Introducing the Futile Recanalization Prediction Score (FRPS): A Novel Approach to Predict and Mitigate Ineffective Recanalization after Endovascular Treatment of Acute Ischemic Stroke
Abstract
1. Introduction
2. Methodology
Development, Optimization, and Modeling Simulation for Risk Prediction Score for Predicting Futile Recanalization Risk Severity after Endovascular Thrombectomy
3. Results
4. Discussion
4.1. Implications of Futile Recanalization and Clinical Need for Risk Prediction
4.2. Rationale and Development of a Futile Recanalization Risk Score
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| AF | Atrial Fibrillation |
| AHA | American Heart Association |
| ASA | American Stroke Association |
| ASPECTS | Alberta Stroke Program Early CT Score |
| BAO | Basilar Artery Occlusion |
| BBB | Blood–Brain Barrier |
| CMR | Clinically Meaningful Recanalization |
| CT | Computed Tomography |
| DALYs | Disability-Adjusted Life Years |
| DM | Diabetes Mellitus |
| EVT | Endovascular Thrombectomy |
| FR | Futile Recanalization |
| FRPS | Futile Recanalization Prediction Score |
| GA | General Anesthesia |
| GBD | Global Burden of Disease |
| HDMCA | Hyperdense Middle Cerebral Artery Sign |
| HL | Hyperlipidemia |
| HT | Hemorrhagic Transformation |
| ICU | Intensive Care Unit |
| IV-rtPA | Intravenous Recombinant Tissue Plasminogen Activator |
| IVT | Intravenous Thrombolysis |
| LVO | Large Vessel Occlusion |
| MCA | Middle Cerebral Artery |
| mRS | Modified Rankin Score |
| mTICI | Thrombolysis in Cerebral Infarction |
| NLR | Neutrophil-to-Lymphocyte Ratio |
| NIHSS | National Institutes of Health Stroke Severity Score |
| NNT | Number Needed to Treat |
| OTT | Onset-to-Treatment Time |
| OTR | Onset-to-Reperfusion Time |
| pc-ASPECTS | Posterior Circulation Alberta Stroke Program Early CT Score |
| PS/TIA | Previous Stroke/Transient Ischemic Attack |
| RCT | Randomized Control Trial |
| SMM | Standard Medical Management |
| sICH | Symptomatic Intracranial Hemorrhage |
| VHF | Vascular Hyperintensities on FLAIR Imaging |
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abedi, V.; et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef] [PubMed]
- Kleindorfer, D.O.; Towfighi, A.; Chaturvedi, S.; Cockroft, K.M.; Gutierrez, J.; Lombardi-Hill, D.; Kamel, H.; Kernan, W.N.; Kittner, S.J.; Leira, E.C.; et al. 2021 Guideline for the Prevention of Stroke in Patients with Stroke and Transient Ischemic Attack: A Guideline from the American Heart Association/American Stroke Association. Stroke 2021, 52, e364–e467. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Wang, B.; Hang, Y.; Liu, S.; Jia, Z.-Y.; Shi, H.-B.; Zhao, L.-B. Predictors of Futile Recanalization in Patients with Intracranial Atherosclerosis-Related Stroke Undergoing Endovascular Treatment. World Neurosurg. 2023, 171, e752–e759. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Lin, C.; Chen, L.; Qiao, Y.; Huang, P.; Liu, B.; Zhu, Y.; Su, J.; Liu, J. Multiple-Factor Analyses of Futile Recanalization in Acute Ischemic Stroke Patients Treated with Mechanical Thrombectomy. Front. Neurol. 2021, 12, 704088. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zheng, X.; Zhang, J.; Cui, X.; Zou, D.; Zhao, Z.; Pan, X.; Jie, Q.; Wu, Y.; Qiu, R.; et al. Machine learning to predict futile recanalization of large vessel occlusion before and after endovascular thrombectomy. Front. Neurol. 2022, 13, 909403. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Killingsworth, M.C.; Bhaskar, S.M.M. Futile Recanalization after Endovascular Thrombectomy for Acute Ischemic Stroke: A Comprehensive Meta-Analysis of Prevalence, Predictive Markers, and Clinical Outcomes. Life 2023, 13, 1965. [Google Scholar] [CrossRef] [PubMed]
- Shahid, A.H.; Abbasi, M.; Larco, J.L.A.; Madhani, S.I.; Liu, Y.; Brinjikji, W.; Savastano, L.E. Risk Factors of Futile Recanalization Following Endovascular Treatment in Patients with Large-Vessel Occlusion: Systematic Review and Meta-Analysis. Stroke Vasc. Interv. Neurol. 2022, 2, e000257. [Google Scholar] [CrossRef]
- Deng, G.; Xiao, J.; Yu, H.; Chen, M.; Shang, K.; Qin, C.; Tian, D.-S. Predictors of futile recanalization after endovascular treatment in acute ischemic stroke: A meta-analysis. J. NeuroInterv. Surg. 2022, 14, 881. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Jia, B.; Huo, X.; Mo, D.; Ma, N.; Gao, F.; Yang, M.; Miao, Z. Predictors of Futile Recanalization after Endovascular Treatment in Patients with Acute Ischemic Stroke in a Multicenter Registry Study. J. Stroke Cerebrovasc. Dis. 2020, 29, 105067. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, P.S.; Butt, W.; Marei, O.; Podlasek, A.; McConachie, N.; Lenthall, R.; Nair, S.; Malik, L.; Bhogal, P.; Makalanda, H.L.D.; et al. Incidence and predictors of poor functional outcome despite complete recanalisation following endovascular thrombectomy for acute ischaemic stroke. J. Stroke Cerebrovasc. Dis. 2023, 32, 107083. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Liu, X.; Hang, Y.; Jia, Z.; Cao, Y.; Shi, H.; Liu, S.; Zhao, L. Predictors of futile recanalization in patients with acute ischemic stroke undergoing mechanical thrombectomy in late time windows. Front. Neurol. 2022, 13, 958236. [Google Scholar] [CrossRef]
- Gilberti, N.; Gamba, M.; Premi, E.; Costa, A.; Vergani, V.; Delrio, I.; Spezi, R.; Dikran, M.; Frigerio, M.; Gasparotti, R.; et al. Leukoaraiosis is a predictor of futile recanalization in acute ischemic stroke. J. Neurol. 2017, 264, 448–452. [Google Scholar] [CrossRef]
- Ouyang, K.; Kang, Z.; Liu, Z.; Hou, B.; Fang, J.; Xie, Y.; Liu, Y. Posterior Circulation ASPECTS on CT Angiography Predicts Futile Recanalization of Endovascular Thrombectomy for Acute Basilar Artery Occlusion. Front. Neurol. 2022, 13, 831386. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.-S.; Loh, Y.; Walker, G.; Duckwiler, G.R. Clinical outcomes in middle cerebral artery trunk occlusions versus secondary division occlusions after mechanical thrombectomy: Pooled analysis of the Mechanical Embolus Removal in Cerebral Ischemia (MERCI) and Multi MERCI trials. Stroke 2010, 41, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Pedraza, M.I.; de Lera, M.; Bos, D.; Calleja, A.I.; Cortijo, E.; Gómez-Vicente, B.; Reyes, J.; Coco-Martín, M.B.; Calonge, T.; Agulla, J.; et al. Brain Atrophy and the Risk of Futile Endovascular Reperfusion in Acute Ischemic Stroke. Stroke 2020, 51, 1514–1521. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Yi, T.; Li, T.; Zhu, L.; Li, Y.; Li, Z.; Wang, M.; Li, Q.; He, Y.; Yang, P.; et al. Predictors of futile recanalization in patients undergoing endovascular treatment in the DIRECT-MT trial. J. NeuroInterv. Surg. 2022, 14, 752. [Google Scholar] [CrossRef] [PubMed]
- Baskar, P.S.; Chowdhury, S.Z.; Bhaskar, S.M.M. In-hospital systems interventions in acute stroke reperfusion therapy: A meta-analysis. Acta Neurol. Scand. 2021, 144, 418–432. [Google Scholar] [CrossRef]
- Venkat, A.; Cappelen-Smith, C.; Askar, S.; Thomas, P.R.; Bhaskar, S.; Tam, A.; McDougall, A.J.; Hodgkinson, S.J.; Cordato, D.J. Factors Associated with Stroke Misdiagnosis in the Emergency Department: A Retrospective Case-Control Study. Neuroepidemiology 2018, 51, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Santana Baskar, P.; Cordato, D.; Wardman, D.; Bhaskar, S. In-hospital acute stroke workflow in acute stroke—Systems-based approaches. Acta Neurol. Scand. 2021, 143, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Vatan, M.B.; Acar, B.A.; Acar, T.; Aras, Y.G. The CHA2DS2-VASc risk score predicts futile recanalization after endovascular treatment in patients with acute ischemic stroke. Neurol. Asia 2023, 28, 89–97. [Google Scholar] [CrossRef]
- Widimsky, P.; Snyder, K.; Sulzenko, J.; Hopkins, L.N.; Stetkarova, I. Acute ischaemic stroke: Recent advances in reperfusion treatment. Eur. Heart J. 2023, 44, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Yang, I.H.; Orru, E.; Krings, T.; Tsang, A.C.O. Endovascular Thrombectomy for Distal Occlusion Using a Semi-Deployed Stentriever: Report of 2 Cases and Technical Note. Neurointervention 2019, 14, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Pilgram-Pastor, S.M.; Piechowiak, E.I.; Dobrocky, T.; Kaesmacher, J.; Den Hollander, J.; Gralla, J.; Mordasini, P. Stroke thrombectomy complication management. J. NeuroInterv. Surg. 2021, 13, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Westphal, L.P.; Lohaus, N.; Winklhofer, S.; Manzolini, C.; Held, U.; Steigmiller, K.; Hamann, J.M.; El Amki, M.; Dobrocky, T.; Panos, L.D.; et al. Circle of Willis variants and their association with outcome in patients with middle cerebral artery-M1-occlusion stroke. Eur. J. Neurol. 2021, 28, 3682–3691. [Google Scholar] [CrossRef] [PubMed]
- Vu-Dang, L.; Nguyen, Q.A.; Nguyen-Thi-Thu, T.; Tran, A.T.; Le-Chi, C.; Le-Hoang, K.; Nguyen-Tat, T.; Nguyen-Huu, A.; Pham-Minh, T.; Chu-Dinh, T.; et al. Endovascular Treatment for Acute Tandem Occlusion Stroke: Results from Case Series of 17 Patients. Ann. Indian Acad. Neurol. 2020, 23, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Compagne, K.C.J.; Boers, A.M.M.; Marquering, H.A.; Berkhemer, O.A.; Yoo, A.J.; Beenen, L.F.M.; van Oostenbrugge, R.J.; van Zwam, W.H.; Roos, Y.; Majoie, C.B.; et al. Follow-up infarct volume as a mediator of endovascular treatment effect on functional outcome in ischaemic stroke. Eur. Radiol. 2019, 29, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Bani-Sadr, A.; Escande, R.; Mechtouff, L.; Pavie, D.; Hermier, M.; Derex, L.; Choc, T.-H.; Eker, O.F.; Nighoghossian, N.; Berthezène, Y. Vascular hyperintensities on baseline FLAIR images are associated with functional outcome in stroke patients with successful recanalization after mechanical thrombectomy. Diagn. Interv. Imaging 2023, 104, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Hong, C.T.; Chung, C.C.; Kuan, Y.C.; Chan, L. Safety and efficacy of endovascular thrombectomy in acute ischemic stroke treated with anticoagulants: A systematic review and meta-analysis. Thromb. J. 2022, 20, 35. [Google Scholar] [CrossRef] [PubMed]
- Linfante, I.; Starosciak, A.K.; Walker, G.R.; Dabus, G.; Castonguay, A.C.; Gupta, R.; Sun, C.H.; Martin, C.; Holloway, W.E.; Mueller-Kronast, N.; et al. Predictors of poor outcome despite recanalization: A multiple regression analysis of the NASA registry. J. NeuroInterv. Surg. 2016, 8, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, D.D.; Lu, G.; Liu, Y.; Zhou, J.S.; Deng, Q.W.; Yan, F.L. Endovascular Treatment with and without Intravenous Thrombolysis in Large Vessel Occlusions Stroke: A Systematic Review and Meta-Analysis. Front. Neurol. 2021, 12, 697478. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ye, Z.; Busse, J.W.; Hill, M.D.; Smith, E.E.; Guyatt, G.H.; Prasad, K.; Lindsay, M.P.; Yang, H.; Zhang, Y.; et al. Endovascular thrombectomy with or without intravenous alteplase for acute ischemic stroke due to large vessel occlusion: A systematic review and meta-analysis of randomized trials. Stroke Vasc. Neurol. 2022, 7, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, J.M.; Zuurbier, S.M.; Bousser, M.G.; Ji, X.; Canhão, P.; Roos, Y.B.; Crassard, I.; Nunes, A.P.; Uyttenboogaart, M.; Chen, J.; et al. Effect of Endovascular Treatment with Medical Management vs Standard Care on Severe Cerebral Venous Thrombosis: The TO-ACT Randomized Clinical Trial. JAMA Neurol. 2020, 77, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Collette, S.L.; Bokkers, R.P.H.; Mazuri, A.; Lycklama À Nijeholt, G.J.; van Oostenbrugge, R.J.; LeCouffe, N.E.; Benali, F.; Majoie, C.; de Groot, J.C.; Luijckx, G.J.R.; et al. Intra-arterial thrombolytics during endovascular thrombectomy for acute ischaemic stroke in the MR CLEAN Registry. Stroke Vasc. Neurol. 2023, 8, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Kaesmacher, J.; Meinel, T.R.; Kurmann, C.; Zaidat, O.O.; Castonguay, A.C.; Zaidi, S.F.; Mueller-Kronast, N.; Kappelhof, M.; Dippel, D.W.J.; Soudant, M.; et al. Safety and efficacy of intra-arterial fibrinolytics as adjunct to mechanical thrombectomy: A systematic review and meta-analysis of observational data. J. NeuroInterv. Surg. 2021, 13, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- van der Steen, W.; van der Sluijs, P.M.; van de Graaf, R.A.; Su, R.; Wolff, L.; van Voorst, H.; den Hertog, H.M.; van Doormaal, P.J.; van Es, A.; Staals, J.; et al. Safety and efficacy of periprocedural antithrombotics in patients with successful reperfusion after endovascular stroke treatment. J. Stroke Cerebrovasc. Dis. 2022, 31, 106726. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Wang, D.; Pu, Y.; Wei, Y.; Lu, Q.; Yan, H.; Liu, X.; Zheng, L.; Liu, J.; Yang, X.; et al. Endovascular treatment with or without intravenous alteplase for acute ischaemic stroke due to basilar artery occlusion. Stroke Vasc. Neurol. 2022, 7, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Zaidat, O.O.; Kasab, S.A.; Sheth, S.; Ortega-Gutierrez, S.; Rai, A.T.; Given, C.A.; Grandhi, R.; Mokin, M.; Katz, J.M.; Maud, A.; et al. TESLA Trial: Rationale, Protocol, and Design. Stroke Vasc. Interv. Neurol. 2023, 3, e000787. [Google Scholar] [CrossRef]
- Bendszus, M.; Fiehler, J.; Subtil, F.; Bonekamp, S.; Aamodt, A.H.; Fuentes, B.; Gizewski, E.R.; Hill, M.D.; Krajina, A.; Pierot, L.; et al. Endovascular thrombectomy for acute ischaemic stroke with established large infarct: Multicentre, open-label, randomised trial. Lancet 2023, 402, 1753–1763. [Google Scholar] [CrossRef]
- Uchida, K.; Shindo, S.; Yoshimura, S.; Toyoda, K.; Sakai, N.; Yamagami, H.; Matsumaru, Y.; Matsumoto, Y.; Kimura, K.; Ishikura, R.; et al. Association Between Alberta Stroke Program Early Computed Tomography Score and Efficacy and Safety Outcomes with Endovascular Therapy in Patients with Stroke from Large-Vessel Occlusion: A Secondary Analysis of the Recovery by Endovascular Salvage for Cerebral Ultra-acute Embolism—Japan Large Ischemic Core Trial (RESCUE-Japan LIMIT). JAMA Neurol. 2022, 79, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Ma, G.; Tong, X.; Zhang, X.; Pan, Y.; Nguyen Thanh, N.; Yuan, G.; Han, H.; Chen, W.; Wei, M.; et al. Trial of Endovascular Therapy for Acute Ischemic Stroke with Large Infarct. N. Engl. J. Med. 2023, 388, 1272–1283. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, S.; Sakai, N.; Yamagami, H.; Uchida, K.; Beppu, M.; Toyoda, K.; Matsumaru, Y.; Matsumoto, Y.; Kimura, K.; Takeuchi, M.; et al. Endovascular Therapy for Acute Stroke with a Large Ischemic Region. N. Engl. J. Med. 2022, 386, 1303–1313. [Google Scholar] [CrossRef] [PubMed]
- Costalat, V.; Lapergue, B.; Albucher, J.F.; Labreuche, J.; Henon, H.; Gory, B.; Sibon, I.; Boulouis, G.; Cognard, C.; Nouri, N.; et al. Evaluation of acute mechanical revascularization in large stroke (ASPECTS ≤ 5) and large vessel occlusion within 7 h of last-seen-well: The LASTE multicenter, randomized, clinical trial protocol. Int. J. Stroke 2024, 19, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Alverne, F.; Lima, F.O.; Rocha, F.A.; Bandeira, D.A.; Lucena, A.F.; Silva, H.C.; Lee, J.S.; Nogueira, R.G. Unfavorable Vascular Anatomy during Endovascular Treatment of Stroke: Challenges and Bailout Strategies. J. Stroke 2020, 22, 185–202. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.M.; Saleem, M.A.; Qureshi, A.I. Rates and predictors of futile recanalization in patients undergoing endovascular treatment in a multicenter clinical trial. Neuroradiology 2018, 60, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.S.; Liebeskind, D.S.; Xiang, B.; Ge, S.G.; Feng, L.; Albers, G.W.; Budzik, R.; Devlin, T.; Gupta, R.; Jansen, O.; et al. Predictors of functional dependence despite successful revascularization in large-vessel occlusion strokes. Stroke 2014, 45, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Dong, A.; Maier, B.; Guillon, B.; Preterre, C.; De Gaalon, S.; Gory, B.; Richard, S.; Kaminsky, A.L.; Tracol, C.; Eugene, F.; et al. TICI-RANKIN mismatch: Poor clinical outcome despite complete endovascular reperfusion in the ETIS Registry. Rev. Neurol. 2023, 179, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Ribo, M.; Molina, C.A.; Cobo, E.; Cerdà, N.; Tomasello, A.; Quesada, H.; De Miquel, M.A.; Millan, M.; Castaño, C.; Urra, X.; et al. Association between Time to Reperfusion and Outcome Is Primarily Driven by the Time from Imaging to Reperfusion. Stroke 2016, 47, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Menon, B.K.; van Zwam, W.H.; Dippel, D.W.J.; Mitchell, P.J.; Demchuk, A.M.; Dávalos, A.; Majoie, C.B.L.M.; van der Lugt, A.; de Miquel, M.A.; et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 2016, 387, 1723–1731. [Google Scholar] [CrossRef] [PubMed]
- Berkhemer, O.A.; Fransen, P.S.; Beumer, D.; van den Berg, L.A.; Lingsma, H.F.; Yoo, A.J.; Schonewille, W.J.; Vos, J.A.; Nederkoorn, P.J.; Wermer, M.J.; et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N. Engl. J. Med. 2015, 372, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.C.; Mitchell, P.J.; Kleinig, T.J.; Dewey, H.M.; Churilov, L.; Yassi, N.; Yan, B.; Dowling, R.J.; Parsons, M.W.; Oxley, T.J.; et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N. Engl. J. Med. 2015, 372, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Saver, J.L.; Goyal, M.; Bonafe, A.; Diener, H.C.; Levy, E.I.; Pereira, V.M.; Albers, G.W.; Cognard, C.; Cohen, D.J.; Hacke, W.; et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N. Engl. J. Med. 2015, 372, 2285–2295. [Google Scholar] [CrossRef] [PubMed]
- Jovin, T.G.; Chamorro, A.; Cobo, E.; de Miquel, M.A.; Molina, C.A.; Rovira, A.; San Román, L.; Serena, J.; Abilleira, S.; Ribó, M.; et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N. Engl. J. Med. 2015, 372, 2296–2306. [Google Scholar] [CrossRef] [PubMed]
- Saver, J.L.; Goyal, M.; van der Lugt, A.; Menon, B.K.; Majoie, C.B.; Dippel, D.W.; Campbell, B.C.; Nogueira, R.G.; Demchuk, A.M.; Tomasello, A.; et al. Time to Treatment With Endovascular Thrombectomy and Outcomes From Ischemic Stroke: A Meta-analysis. JAMA 2016, 316, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Wassélius, J.; Arnberg, F.; von Euler, M.; Wester, P.; Ullberg, T. Endovascular thrombectomy for acute ischemic stroke. J. Intern. Med. 2022, 291, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Lina, P.; Amrou, S.; Apostolos, S.; Georgios, M.; Robin, L.; Else Charlotte, S.; Guillaume, T.; Marios, P.; Georgios, T. Endovascular treatment for large-core ischaemic stroke: A meta-analysis of randomised controlled clinical trials. J. Neurol. Neurosurg. Psychiatry 2023, 94, 781. [Google Scholar] [CrossRef]
- Smith, W.S.; Sung, G.; Starkman, S.; Saver, J.L.; Kidwell, C.S.; Gobin, Y.P.; Lutsep, H.L.; Nesbit, G.M.; Grobelny, T.; Rymer, M.M.; et al. Safety and Efficacy of Mechanical Embolectomy in Acute Ischemic Stroke. Stroke 2005, 36, 1432–1438. [Google Scholar] [CrossRef] [PubMed]
- Bathla, G.; Ajmera, P.; Mehta, P.M.; Benson, J.C.; Derdeyn, C.P.; Lanzino, G.; Agarwal, A.; Brinjikji, W. Advances in Acute Ischemic Stroke Treatment: Current Status and Future Directions. AJNR Am. J. Neuroradiol. 2023, 44, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Chartrain, A.G.; Awad, A.J.; Mascitelli, J.R.; Shoirah, H.; Oxley, T.J.; Feng, R.; Gallitto, M.; De Leacy, R.; Fifi, J.T.; Kellner, C.P. Novel and emerging technologies for endovascular thrombectomy. Neurosurg. Focus. 2017, 42, E12. [Google Scholar] [CrossRef] [PubMed]
- Fugate, J.E.; Klunder, A.M.; Kallmes, D.F. What is meant by “TICI”? Am. J. Neuroradiol. 2013, 34, 1792–1797. [Google Scholar] [CrossRef]
- Adcock, A.K.; Schwamm, L.H.; Smith, E.E.; Fonarow, G.C.; Reeves, M.J.; Xu, H.; Matsouaka, R.A.; Xian, Y.; Saver, J.L. Trends in Use, Outcomes, and Disparities in Endovascular Thrombectomy in US Patients With Stroke Aged 80 Years and Older Compared With Younger Patients. JAMA Netw. Open 2022, 5, e2215869. [Google Scholar] [CrossRef] [PubMed]
- Sohn, J.-H.; Kim, C.; Lee, M.; Kim, Y.; Jung Mo, H.; Yu, K.-H.; Lee, S.-H. Effects of prior antiplatelet use on futile reperfusion in patients with acute ischemic stroke receiving endovascular treatment. Eur. Stroke J. 2022, 23969873221144814. [Google Scholar] [CrossRef] [PubMed]
- Brinjikji, W.; Lanzino, G. Regarding: “Localized Marked Elongation of the Distal Internal Carotid Artery with or without PHACE Syndrome: Segmental Dolichoectasia of the Distal Internal Carotid Artery”. Am. J. Neuroradiol. 2018, 39, E95. [Google Scholar] [CrossRef]
- Broeg-Morvay, A.; Mordasini, P.; Bernasconi, C.; Bühlmann, M.; Pult, F.; Arnold, M.; Schroth, G.; Jung, S.; Mattle, H.P.; Gralla, J.; et al. Direct Mechanical Intervention Versus Combined Intravenous and Mechanical Intervention in Large Artery Anterior Circulation Stroke. Stroke 2016, 47, 1037–1044. [Google Scholar] [CrossRef]
- Froehler, M.T.; Saver, J.L.; Zaidat, O.O.; Jahan, R.; Aziz-Sultan, M.A.; Klucznik, R.P.; Haussen, D.C.; Hellinger, F.R., Jr.; Yavagal, D.R.; Yao, T.L.; et al. Interhospital Transfer Before Thrombectomy Is Associated With Delayed Treatment and Worse Outcome in the STRATIS Registry (Systematic Evaluation of Patients Treated With Neurothrombectomy Devices for Acute Ischemic Stroke). Circulation 2017, 136, 2311–2321. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-M.; Bae, J.-H.; Park, K.-Y.; Lee, W.J.; Byun, J.S.; Ahn, S.-W.; Shin, H.-W.; Han, S.-H.; Yoo, I.-H. Incidence and mechanism of early neurological deterioration after endovascular thrombectomy. J. Neurol. 2019, 266, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Lattanzi, S.; Norata, D.; Divani, A.A.; Di Napoli, M.; Broggi, S.; Rocchi, C.; Ortega-Gutierrez, S.; Mansueto, G.; Silvestrini, M. Systemic Inflammatory Response Index and Futile Recanalization in Patients with Ischemic Stroke Undergoing Endovascular Treatment. Brain Sci. 2021, 11, 1164. [Google Scholar] [CrossRef] [PubMed]
- Ritvonen, J.; Sairanen, T.; Silvennoinen, H.; Virtanen, P.; Salonen, O.; Lindsberg, P.J.; Strbian, D. Comatose With Basilar Artery Occlusion: Still Odds of Favorable Outcome With Recanalization Therapy. Front. Neurol. 2021, 12, 665317. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.; Wu, X.; Payabvash, S.; Matouk, C.C.; Forman, H.P.; Gandhi, D.; Sanelli, P.; Schindler, J. Comparative Effectiveness of Endovascular Thrombectomy in Elderly Stroke Patients. Stroke 2019, 50, 963–969. [Google Scholar] [CrossRef]
- Hilditch, C.A.; Nicholson, P.; Murad, M.H.; Rabinstein, A.; Schaafsma, J.; Pikula, A.; Krings, T.; Pereira, V.M.; Agid, R.; Brinjikji, W. Endovascular Management of Acute Stroke in the Elderly: A Systematic Review and Meta-Analysis. Am. J. Neuroradiol. 2018, 39, 887–891. [Google Scholar] [CrossRef] [PubMed]
- McDonough, R.V.; Ospel, J.M.; Campbell, B.C.V.; Hill, M.D.; Saver, J.L.; Dippel, D.W.J.; Demchuk, A.M.; Majoie, C.; Brown, S.B.; Mitchell, P.J.; et al. Functional Outcomes of Patients ≥85 Years With Acute Ischemic Stroke Following EVT: A HERMES Substudy. Stroke 2022, 53, 2220–2226. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Kim, B.J.; Han, M.-K.; Park, T.H.; Lee, K.B.; Lee, B.-C.; Yu, K.-H.; Oh, M.S.; Cha, J.K.; Kim, D.-H.; et al. Futile reperfusion and predicted therapeutic benefits after successful endovascular treatment according to initial stroke severity. BMC Neurol. 2019, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- Chalos, V.; de Ridder, I.R.; Lingsma, H.F.; Brown, S.; van Oostenbrugge, R.J.; Goyal, M.; Campbell, B.C.V.; Muir, K.W.; Guillemin, F.; Bracard, S.; et al. Does Sex Modify the Effect of Endovascular Treatment for Ischemic Stroke? Stroke 2019, 50, 2413–2419. [Google Scholar] [CrossRef] [PubMed]
- Bradley, S.A.; Smokovski, I.; Bhaskar, S.M.M. Impact of diabetes on clinical and safety outcomes in acute ischemic stroke patients receiving reperfusion therapy: A meta-analysis. Adv. Clin. Exp. Med. 2022, 31, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.S.; Kim, B.M. Advance of Thrombolysis and Thrombectomy in Acute Ischemic Stroke. J. Clin. Med. 2023, 12, 720. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xie, Z.; Li, B.; Zhang, P. Renal impairment and the prognosis of endovascular thrombectomy: A meta-analysis and systematic review. Ther. Adv. Neurol. Disord. 2022, 15, 17562864221083620. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, R.; Cordato, D.J.; Wardman, D.; Thomas, P.; Bhaskar, S.M.M. Clinical outcomes following reperfusion therapy in acute ischemic stroke patients with infective endocarditis: A systematic review. J. Cent. Nerv. Syst. Dis. 2022, 14, 11795735221081597. [Google Scholar] [CrossRef]
- Brott, T.; Adams, H.P., Jr.; Olinger, C.P.; Marler, J.R.; Barsan, W.G.; Biller, J.; Spilker, J.; Holleran, R.; Eberle, R.; Hertzberg, V.; et al. Measurements of acute cerebral infarction: A clinical examination scale. Stroke 1989, 20, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L.; Zhang, Y.; Li, Z.; Zhang, Y.; Xing, P.; Chen, W.; Wang, S.; Li, T.; Yang, P.; et al. Endovascular Recanalization for Acute Internal Carotid Artery Terminus Occlusion: A Subgroup Analysis From the Direct-MT Trial. Neurosurgery 2022, 91, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Zang, N.; Lin, Z.; Huang, K.; Pan, Y.; Wu, Y.; Wu, Y.; Wang, S.; Wang, D.; Ji, Z.; Pan, S. Biomarkers of Unfavorable Outcome in Acute Ischemic Stroke Patients with Successful Recanalization by Endovascular Thrombectomy. Cerebrovasc. Dis. 2020, 49, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Zhou, Y.; Chen, Z.; Pu, M.; Li, Z.; Du, H.; Xu, G. Cystatin C predicts futile recanalization in patients with acute ischemic stroke after endovascular treatment. J. Neurol. 2022, 269, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Hervella, P.; Sampedro-Viana, A.; Rodríguez-Yáñez, M.; López-Dequidt, I.; Pumar, J.M.; Mosqueira, A.J.; Fernández-Rodicio, S.; Bazarra-Barreiros, M.; Serena, J.; Silva-Blas, Y.; et al. Systemic biomarker associated with poor outcome after futile reperfusion. Eur. J. Clin. Investig. 2024, 54, e14181. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.s.; Guo, C.; Zhang, B.; Yang, J.; Zi, W.; Li, J.l. Low neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios predict favorable outcomes after endovascular treatment in acute basilar artery occlusion: Subgroup analysis of the BASILAR registry. BMC Neurol. 2023, 23, 113. [Google Scholar] [CrossRef] [PubMed]
- Yeh, S.-J.; Chen, C.-H.; Lin, Y.-H.; Tsai, L.-K.; Lee, C.-W.; Tang, S.-C.; Jeng, J.-S. Serum amyloid A predicts poor functional outcome in patients with ischemic stroke receiving endovascular thrombectomy: A case control study. J. NeuroInterv. Surg. 2023, 15, 75. [Google Scholar] [CrossRef] [PubMed]
- Ryu, C.W.; Shin, H.S.; Park, S.; Suh, S.H.; Koh, J.S.; Choi, H.Y. Alberta Stroke Program Early CT Score in the Prognostication after Endovascular Treatment for Ischemic Stroke: A Meta-analysis. Neurointervention 2017, 12, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Scalzo, F.; Rao, N.M.; Hinman, J.D.; Kim, D.; Ali, L.K.; Saver, J.L.; Sun, W.; Dai, Q.; Liu, X.; et al. Fluid-Attenuated Inversion Recovery Vascular Hyperintensity Topography, Novel Imaging Marker for Revascularization in Middle Cerebral Artery Occlusion. Stroke 2016, 47, 2763–2769. [Google Scholar] [CrossRef] [PubMed]
- Pfaff, J.; Herweh, C.; Schieber, S.; Schönenberger, S.; Bösel, J.; Ringleb, P.A.; Möhlenbruch, M.; Bendszus, M.; Nagel, S. e-ASPECTS Correlates with and Is Predictive of Outcome after Mechanical Thrombectomy. Am. J. Neuroradiol. 2017, 38, 1594–1599. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.Z.; Lin, H.A.; Bai, C.H.; Lin, S.F. Posterior circulation acute stroke prognosis early CT scores in predicting functional outcomes: A meta-analysis. PLoS ONE 2021, 16, e0246906. [Google Scholar] [CrossRef]
- Karatzetzou, S.; Tsiptsios, D.; Sousanidou, A.; Christidi, F.; Psatha, E.A.; Chatzaki, M.; Kitmeridou, S.; Giannakou, E.; Karavasilis, E.; Kokkotis, C.; et al. Elucidating the Role of Baseline Leukoaraiosis on Forecasting Clinical Outcome of Acute Ischemic Stroke Patients Undergoing Reperfusion Therapy. Neurol. Int. 2022, 14, 923–942. [Google Scholar] [CrossRef] [PubMed]
- Diprose, W.K.; Diprose, J.P.; Wang, M.T.M.; Tarr, G.P.; McFetridge, A.; Barber, P.A. Automated Measurement of Cerebral Atrophy and Outcome in Endovascular Thrombectomy. Stroke 2019, 50, 3636–3638. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Wang, Y.; Yuan, J.; Chen, Y.; Luo, H. Small Vessel Disease Burden and Outcomes of Mechanical Thrombectomy in Ischemic Stroke: A Systematic Review and Meta-Analysis. Front. Neurol. 2021, 12, 602037. [Google Scholar] [CrossRef] [PubMed]
- Protto, S.; Pienimäki, J.-P.; Seppänen, J.; Numminen, H.; Sillanpää, N. Low Cerebral Blood Volume Identifies Poor Outcome in Stent Retriever Thrombectomy. CardioVasc. Interv. Radiol. 2017, 40, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Zaidat, O.O.; Castonguay, A.C.; Linfante, I.; Gupta, R.; Martin, C.O.; Holloway, W.E.; Mueller-Kronast, N.; English, J.D.; Dabus, G.; Malisch, T.W.; et al. First Pass Effect: A New Measure for Stroke Thrombectomy Devices. Stroke 2018, 49, 660–666. [Google Scholar] [CrossRef]
- Brinjikji, W.; Robert, M.S.; Murad, M.H.; David, F.; Vitor, M.P.; Mayank, G.; David, F.K. Impact of balloon guide catheter on technical and clinical outcomes: A systematic review and meta-analysis. J. NeuroInterv. Surg. 2018, 10, 335. [Google Scholar] [CrossRef] [PubMed]
- van Horn, N.; Kniep, H.; Leischner, H.; McDonough, R.; Deb-Chatterji, M.; Broocks, G.; Thomalla, G.; Brekenfeld, C.; Fiehler, J.; Hanning, U.; et al. Predictors of poor clinical outcome despite complete reperfusion in acute ischemic stroke patients. J. NeuroInterv. Surg. 2021, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Uniken Venema, S.M.; Wolff, L.; van den Berg, S.A.; Reinink, H.; Luijten, S.P.R.; Lingsma, H.F.; Marquering, H.A.; Boers, A.M.M.; Bot, J.; Hammer, S.; et al. Time Since Stroke Onset, Quantitative Collateral Score, and Functional Outcome After Endovascular Treatment for Acute Ischemic Stroke. Neurology 2022, 99, e1609–e1618. [Google Scholar] [CrossRef] [PubMed]
- Binder, N.F.; El Amki, M.; Glück, C.; Middleham, W.; Reuss, A.M.; Bertolo, A.; Thurner, P.; Deffieux, T.; Lambride, C.; Epp, R.; et al. Leptomeningeal collaterals regulate reperfusion in ischemic stroke and rescue the brain from futile recanalization. Neuron 2024, 112, 1456–1472.e1456. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, A.V.; Killingsworth, M.C.; Bhaskar, S. Cerebral collaterals in acute ischaemia: Implications for acute ischaemic stroke patients receiving reperfusion therapy. Eur. J. Neurosci. 2021, 53, 1238–1261. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.H.; Kim, B.M.; Heo, J.H.; Nam, H.S.; Kim, Y.D.; Park, H.; Bang, O.Y.; Yoo, J.; Kim, D.J.; Jeon, P.; et al. Number of Stent Retriever Passes Associated With Futile Recanalization in Acute Stroke. Stroke 2018, 49, 2088–2095. [Google Scholar] [CrossRef]
- Kitano, T.; Todo, K.; Yoshimura, S.; Uchida, K.; Yamagami, H.; Sakai, N.; Sakaguchi, M.; Nakamura, H.; Kishima, H.; Mochizuki, H.; et al. Futile complete recanalization: Patients characteristics and its time course. Sci. Rep. 2020, 10, 4973. [Google Scholar] [CrossRef] [PubMed]
- Jahan, R.; Saver, J.L.; Schwamm, L.H.; Fonarow, G.C.; Liang, L.; Matsouaka, R.A.; Xian, Y.; Holmes, D.N.; Peterson, E.D.; Yavagal, D.; et al. Association Between Time to Treatment With Endovascular Reperfusion Therapy and Outcomes in Patients With Acute Ischemic Stroke Treated in Clinical Practice. JAMA 2019, 322, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Meinel, T.R.; Kaesmacher, J.; Chaloulos-Iakovidis, P.; Panos, L.; Mordasini, P.; Mosimann, P.J.; Michel, P.; Hajdu, S.; Ribo, M.; Requena, M.; et al. Mechanical thrombectomy for basilar artery occlusion: Efficacy, outcomes, and futile recanalization in comparison with the anterior circulation. J. NeuroInterv. Surg. 2019, 11, 1174–1180. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Shu, H.; Meng, Y.; Zhang, H.; Wang, H.; He, S. Factors Promoting Futile Recanalization After Stent Retriever Thrombectomy for Stroke Affecting the Anterior Circulation: A Retrospective Analysis. World Neurosurg. 2020, 133, e576–e582. [Google Scholar] [CrossRef] [PubMed]
- Kharouba, R.; Gavriliuc, P.; Yaghmour, N.E.; Gomori, J.M.; Cohen, J.E.; Leker, R.R. Number of stentriever passes and outcome after thrombectomy in stroke. J. Neuroradiol. 2019, 46, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Tornel Garcia-Camba, A.; Requena, M.; Rubiera, M.; Muchada, M.; Pagola, J.; Rodriguez-Luna, D.; Deck, M.; Juega, J.; Rodríguez-Villatoro, N.; Boned Riera, S.; et al. When to Stop: Detrimental Effect of Device Passes in Acute Ischemic Stroke Secondary to Large Vessel Occlusion. Stroke 2019, 50, 1781–1788. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, M.; Liu, Y.; Fitzgerald, S.; Mereuta, O.M.; Arturo Larco, J.L.; Rizvi, A.; Kadirvel, R.; Savastano, L.; Brinjikji, W.; Kallmes, D.F. Systematic review and meta-analysis of current rates of first pass effect by thrombectomy technique and associations with clinical outcomes. J. NeuroInterv. Surg. 2021, 13, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Flottmann, F.; Leischner, H.; Broocks, G.; Nawabi, J.; Bernhardt, M.; Faizy, T.D.; Deb-Chatterji, M.; Thomalla, G.; Fiehler, J.; Brekenfeld, C. Recanalization Rate per Retrieval Attempt in Mechanical Thrombectomy for Acute Ischemic Stroke. Stroke 2018, 49, 2523–2525. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Fan, L. Does stress hyperglycemia in diabetic and non-diabetic acute ischemic stroke patients predict unfavorable outcomes following endovascular treatment? Neurol. Sci. 2023, 44, 1695–1702. [Google Scholar] [CrossRef] [PubMed]
- Filioglo, A.; Cohen, J.E.; Honig, A.; Simaan, N.; Gomori, J.M.; Leker, R.R. More than five stentriever passes: Real benefit or futile recanalization? Neuroradiology 2020, 62, 1335–1340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xing, P.; Tang, J.; Shi, L.; Yang, P.; Zhang, Y.; Zhang, L.; Peng, Y.; Liu, S.; Zhang, L.; et al. Predictors and outcome of early neurological deterioration after endovascular thrombectomy: A secondary analysis of the DIRECT-MT trial. J. NeuroInterv. Surg. 2022, 15, e9–e16. [Google Scholar] [CrossRef] [PubMed]
- Heitkamp, C.; Winkelmeier, L.; Heit, J.J.; Albers, G.W.; Lansberg, M.G.; Wintermark, M.; Broocks, G.; van Horn, N.; Kniep, H.C.; Sporns, P.B.; et al. Unfavorable cerebral venous outflow is associated with futile recanalization in acute ischemic stroke patients. Eur. J. Neurol. 2023, 30, 2684–2692. [Google Scholar] [CrossRef] [PubMed]
- Mohammaden, M.H.; Stapleton, C.J.; Brunozzi, D.; Hussein, A.E.; Khedr, E.M.; Atwal, G.; Alaraj, A. Predictors of Poor Outcome Despite Successful Mechanical Thrombectomy of Anterior Circulation Large Vessel Occlusions Within 6 h of Symptom Onset. Front. Neurol. 2020, 11, 907. [Google Scholar] [CrossRef]
- Spronk, E.; Sykes, G.; Falcione, S.; Munsterman, D.; Joy, T.; Kamtchum-Tatuene, J.; Jickling, G.C. Hemorrhagic Transformation in Ischemic Stroke and the Role of Inflammation. Front. Neurol. 2021, 12, 661955. [Google Scholar] [CrossRef] [PubMed]
- Boisseau, W.; Jean-Philippe, D.; Robert, F.; Maeva, K.; Kevin, Z.; Candice, S.; Guillaume, T.; Malek Ben, M.; Benjamin, M.; Daniele, B.; et al. Neutrophil count predicts poor outcome despite recanalization after endovascular therapy. Neurology 2019, 93, e467. [Google Scholar] [CrossRef] [PubMed]
- Mechtouff, L.; Bochaton, T.; Paccalet, A.; Da Silva, C.C.; Buisson, M.; Amaz, C.; Derex, L.; Ong, E.; Berthezene, Y.; Eker, O.F.; et al. Association of Interleukin-6 Levels and Futile Reperfusion After Mechanical Thrombectomy. Neurology 2021, 96, e752–e757. [Google Scholar] [CrossRef] [PubMed]
- Tajima, Y.; Hayasaka, M.; Ebihara, K.; Kubota, M.; Suda, S. Predictors of poor outcome after successful mechanical thrombectomy in patients with acute anterior circulation stroke. J. Clin. Interv. Radiol. 2017, 1, 139–143. [Google Scholar] [CrossRef]
- Phuong, N.V.; Cong Thanh, N.; Keserci, B.; Sang, N.V.; Minh Duc, N. Mechanical thrombectomy treatment of basilar artery occlusion within 24 hours of symptom onset: A Single-Center Experience. Clin. Ter. 2022, 173, 400–406. [Google Scholar] [CrossRef]
- Aguirre, C.; Trillo, S.; Ramos, C.; Zapata-Wainberg, G.; Sanz-García, A.; Ximénez-Carrillo, Á.; Barbosa, A.; Caniego, J.L.; Vivancos, J. Predictive value of ischemia location on multimodal CT in thrombectomy-treated patients. Neuroradiol. J. 2023, 36, 319–328. [Google Scholar] [CrossRef] [PubMed]
- de Havenon, A.; Elhorany, M.; Boulouis, G.; Naggara, O.; Darcourt, J.; Clarençon, F.; Richard, S.; Marnat, G.; Bourcier, R.; Sibon, I.; et al. Thrombectomy in basilar artery occlusions: Impact of number of passes and futile reperfusion. J. NeuroInterv. Surg. 2023, 15, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Seker, F.; Qureshi, M.M.; Möhlenbruch, M.A.; Nogueira, R.G.; Abdalkader, M.; Ribo, M.; Caparros, F.; Haussen, D.C.; Mohammaden, M.H.; Sheth, S.A.; et al. Reperfusion Without Functional Independence in Late Presentation of Stroke With Large Vessel Occlusion. Stroke 2022, 53, 3594–3604. [Google Scholar] [CrossRef] [PubMed]
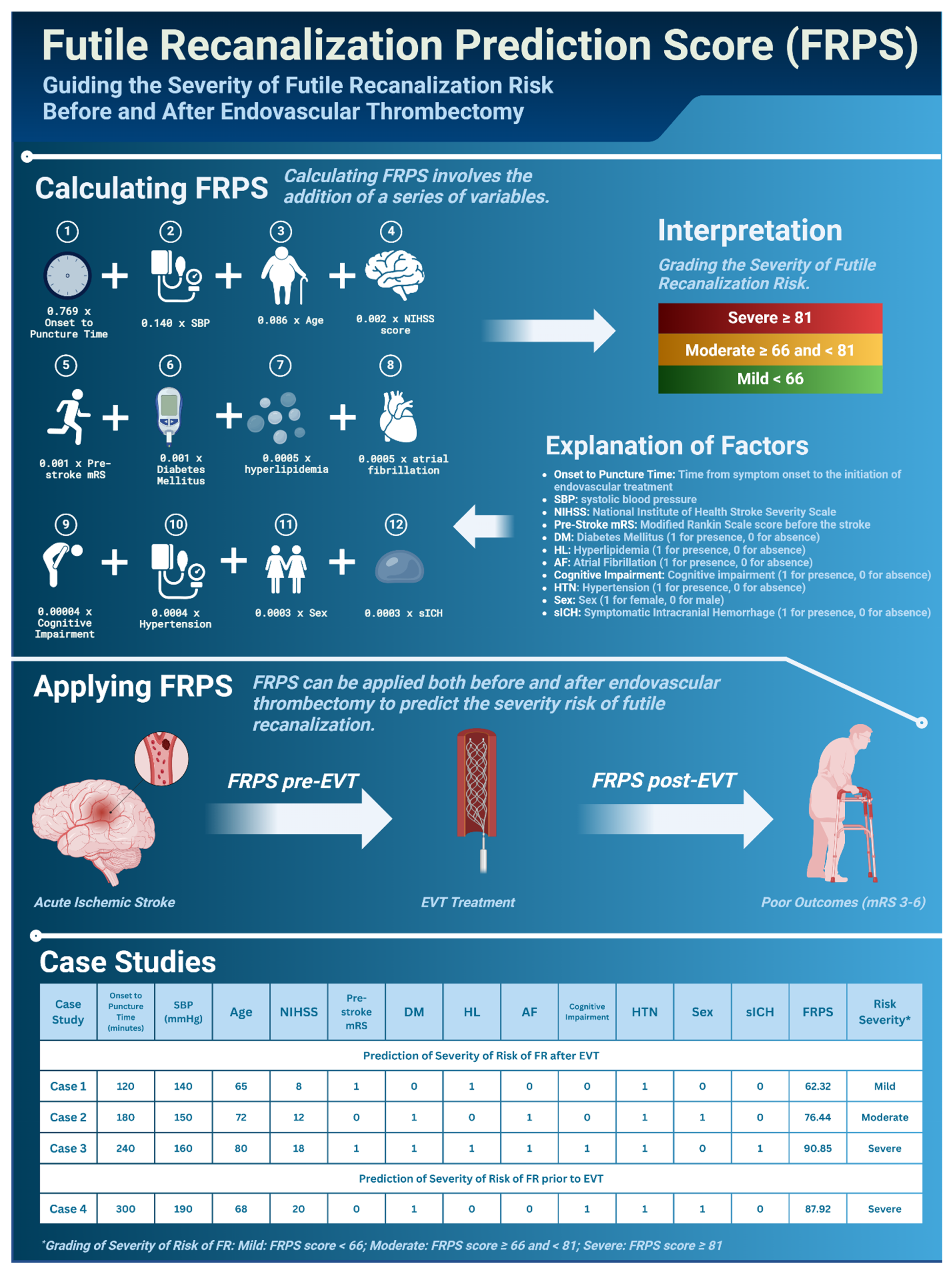
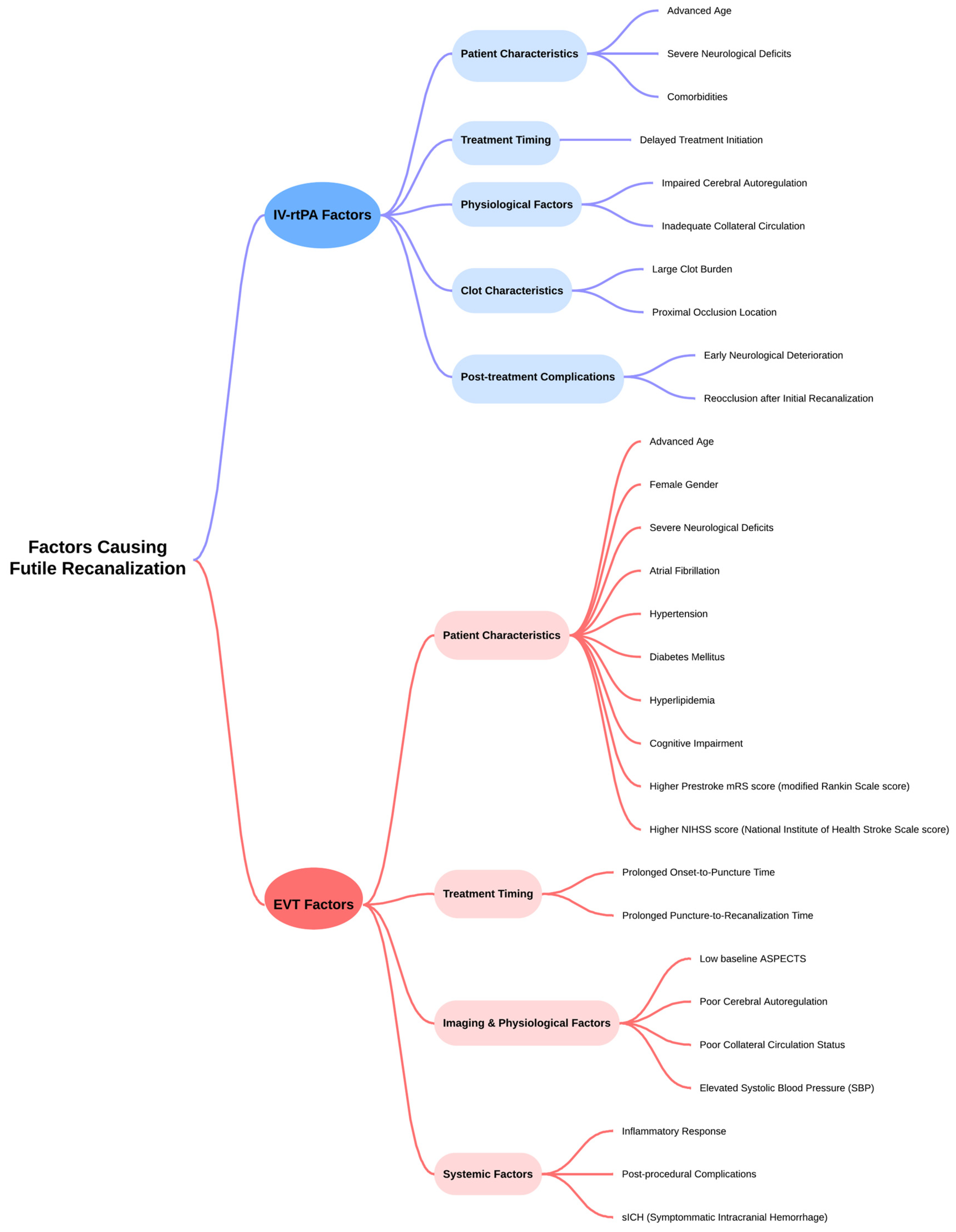
| Author, y | Shahid et al., 2022 [8] | Deng et al., 2022 [9] | Shen et al., 2023 [7] | |
|---|---|---|---|---|
| No. of studies | N | 22 | 12 | 39 |
| No. of patients | n | 3037 | 2138 | 11,700 |
| Search strategy until | - | February 2021 | April 2021 | May 2023 |
| FR prevalence | ||||
| Prevalence | Percentage [95% CI; p-value] | 51% [45.8–54.7%] | 48.7% (only crude prevalence reported) | 51% [48–54%; p < 0.001] |
| FR predictors | ||||
| Age | SMD [95% CI; p-value] | 5.6 [4.7–6.6; p < 0.001] | 5.81 [4.16–7.46; p < 0.00001] | 0.49 [0.42–0.56; p < 0.0001] |
| AF | OR [95% CI; p-value] | 1.5 [1.2–1.8; p < 0.001] | 1.24 [1.01–1.51; p < 0.00001] | 1.39 [1.22–1.59; p < 0.001] |
| Alcohol | OR [95% CI; p-value] | NR | NR | 0.80 [0.581–1.101; p = 0.170] |
| CVD | OR [95% CI; p-value] | 1.4 [1.1–1.8; p < 0.01] | NR | 1.15 [0.795–1.671; p = 0.454] |
| HTN | OR [95% CI; p-value] | 1.5 [1.3–1.9; p < 0.001] | 1.73 [1.43–2.09; p < 0.00001] | 1.65 [1.41–1.92; p < 0.001] |
| HL | OR [95% CI; p-value] | 1.1 [0.9–1.3; p = 0.20] | 1.01 [0.80–1.28; p = 0.92] | 0.97 [0.870–1.088; p = 0.627] |
| DM | OR [95% CI; p-value] | 1.5 [1.1–2.1; p = 0.1] | 1.78 [1.41–2.24; p < 0.00001] | 1.71 [1.47–1.99; p < 0.001] |
| Male sex | OR [95% CI; p-value] | NR | NR | 0.87 [0.77–0.97; p = 0.016] |
| Female sex | OR [95% CI; p-value] | 1.3 [1.1–1.6; p < 0.01] | 1.40 [1.16–1.68; p < 0.0004] | NR |
| PS/TIA | OR [95% CI; p-value] | 1.4 [1.03–2.04; p < 0.03] | NR | 1.30 [1.06–1.59; p = 0.012] |
| Smoking | OR [95% CI; p-value] | 0.6 [0.5–0.7; p < 0.01] | NR | 0.66 [0.57–0.77; p < 0.001] |
| GC | OR [95% CI; p-value] | NR | NR | 0.33 [0.23–0.49; p < 0.001] |
| APU | OR [95% CI; p-value] | 1.1 [0.8–1.4; p = 0.58] | NR | 1.16 [0.976–1.386; p = 0.094] |
| ACU | OR [95% CI; p-value] | 0.5 [0.1–1.6; p = 0.23] | NR | 1.33 [1.08–1.63; p = 0.007] |
| LAA | OR [95% CI; p-value] | NR | 0.92 [0.70–1.21; p = 0.54] | 0.83 [0.671–1.018; p = 0.073] |
| CE | OR [95% CI; p-value] | NR | 1.06 [0.85–1.33; p = 0.60] | 1.34 [1.10–1.63; p = 0.003] |
| GA | OR [95% CI; p-value] | 1.2 [0.78–2.01; p = 0.34] | NR | 1.53 [1.35–1.74; p < 0.001] |
| IVT | OR [95% CI; p-value] | 0.7 [0.5–0.8; p < 0.001] | 0.67 [0.55–0.83; p < 0.0001] | 0.75 [0.66–0.86; p < 0.001] |
| BG | SMD [95% CI; p-value] | NR | 0.59 [0.37–0.81; p < 0.00001] | 0.31 [0.22–0.41; p < 0.001] |
| SBP | SMD [95% CI; p-value] | 6.9 [3.6–8.7; p < 0.001] | 4.98 [1.87–8.09; p < 0.002] | 0.20 [0.13–0.27; p < 0.001] |
| DBP | SMD [95% CI; p-value] | 1.31 [−1.0–3.6; p = 0.26] | −0.36 [−3.14–2.42; p = 0.80] | NR |
| NIHSS | SMD [95% CI; p-value] | 4.2 [3.2–5.1; p < 0.001] | 4.22 [3.38–5.07; p < 0.00001] | 0.75 [0.65–0.86; p < 0.001] |
| ASPECTS | SMD [95% CI; p-value] | −0.5 [−0.8– −0.3; p < 0.001] | −0.71 [−1.23–−0.19; p = 0.007] | −0.37 [−0.46–−0.27; p < 0.001] |
| OTT | SMD [95% CI; p-value] | 24.3 [9.9–38.7; p < 0.001] | 16.92 [6.52–27.31; p < 0.001] | 0.22 [0.13–0.30; p < 0.001] |
| PTR | SMD [95% CI; p-value] | 9.58 [5.3–13.8; p < 0.001] | 12.37 [7.96–16.79; p < 0.00001] | NR |
| OTR | SMD [95% CI; p-value] | 32.1 [6.5–47.7; p < 0.001] | 13.97 [−7.85–35.80; p = 0.21] | 0.38 [0.19–0.57; p < 0.001] |
| OTED | SMD [95% CI; p-value] | 20.1 [4.4–35.8; p < 0.01] | NR | NR |
| ICA occlusion | OR [95% CI; p-value] | NR | 1.85 [1.17–2.95; p = 0.009] | NR |
| MCA-MI occlusion | OR [95% CI; p-value] | NR | 0.81 [0.51–1.28; p = 0.37] | NR |
| MCA-M2 occlusion | OR [95% CI; p-value] | NR | 0.70 [0.42–1.18; p = 0.19] | NR |
| Tandem occlusion | OR [95% CI; p-value] | NR | 1.30 [0.72–2.33; p = 0.38] | NR |
| Procedure complications | OR [95% CI; p-value] | 0.8 [0.4–1.8; p = 0.61] | NR | NR |
| FR outcomes | ||||
| sICH | OR [95% CI; p-value] | 5.7 [2.8–11.65; p < 0.01] | 6.09 [3.18–11.68; p < 0.00001] | 7.37 [4.89–11.12; p < 0.001] |
| HT | OR [95% CI; p-value] | NR | NR | 2.98 [2.37–3.75; p < 0.001] |
| 90-day mortality | OR [95% CI; p-value] | NR | NR | 19.24 [1.57–235.18; p = 0.021] |
| Data processing and evaluation | ||||
| Meta-regression | - | Applied | Applied | Applied |
| Sensitivity analysis | - | NP | NP | Applied |
| Trails sequential analysis | - | NP | NP | NP |
| Evidence of effect | - | NP | NP | NP |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, H.; Huasen, B.B.; Killingsworth, M.C.; Bhaskar, S.M.M. Introducing the Futile Recanalization Prediction Score (FRPS): A Novel Approach to Predict and Mitigate Ineffective Recanalization after Endovascular Treatment of Acute Ischemic Stroke. Neurol. Int. 2024, 16, 605-619. https://doi.org/10.3390/neurolint16030045
Shen H, Huasen BB, Killingsworth MC, Bhaskar SMM. Introducing the Futile Recanalization Prediction Score (FRPS): A Novel Approach to Predict and Mitigate Ineffective Recanalization after Endovascular Treatment of Acute Ischemic Stroke. Neurology International. 2024; 16(3):605-619. https://doi.org/10.3390/neurolint16030045
Chicago/Turabian StyleShen, Helen, Bella B. Huasen, Murray C. Killingsworth, and Sonu M. M. Bhaskar. 2024. "Introducing the Futile Recanalization Prediction Score (FRPS): A Novel Approach to Predict and Mitigate Ineffective Recanalization after Endovascular Treatment of Acute Ischemic Stroke" Neurology International 16, no. 3: 605-619. https://doi.org/10.3390/neurolint16030045
APA StyleShen, H., Huasen, B. B., Killingsworth, M. C., & Bhaskar, S. M. M. (2024). Introducing the Futile Recanalization Prediction Score (FRPS): A Novel Approach to Predict and Mitigate Ineffective Recanalization after Endovascular Treatment of Acute Ischemic Stroke. Neurology International, 16(3), 605-619. https://doi.org/10.3390/neurolint16030045








