsen, G.M.; Ashina, M. Serotonergic mechanisms in the migraine brain—A systematic review. Cephalalgia 2017, 37, 251–264. [Google Scholar] [CrossRef]
- Gasparini, C.F.; Smith, R.A.; Griffiths, L.R. Genetic and biochemical changes of the serotonergic system in migraine pathobiology. J. Headache Pain 2017, 18, 20. [Google Scholar] [CrossRef]
- Curto, M.; Lionetto, L.; Fazio, F.; Mitsikostas, D.D.; Martelletti, P. Fathoming the kynurenine pathway in migraine: Why understanding the enzymatic cascades is still critically important. Intern. Emerg. Med. 2015, 10, 413–421. [Google Scholar] [CrossRef]
- Nagy-Grócz, G.; Laborc, K.F.; Veres, G.; Bajtai, A.; Bohár, Z.; Zádori, D.; Fejes-Szabó, A.; Spekker, E.; Vécsei, L.; Párdutz, Á. The Effect of Systemic Nitroglycerin Administration on the Kynurenine Pathway in the Rat. Front. Neurol. 2017, 8, 278. [Google Scholar] [CrossRef] [PubMed]
- Sicuteri, F. The ingestion of serotonin precursors (L-5-hydroxytryptophan and L-tryptophan) improves migraine headache. Headache 1973, 13, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Gedye, A. Hypothesized treatment for migraines using low doses of tryptophan, niacin, calcium, caffeine, and acetylsalicylic acid. Med. Hypotheses 2001, 56, 91–94. [Google Scholar] [CrossRef]
- Roberfroid, M. Prebiotics: The concept revisited. J. Nutr. 2007, 137 (Suppl. 2), 830S–837S. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Gazerani, P. Probiotics for Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 4121. [Google Scholar] [CrossRef]
- Galland, L. The Gut Microbiome and the Brain. J. Med. Food 2014, 17, 1261–1272. [Google Scholar] [CrossRef]
- Saberi, A.; Nemati, S.; Shakib, R.J.; Kazemnejad, E.; Maleki, M. Association between allergic rhinitis and migraine. J. Res. Med. Sci. 2012, 17, 508–512. [Google Scholar]
- Kim, S.Y.; Min, C.; Oh, D.J.; Lim, J.-S.; Choi, H.-G. Bidirectional association between asthma and migraines in adults: Two longitudinal follow-up studies. Sci. Rep. 2019, 9, 18343–18349. [Google Scholar] [CrossRef]
- Ghavami, A.; Khorvash, F.; Heidari, Z.; Khalesi, S.; Askari, G. Effect of synbiotic supplementation on migraine characteristics and inflammatory biomarkers in women with migraine: Results of a randomized controlled trial. Pharmacol. Res. 2021, 169, 105668. [Google Scholar] [CrossRef]
- Martami, F.; Togha, M.; Seifishahpar, M.; Ghorbani, Z.; Ansari, H.; Karimi, T.; Jahromi, S.R. The effects of a multispecies probiotic supplement on inflammatory markers and episodic and chronic migraine characteristics: A randomized double-blind controlled trial. Cephalalgia 2019, 39, 841–853. [Google Scholar] [CrossRef] [PubMed]
- De Roos, N.M.; van Hemert, S.; Rovers, J.M.P.; Smits, M.G.; Witteman, B.J.M. The effects of a multispecies probiotic on migraine and markers of intestinal permeability—Results of a randomized placebo-controlled study. Eur. J. Clin. Nutr. 2017, 71, 1455–1462. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spekker, E.; Nagy-Grócz, G. All Roads Lead to the Gut: The Importance of the Microbiota and Diet in Migraine. Neurol. Int. 2023, 15, 1174-1190. https://doi.org/10.3390/neurolint15030073
Spekker E, Nagy-Grócz G. All Roads Lead to the Gut: The Importance of the Microbiota and Diet in Migraine. Neurology International. 2023; 15(3):1174-1190. https://doi.org/10.3390/neurolint15030073
Chicago/Turabian StyleSpekker, Eleonóra, and Gábor Nagy-Grócz. 2023. "All Roads Lead to the Gut: The Importance of the Microbiota and Diet in Migraine" Neurology International 15, no. 3: 1174-1190. https://doi.org/10.3390/neurolint15030073
APA StyleSpekker, E., & Nagy-Grócz, G. (2023). All Roads Lead to the Gut: The Importance of the Microbiota and Diet in Migraine. Neurology International, 15(3), 1174-1190. https://doi.org/10.3390/neurolint15030073






