An Ecchordosis Physaliphora, a Rare Entity, Involving the Central Nervous System: A Systematic Review of the Literature
Abstract
1. Introduction
2. Methods
2.1. Study Protocol and Search Strategy
2.2. Study Selection Process
2.3. Quality Assessment and Data Acquisition
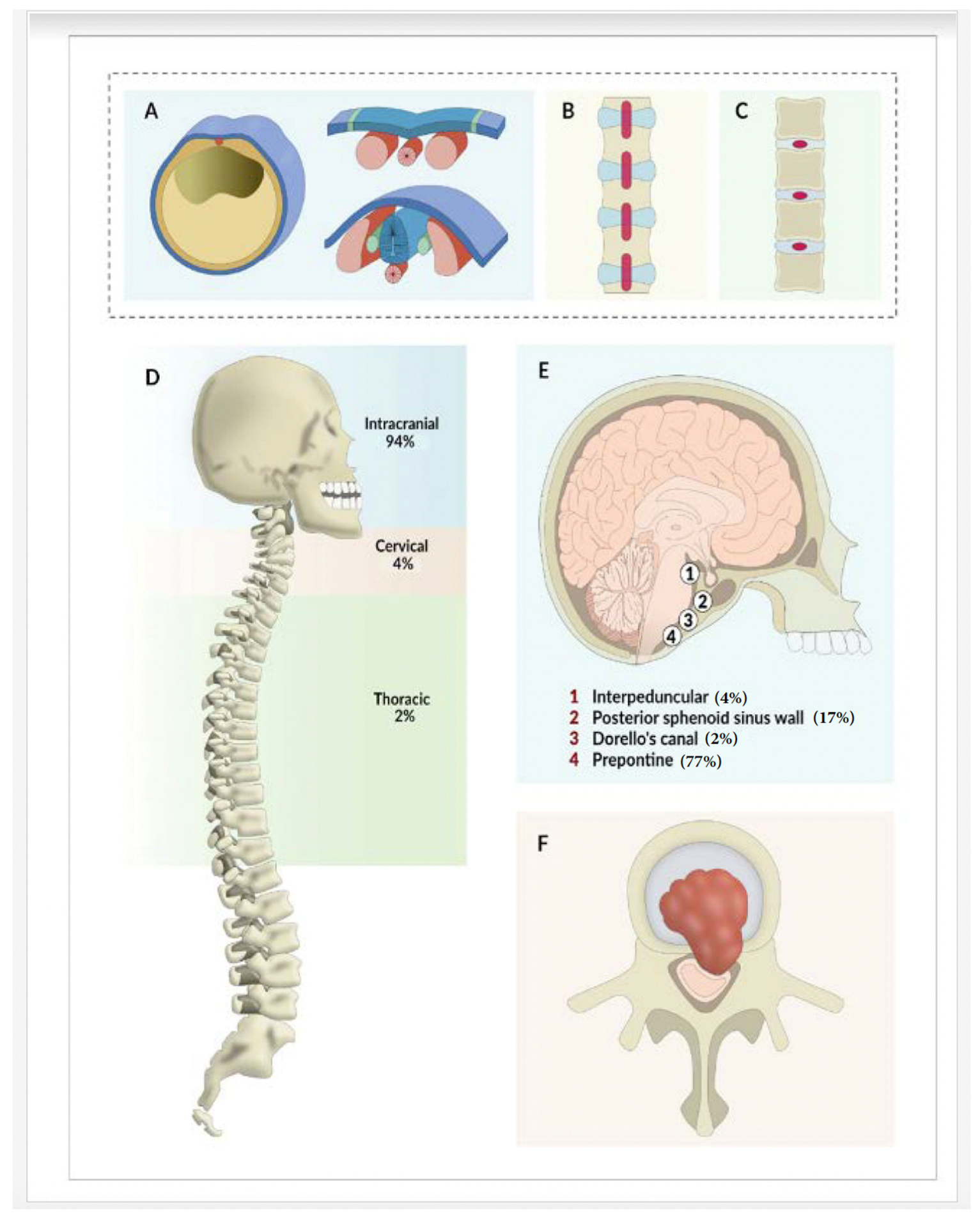
3. Results
4. Discussion
4.1. Classification
4.2. Common Presentations
4.3. Diagnosis of EP
4.3.1. Imaging
4.3.2. Immunohistochemical and Histopathological Analysis
4.3.3. Management and Outcomes
4.3.4. Differential Diagnoses
4.3.5. Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Macdonald, R.L.; Cusimano, M.D.; Deck, J.H.; Gullane, P.J.; Dolan, E.J. Cerebrospinal fluid fistula secondary to Ecchordosis Physaliphora. Neurosurgery 1990, 26, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Stam, F.C.; Kamphorst, W. Ecchordosis Physaliphora as a cause of fatal pontine hemorrhage. Eur. Neurol. 1982, 21, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Lagman, C.; Varshneya, K.; Sarmiento, J.M.; Turtz, A.R.; Chitale, R.V. Proposed Diagnostic Criteria, Classification Schema, and Review of Literature of Notochord-Derived Ecchordosis Physaliphora. Cureus 2016, 8, e547. [Google Scholar] [CrossRef] [PubMed]
- Park, H.H.; Lee, K.-S.J.; Ahn, S.H.; Suh, S.; Hong, C.K. Ecchordosis Physaliphora: Typical and atypical radiologic features. Neurosurg. Rev. 2016, 40, 87–94. [Google Scholar] [CrossRef]
- Ahn, S.S.; Han, J. Ecchordosis Physaliphora presenting with abducens nerve palsy. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2016, 20, 266–268. [Google Scholar] [CrossRef]
- Bolzoni-Villaret, A.; Stefini, R.; Fontanella, M.; Bottazzoli, M.; Turri Zanoni, M.; Pistochini, A.; Castelnuovo, P.; Nicolai, P. Transnasal endoscopic resection of symptomatic Ecchordosis Physaliphora. Laryngoscope 2014, 124, 1325–1328. [Google Scholar] [CrossRef]
- Reddy, T.; Perez, C.; Haque, A.; Samant, R.; Gupta, R.K. Steroid-Responsive Ecchordosis Physaliphora Diagnosed as CLIPPERS: A Systematic Review of Literature and Case Report (P1-1.Virtual). Neurology 2022, 98 (Suppl. S18), 3339. [Google Scholar]
- Adib, S.D.; Bisdas, S.; Bornemann, A.; Schuhmann, M.U. Neuroendoscopic Trans-Third Ventricular Approach for Surgical Management of Ecchordosis Physaliphora. World Neurosurg. 2016, 90, 701. [Google Scholar] [CrossRef]
- Akimoto, J.; Takeda, H.; Hashimoto, T.; Haraoka, J.; Ito, H. A surgical case of Ecchordosis Physaliphora. No ShinkeiGeka. Neurol. Surg. 1996, 24, 1021–1025. (In Japanese) [Google Scholar]
- Alkan, O.; Yildirim, T.; Kizilkiliç, O.; Tan, M.; Cekinmez, M. A case of Ecchordosis Physaliphora presenting with an intratumoralhemorrhage. Turk. Neurosurg. 2009, 19, 293–296. [Google Scholar]
- Alli, A.; Clark, M.; Mansell, N. Cerebrospinal Fluid Rhinorrhea Secondary to Ecchordosis Physaliphora. Skull Base 2008, 18, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.T.; Jarrahy, R.; Yong, W.H.; Eby, T.; Shahinian, H.K. A Rare Symptomatic Presentation of Ecchordosis Physaliphora and Unique Endoscope-Assisted Surgical Management. Minim. Invasive Neurosurg. 2002, 45, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Choudhri, O.; Feroze, A.; Hwang, P.; Vogel, H.; Ajlan, A.; Harsh, G. Endoscopic Resection of a Giant Intradural RetroclivalEcchordosis Physaliphora: Surgical Technique and Literature Review. World Neurosurg. 2014, 82, 912.e21. [Google Scholar] [CrossRef] [PubMed]
- Derakhshani, A.; Livingston, S.; William, C.; Lieberman, S.; Young, M.; Pacione, D.; Dehkharghani, S. Spontaneous, Intrasphenoidal Rupture of Ecchordosis Physaliphora with Pneumocephalus Captured During Serial Imaging and Clinical Follow-Up: Pathoanatomic Features and Management. World Neurosurg. 2020, 141, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Dias, L.A.; Nakanishi, M.; Mangussi-Gomes, J.; Canuto, M.; Takano, G.; Oliveira, C.A. Successful endoscopic endonasal management of a transclival cerebrospinal fluid fistula secondary to Ecchordosis Physaliphora—An ectopic remnant of primitive notochord tissue in the clivus. Clin. Neurol. Neurosurg. 2014, 117, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, C.; Clarke, D.B.; Sinha, N.; Shankar, J.J.S. A Case Study of Symptomatic RetroclivalEcchordosis Physaliphora: CT and MR Imaging. Can. J. Neurol. Sci./J. Can. Des. Sci. Neurol. 2016, 43, 210–212. [Google Scholar] [CrossRef]
- Filis, A.; Kalakoti, P.; Nanda, A. Symptomatic Ecchordosis Physaliphora mimicking as an intracranial arachnoid cyst. J. Clin. Neurosci. 2016, 28, 171–174. [Google Scholar] [CrossRef]
- Fracasso, T.; Brinkmann, B.; Paulus, W. Sudden death due to subarachnoid bleeding from ecchordosis physaliphora. Int. J. Leg. Med. 2008, 122, 225–227. [Google Scholar] [CrossRef]
- Galloway, L.; Hayhurst, C. Spontaneous cerebrospinal fluid rhinorrhoea with meningitis secondary to Ecchordosis Physaliphora. Br. J. Neurosurg. 2017, 33, 99–100. [Google Scholar] [CrossRef]
- Georgalas, C.; Terzakis, D.; Tsikna, M.; Alatzidou, Z.; de Santi, S.; Seccia, V.; Dallan, I. Ecchordosis Physaliphora: A cautionary tale. J. Laryngol. Otol. 2020, 134, 46–51. [Google Scholar] [CrossRef]
- Ghimire, P.; Shapey, J.; Bodi, I.; Connor, S.; Thomas, N.; Barkas, K. Spontaneous tension pneumocephalus and pneumoventricle in Ecchordosis Physaliphora: Case report of a rare presentation and review of the literature. Br. J. Neurosurg. 2020, 34, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Ilorah, C.; Bond, B.; Kattah, J.; Hassanzadeh, B. A case of abducens nerve palsy in the context of Ecchordosis Physaliphora. Neurology 2017, 88, P3.154. [Google Scholar]
- Indiran, V. Ecchordosis Physaliphora—Classical MRI Image. Neurol. India 2022, 70, 834–835. [Google Scholar] [CrossRef] [PubMed]
- Kaul, S.; Khan, O.H.; Edem, I.; Lwu, S.; Willinsky, R.; Vescan, A.; Zadeh, G. TransclivalPseudomeningocele Secondary to Ecchordosis Physaliphora: Case Report and Literature Review. J. Neurol. Surg. Rep. 2013, 74, 92–95. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Krisht, K.M.; Palmer, C.A.; Osborn, A.G.; Couldwell, W.T. Giant Ecchordosis Physaliphora in an adolescent girl. J. Neurosurg. Pediatr. 2013, 12, 328–333. [Google Scholar] [CrossRef]
- Kurokawa, H.; Miura, S.; Goto, T. Ecchordosis Physaliphora arising from the cervical vertebra, the CT and MRI appearance. Neuroradiology 1988, 30, 81–83. [Google Scholar] [CrossRef]
- Lakhani, D.A.; Martin, D. Ecchordosis Physaliphora: Case report and brief review of the literature. Radiol. Case Rep. 2021, 16, 3937–3939. [Google Scholar] [CrossRef]
- Ling, S.S.; Sader, C.; Robbins, P.; Rajan, G.P. A Case of Giant Ecchordosis Physaliphora. Otol. Neurotol. 2007, 28, 931–933. [Google Scholar] [CrossRef]
- Ang, L.N.; Kew, T.Y.; Toh, C.J.; Isa, M.R. Ecchordosis Physaliphora Masquerading as Chordoma: A Case Report. Hong Kong J. Radiol. 2020, 23, 223–226. [Google Scholar] [CrossRef]
- Magro, G.; Lanza, P.; Bono, F. Ecchordosis Physaliphora presenting as hypnic headache. Neuroradiol. J. 2023, 6, 19714009221150852. [Google Scholar] [CrossRef]
- Miki, T.; Nakajima, N.; Akimoto, J.; Wada, J.; Haraoka, J. Neuroendoscopic Trans-third Ventricle Approach for Lesions of the Ventral Brainstem Surface. Minim. Invasive Neurosurg. 2008, 51, 313–318. [Google Scholar] [CrossRef]
- Miki, K.; Yoshimoto, K.; Nishimura, A.; Suzuki, S.O.; Hiwatashi, A.; Iihara, K. A Case of Ecchordosis Physaliphora in the Prepontine Cistern: A Rare Entity in the Differential Diagnosis of an Epidermoid Cyst. World Neurosurg. 2017, 105, 1033.e11. [Google Scholar] [CrossRef]
- Ng, S.H.; Ko, S.F.; Wan, Y.L.; Tang, L.M.; Ho, Y.S. Cervical Ecchordosis Physaliphora: CT and MR features. Br. J. Radiol. 1998, 71, 329–331. [Google Scholar] [CrossRef]
- Raffa, A. Atypical Presentation and Neuroradiological Features of Giant Ecchordosis Physalyphora in a Seven-Year-Old Patient: A Case Report. Cureus 2022, 14, e23544. [Google Scholar] [CrossRef]
- Rengachary, S.S.; Grotte, D.A.; Swanson, P.E. Extradural Ecchordosis Physalyphora of the Thoracic Spine: Case Report. Neurosurgery 1997, 41, 1198–1202. [Google Scholar] [CrossRef]
- Rodriguez, L.; Colina, J.; Lopez, J.; Molina, O.; Cardozo, J. Intradural prepontine growth: Giant Ecchordosis Physaliphora or extraosseous chordoma? Neuropathology 1999, 19, 336–340. [Google Scholar] [CrossRef]
- Rotondo, M.; Natale, M.; Mirone, G.; Cirillo, M.; Conforti, R.; Scuotto, A. A rare symptomatic presentation of Ecchordosis Physaliphora: Neuroradiological and surgical management. J. Neurol. Neurosurg. Psychiatry 2007, 78, 647–649. [Google Scholar] [CrossRef]
- Castello Ruiz, M.J.; Alsavaf, M.B.; Fadel, M.; Salem, E.H.; Mongkolkul, K.; Naksen, P.; Godil, S.S.; Otto, B.A.; Carrau, R.L.; Prevedello, D.M. Spontaneous rhinorrhea: A possible concealing initial symptom of Ecchordosis Physaliphora. Illustrative case. J. Neurosurg. Case Lessons 2023, 5, CASE236. [Google Scholar] [CrossRef]
- Sarkar, N.; Chakravarthy, S.; Chakravarty, R.; Mukhopadhyay, S. Radiological Diagnosis of a Rare Prepontine Lesion: Ecchordosis Physaliphora. Cureus 2022, 14, e24335. [Google Scholar] [CrossRef]
- Sooltangos, A.; Bodi, I.; Ghimire, P.; Barkas, K.; Al-Barazi, S.; Thomas, N.; Maratos, E.C. Do All Notochordal Lesions Require Proton Beam Radiotherapy? A Proposed Reclassification of Ecchordosis Physaliphora as Benign Notochord Cell Tumor. J. Neurol. Surg. Part B Skull Base 2021, 83 (Suppl. S2), e96–e104. [Google Scholar] [CrossRef]
- Srinivasan, A.; Goyal, M.; Kingstone, M. Case 133: Ecchordosis Physaliphora. Radiology 2008, 247, 585–588. [Google Scholar] [CrossRef]
- Sun, R.; Ajam, Y.; Campbell, G.; Masel, T. A Rare Case of Ecchordosis Physaliphora Presenting with Headache, Abducens Nerve Palsy, and Intracranial Hypertension. Cureus 2020, 12, e8843. [Google Scholar] [CrossRef] [PubMed]
- Takeyama, J.; Hayashi, T.; Shirane, R. Notochordal remnant-derived mass: Ecchordosis Physaliphora or chordoma? Pathology 2006, 38, 599–600. [Google Scholar] [CrossRef] [PubMed]
- Toda, H.; Kondo, A.; Iwasaki, K. Neuroradiological characteristics of Ecchordosis Physaliphora. J. Neurosurg. 1998, 89, 830–834. [Google Scholar] [CrossRef]
- Touska, P.; Skillbeck, C.J.; Clarke, P.M. To scan or not to scan: An unusual case of tinnitus. J. Laryngol. Otol. 2014, 128, 1179–1180. [Google Scholar]
- Veiceschi, P.; Arosio, A.D.; Agosti, E.; Bignami, M.; Pistochini, A.; Cerati, M.; Castelnuovo, P.; Locatelli, D. Symptomatic Ecchordosis Physaliphora of the upper clivus: An exceedingly rare entity. Acta Neurochir. 2021, 163, 2475–2486. [Google Scholar] [CrossRef]
- Watanabe, A.; Yanagita, M.; Ishii, R.; Shirabe, T. Magnetic Resonance Imaging of Ecchordosis Physaliphora—Case Report. Neurol. Med.-Chir. 1994, 34, 448–450. [Google Scholar] [CrossRef]
- Wells, S.; Srinivasan, A. Teaching neuroImages: Incidental retroclival mass in a patient with headache. Neurology. 2010, 75, e93. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yano, S.; Hide, T.; Kuratsu, J.-I. A case of Ecchordosis Physaliphora presenting with an abducens nerve palsy: A rare symptomatic case managed with endoscopic endonasal transsphenoidal surgery. Surg. Neurol. Int. 2013, 4, 13. [Google Scholar]
- Zhong, X.-L.; Huang, B.; Liu, C.; Zhan, S.-Q. Multiple Ecchordosis Physaliphora. Chin. Med. J. 2015, 128, 2826–2828. [Google Scholar] [CrossRef]
- Luschka, H.V. Die altersveränderungen der Zwischenwirbelknorpel. Arch. Pathol. Anat. Physiol. Klin. Med. 1856, 9, 311–327. [Google Scholar] [CrossRef]
- Virchow, R. Untersuschungen über die Entwickelung des Schädelgrundes im Gesunden und Krankhaften zur Tande und über den Einfluss Derselbe auf Schädelform, Gesichtsbildung und Gehirnbau; G Reimer: Berlin, Germany, 1857; p. 47. [Google Scholar]
- Müller, H. Über das Vorkommen von Resten der Chorda Dorsalis bei Menschen nach der Geburt und über ihr Verhältnis zu den Gallertgeschwulsten am Clivus. Z Rat Med. 1858, 2, 202–229. [Google Scholar]
- Ribbert, H. Über die Ecchondrosis Physalifora Sphenooccipitalis. Zentralbl Allg Path. 1894, 5, 457–461. [Google Scholar]
- Chihara, C.; Korogi, Y.; Kakeda, S.; Nishimura, J.; Murakami, Y.; Moriya, J.; Ohnari, N. Ecchordosis Physaliphora and its variants: Proposed new classification based on high-resolution fast MR imaging employing steady-state acquisition. Eur. Radiol. 2013, 23, 2854–2860. [Google Scholar] [CrossRef] [PubMed]
- Ezer, H.; Banerjee, A.D.; Thakur, J.D.; Nanda, A. Dorello’s Canal for Laymen: A Lego-Like Presentation. J. Neurol. Surg. Part B Skull Base 2012, 73, 183–189. [Google Scholar] [CrossRef] [PubMed]
- de Notaris, M.; Cavallo, L.M.; Prats-Galino, A.; Esposito, I.; Benet, A.; Poblete, J.; Valente, V.; Gonzalez, J.B.; Ferrer, E.; Cappabianca, P. Endoscopic endonasal transclival approach and retrosigmoid approach to the clival and petroclivalregions. Neurosurgery 2009, 65 (Suppl. S6), 42–50; discussion 50–52. [Google Scholar] [PubMed]
- Lantos, P.L.; Louis, D.N.; Rosenblum, M.K.; Kleihues, P. Tumours of the nervoussystem. In Greenfield’s Neuropathology, 7th ed.; Graham, D.I., Lantos, P.L., Eds.; Arnold: London, UK, 2002; Volume 2, pp. 767–1052. [Google Scholar]
- Ho, K.L. Ecchordosis Physaliphora and chordoma: A comparative ultrastructural study. Clin. Neuropathol. 1985, 4, 77–86. [Google Scholar] [PubMed]
- Nishigaya, K.; Kaneko, M.; Ohashi, Y.; Nukui, H. Intradural retroclival chordoma without bone involvement: No tumor regrowth 5 years after operation—Case report. J. Neurosurg. 1998, 88, 764–768. [Google Scholar] [CrossRef]
- Katayama, Y.; Tsubokawa, T.; Hirasawa, T.; Takahata, T.; Nemoto, N. Intradural extraosseous chordoma in the foramen magnum region: Case report. J. Neurosurg. 1991, 75, 976–979. [Google Scholar] [CrossRef]
- Mapstone, T.B.; Kaufman, B.; Ratcheson, R.A. Intradural chordoma without bone involvement: Nuclear magnetic resonance (NMR) appearance. Case report. J. Neurosurg. 1983, 59, 535–537. [Google Scholar] [CrossRef]
- Mehnert, F.; Beschorner, R.; Küker, W.; Hahn, U.; Nägele, T. RetroclivalEcchordosis Physaliphora: MR imaging and review of the literature. Am. J. Neuroradiol. 2004, 25, 1851–1855. [Google Scholar] [PubMed]
- Iorgulescu, J.B.; Laufer, I.; Hameed, M.; Boland, P.; Yamada, Y.; Lis, E.; Bilsky, M. Benign notochordal cell tumors of the spine: Natural history of 8 patients with histologically confirmed lesions. Neurosurgery 2013, 73, 411–416. [Google Scholar] [CrossRef] [PubMed]


| Authors | Sex | Age | Symptom and Clinical Findings at Presentation(s) | EP Location | Management | Outcome |
|---|---|---|---|---|---|---|
| Adib et al. 2016 [8] | M | 57 | CN VI palsy, Diplopia, paresthesia | Prepontine | Surgical | RD |
| Ahn et al. 2016 [5] | M | 15 | CN VI palsy, diplopia | Prepontine | Conservative | SR |
| Akimoto et al. [9] 1996 | F | 51 | Diplopia, headache | Prepontine | Surgical | ND |
| Alkan et al. 2009 [10] | M | 22 | Confusion, headache | Prepontine | Conservative | SF |
| Alli et al. 2008 [11] | F | 52 | CSF-L | Posterior SS wall | Surgical | NED |
| Bolzoni-Villaret et al. 2014 [6] #A | F | 51 | CSF-L | Posterior SS wall | Surgical | NED |
| #B | F | 39 | Diplopia, CN VI palsy | Prepontine | Surgical | NED |
| Cha et al. 2002 [12] | M | 49 | Dizziness, headache | Prepontine | Surgical | NED |
| Choudhri et al. 2014 [13] | M | 63 | Headache, tremor | Prepontine | Surgical | NED |
| Derakhshani et al. 2020 [14] | F | 68 | CSF-L, headache | Posterior SS wall | Surgical | NED |
| Dias et al. 2014 [15] | F | 54 | CSF-L, headache * | Posterior SS wall | Surgical | NED |
| Ferguson et al. 2016 [16] | F | ND | CSF-L, headache * | Prepontine | Surgical | ND |
| Filis et al. 2016 [17] | F | 44 | Headache | Prepontine | Surgical | NED |
| Fracasso et al. 2008 [18] | F | 48 | Sudden death ** | Prepontine | Conservative | Death |
| Galloway et al. 2017 [19] | F | 40 | CSF-L, headache * | Posterior SS wall | Surgical | NED |
| Georgalas et al. 2020 [20] #A | M | 81 | CSF-L, headache * | Prepontine | Surgical | SF |
| #B | M | 60 | CSF-L, headache * | Posterior SS wall | Surgical | SF |
| #C | F | 64 | Headache * | Posterior SS wall | Surgical | SF |
| Ghimire et al. 2020 [21] | F | 65 | Hemiparesis, mutism | Prepontine | Surgical | NED |
| Ilorah et al. 2017 [22] | M | 42 | CN VI palsy, diplopia | Prepontine | Conservative | SF |
| Indiran V 2022 [23] | F | 44 | Headache | Prepontine | NA | NA |
| Kaul et al. 2013 [24] | F | 52 | Headache *, otalgia, tinnitus | Posterior SS wall | Surgical | SF |
| Krisht et al. 2013 [25] | F | 17 | Diplopia, headache CN VI palsy | Prepontine | Surgical | RD |
| Kurokawa et al. 1988 [26] | M | 84 | Hemiparesis, hyperalgesia, thermohypesthesia | C2, extradura | Surgical | ND |
| Lakhani et al. 2021 [27] | M | 30 | Headache | Prepontine | Conservative | SF |
| Ling et al. 2007 [28] | F | 45 | Hearing loss, tinnitus | Prepontine | Surgical | NED |
| LN Ang et al. 2020 [29] | F | 43 | Headache | Prepontine | Surgical | SF |
| MacDonald et al. 1990 [1] | F | 66 | CSF-L | Prepontine | Surgical | NED |
| Margo G et al. 2023 [30] #A | F | 61 | Headache | Prepontine | Conservative | SF |
| #B | M | 41 | Headache | Prepontine | Conservative | SF |
| Miki et al. 2017 [31] | F | 44 | Facial pain | Prepontine | Surgical | NED |
| Miki et al. 2008 [32] | M | 59 | Dizziness, gait disturbance | Prepontine | Surgical | NED |
| Ng et al. 1998 [33] | M | ND | Hemihypoesthesia, Hemiparesis | Odontoid process | ND | ND |
| Raffa A 2022 [34] | M | 7 | Headache | Prepontine | Surgical | SF |
| Reddy et al. 2022 [7] | F | 55 | Headache, paresthesia, weakness | Prepontine | Conservative | RD |
| Rengachary et al. 1997 [35] | F | 34 | Interscapular back pain | T8-T9 intervertebral foramen | Surgical | SF |
| Rodriguez et al. 1999 [36] | F | 54 | Dizziness | Prepontine | Surgical | ND |
| Rotondo et al. 2007 [37] | F | 47 | Facial pain, headache | Prepontine | Surgical | NED |
| Ruiz Castello MJ et al. 2023 [38] | F | 46 | Headache *, CSF L | Posterior SS wall | Surgical | NA |
| Sarkar N et al. 2022 [39] | M | 16 | Headache | Prepontine | Conservative | SF |
| Sooltangos et al. 2021 [40] #A | F | 65 | Hemiparesis, Confusion CSF L | Prepontine | Surgical | NED |
| #B | F | 39 | Headache, CSF L | Prepontine | Surgical | SF |
| #C | F | 43 | Headache *, CSF L | Prepontine | Surgical | NED |
| #D | F | 39 | Headache *, CSF L | Prepontine | Surgical | NED |
| #E | F | 45 | CSF L | Prepontine | Surgical | NA |
| Srinivasan et al. 2008 [41] | F | 31 | Headache | Prepontine | ND | ND |
| Stam et al. 1982 [2] | M | 75 | Sudden death ** | Prepontine | ND | Death |
| Sun et al. 2020 [42] | F | 22 | CN VI palsy, Diplopia headache | Prepontine | Surgical | NED |
| Takeyama et al. 2006 [43] | M | 12 | Diplopia, hemiparesis | Prepontine | Surgical | NED |
| Toda et al. 1998 [44] | F | 56 | Headache | Prepontine | Surgical | NED |
| Touska et al. 2014 [45] | M | ND | Tinnitus | Prepontine | Conservative | ND |
| Veiceschi et al. 2021 [46] #A | M | 59 | CSF-L, headache * | Prepontine | Surgical | NED |
| #B | F | 64 | CSF-L | Prepontine | Surgical | NED |
| #C | M | 41 | CSF-L | Prepontine | Surgical | NED |
| #D | F | 39 | CN VI palsy, diplopia | Prepontine | Surgical | NED |
| #E | M | 57 | CSF-L | Prepontine | Surgical | NED |
| Watanabe et al. 1994 [47] | F | 51 | Hearing loss, facial hemihypoesthesia | Posterior SS wall | Surgical | RD |
| Wells et al. 2010 [48] | F | 46 | Headache, Facial numbness | Prepontine | NA | NA |
| Yamamoto et al. 2013 [49] | M | 20 | CN VI palsy, Diplopia | Dorello’s canal | Surgical | SF |
| Zhong et al. 2015 [50] | M | 34 | Diplopia, headache, CN VI palsy | Prepontine | Surgical | SF |
| Symptoms and Clinical Findings at Presentation | Frequency (Case Count) | Frequency (% of Patients) * |
|---|---|---|
| Headache | 33 | 55 |
| CSF rhinorrhea | 19 | 32 |
| Diplopia | 11 | 18 |
| CN VI palsy | 9 | 15 |
| Other ** | 8 | 13 |
| Hemiparesis | 5 | 8 |
| Dizziness | 3 | 5 |
| Tinnitus | 3 | 5 |
| Facial pain | 2 | 3 |
| Hearing loss | 2 | 3 |
| Hemihypoesthesia | 2 | 3 |
| Paresthesias | 2 | 3 |
| Confusion | 2 | 3 |
| Sex | Location of EP | Total | ||
|---|---|---|---|---|
| Prepontine | Posterior Sphenoidal Sinus Wall | Others * | ||
| Male | 19 | 1 | 3 | 23 |
| Female | 27 | 9 | 1 | 37 |
| Total | 46 | 10 | 4 | 60 |
| Total | Outcomes | Total | |||||
|---|---|---|---|---|---|---|---|
| No Evidence of Disease | Symptom Free | Symptom Recurrence | Residual Disease | Not Documented | Death | ||
| Surgical | 26 | 10 | 0 | 3 | 6 | 0 | 45 |
| Conservative | 0 | 6 | 1 | 1 | 1 | 1 | 10 |
| Not defined | 0 | 0 | 0 | 0 | 4 | 1 | 5 |
| Total | 26 | 16 | 1 | 4 | 11 | 2 | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, R.K.; Reddy, T.A.; Gupta, A.; Samant, R.; Perez, C.A.; Haque, A. An Ecchordosis Physaliphora, a Rare Entity, Involving the Central Nervous System: A Systematic Review of the Literature. Neurol. Int. 2023, 15, 1200-1211. https://doi.org/10.3390/neurolint15040075
Gupta RK, Reddy TA, Gupta A, Samant R, Perez CA, Haque A. An Ecchordosis Physaliphora, a Rare Entity, Involving the Central Nervous System: A Systematic Review of the Literature. Neurology International. 2023; 15(4):1200-1211. https://doi.org/10.3390/neurolint15040075
Chicago/Turabian StyleGupta, Rajesh K., Thejasvi A. Reddy, Ashutosh Gupta, Rohan Samant, Carlos A. Perez, and Anam Haque. 2023. "An Ecchordosis Physaliphora, a Rare Entity, Involving the Central Nervous System: A Systematic Review of the Literature" Neurology International 15, no. 4: 1200-1211. https://doi.org/10.3390/neurolint15040075
APA StyleGupta, R. K., Reddy, T. A., Gupta, A., Samant, R., Perez, C. A., & Haque, A. (2023). An Ecchordosis Physaliphora, a Rare Entity, Involving the Central Nervous System: A Systematic Review of the Literature. Neurology International, 15(4), 1200-1211. https://doi.org/10.3390/neurolint15040075






