Perspectives on Stem Cell Therapy in Diabetic Neuropathic Pain
Abstract
1. Introduction
2. Diabetic Neuropathy and Diabetic Neuropathic Pain
3. Mesenchymal Stromal Cells as Alternative Therapy for Diabetic Neuropathy
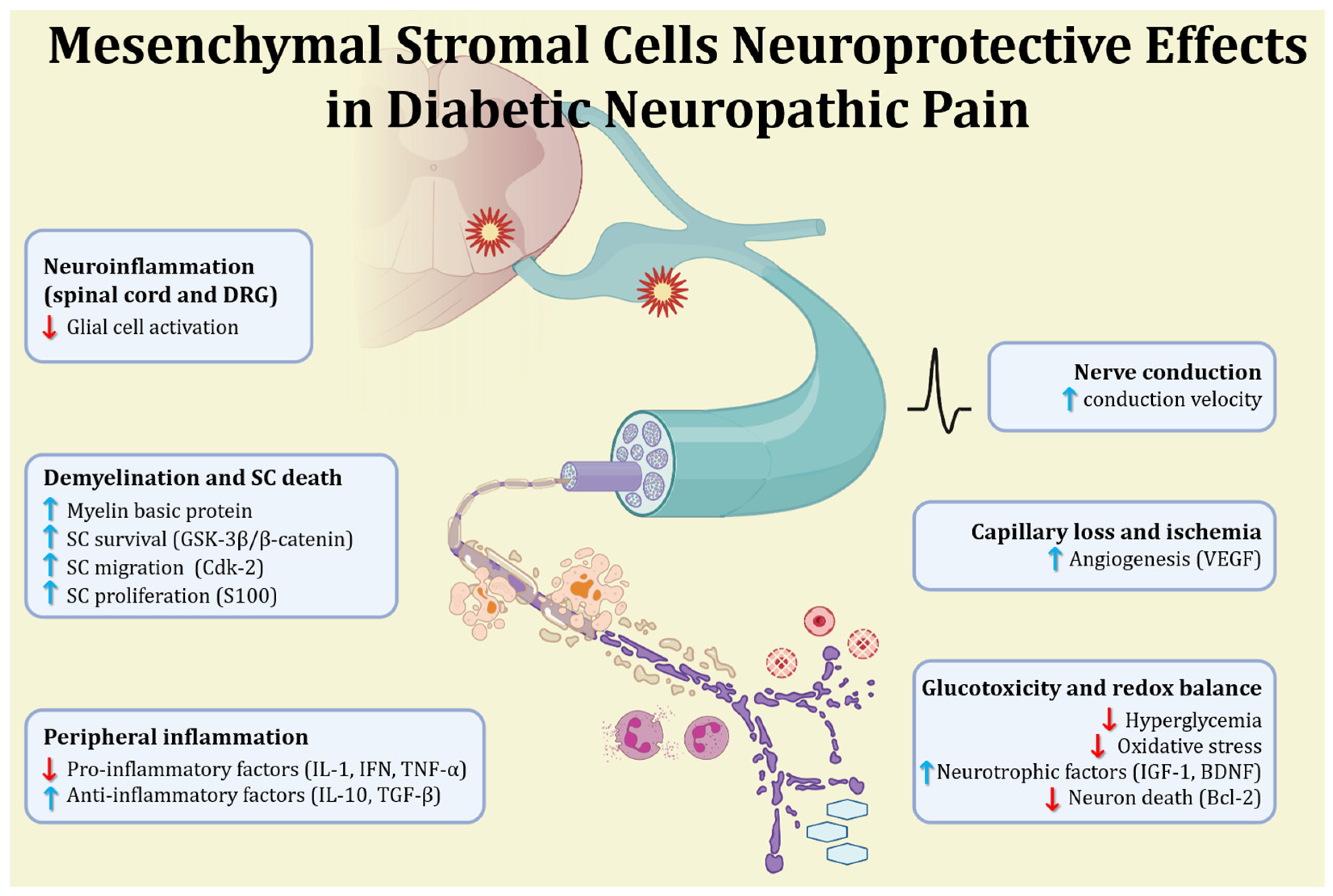
4. Mesenchymal Stromal Cells in Diabetic Neuropathic Pain
5. Clinical Translation of Mesenchymal Stromal Cells Therapy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamenov, Z.A.; Traykov, L.D. Diabetic autonomic neuropathy. Adv. Exp. Med. Biol. 2012, 771, 176–193. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, A.K. Diabetic neuropathic pain: Physiopathology and treatment. World J. Diabetes 2015, 6, 432–444. [Google Scholar] [CrossRef]
- Scholz, J.; Woolf, C.J. The neuropathic pain triad: Neurons, immune cells and glia. Nat. Neurosci. 2007, 10, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Ali, A.; Katare, R. Molecular complexities underlying the vascular complications of diabetes mellitus - A comprehensive review. J Diabetes Complicat. 2020, 34, 107613. [Google Scholar] [CrossRef] [PubMed]
- Stoltz, J.-F.; De Isla, N.; Li, Y.P.; Bensoussan, D.; Zhang, L.; Huselstein, C.; Chen, Y.; Decot, V.; Magdalou, J.; Li, N.; et al. Stem Cells and Regenerative Medicine: Myth or Reality of the 21th Century. Stem Cells Int. 2015, 2015, 734731. [Google Scholar] [CrossRef]
- Li, F.-X.-Z.; Lin, X.; Xu, F.; Shan, S.-K.; Guo, B.; Lei, L.-M.; Zheng, M.-H.; Wang, Y.; Xu, Q.-S.; Yuan, L.-Q. The Role of Mesenchymal Stromal Cells-Derived Small Extracellular Vesicles in Diabetes and Its Chronic Complications. Front. Endocrinol. 2021, 12, 780974. [Google Scholar] [CrossRef]
- Lachaud, C.C.; Cobo-Vuilleumier, N.; Fuente-Martin, E.; Diaz, I.; Andreu, E.; Cahuana, G.M.; Tejedo, J.R.; Hmadcha, A.; Gauthier, B.R.; Soria, B. Umbilical cord mesenchymal stromal cells transplantation delays the onset of hyperglycemia in the RIP-B7.1 mouse model of experimental autoimmune diabetes through multiple immunosuppressive and anti-inflammatory responses. Front. Cell Dev. Biol. 2023, 11, 1089817. [Google Scholar] [CrossRef]
- Fan, B.; Li, C.; Szalad, A.; Wang, L.; Pan, W.; Zhang, R.; Chopp, M.; Zhang, Z.G.; Liu, X.S. Mesenchymal stromal cell-derived exosomes ameliorate peripheral neuropathy in a mouse model of diabetes. Diabetologia 2020, 63, 431–443. [Google Scholar] [CrossRef]
- Gao, F.; Chiu, S.M.; Motan, D.A.L.; Zhang, Z.; Chen, L.; Ji, H.-L.; Tse, H.-F.; Fu, Q.-L.; Lian, Q. Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death Dis. 2016, 7, e2062. [Google Scholar] [CrossRef]
- Bain, B.J. Bone marrow biopsy morbidity and mortality. Br. J. Haematol. 2003, 121, 949–951. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Guan, H.; Oishi, H.; Takahashi, S.; Zhang, C. Human umbilical cord mesenchymal stem cells in diabetes mellitus and its complications: Applications and research advances. Int. J. Med. Sci. 2023, 20, 1492–1507. [Google Scholar] [CrossRef] [PubMed]
- Scuteri, A.; Monfrini, M. Mesenchymal Stem Cells as New Therapeutic Approach for Diabetes and Pancreatic Disorders. Int. J. Mol. Sci. 2018, 19, 2783. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.; Kahlenberg, S.; Hornsby, P. Therapeutic potential of mesenchymal stem cells for diabetes. J. Mol. Endocrinol. 2017, 59, R109–R120. [Google Scholar] [CrossRef]
- Feldman, E.L.; Callaghan, B.C.; Pop-Busui, R.; Zochodne, D.W.; Wright, D.E.; Bennett, D.L.; Bril, V.; Russell, J.W.; Viswanathan, V. Diabetic neuropathy. Nat. Rev. Dis. Prim. 2019, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Calcutt, N.A. Diabetic neuropathy and neuropathic pain: A (con)fusion of pathogenic mechanisms? Pain 2020, 161, S65–S86. [Google Scholar] [CrossRef]
- Hicks, C.W.; Selvin, E. Epidemiology of Peripheral Neuropathy and Lower Extremity Disease in Diabetes. Curr. Diabetes Rep. 2019, 19, 86. [Google Scholar] [CrossRef]
- Lund, J.; Ouwens, D.M.; Wettergreen, M.; Bakke, S.S.; Thoresen, G.H.; Aas, V. Increased glycolysis and higher lactate production in hyperglycemic myotubes. Cell 2019, 8, 1101. [Google Scholar] [CrossRef]
- Vincent, A.M.; Hayes, J.M.; McLean, L.L.; Vivekanandan-Giri, A.; Pennathur, S.; Feldman, E.L. Dyslipidemia-induced neuropathy in mice: The role of oxLDL/LOX-1. Diabetes 2009, 58, 2376–2385. [Google Scholar] [CrossRef]
- Ishibashi, F.; Taniguchi, M.; Kosaka, A.; Uetake, H.; Tavakoli, M. Improvement in Neuropathy Outcomes with Normalizing HbA1c in Patients with Type 2 Diabetes. Diabetes Care 2019, 42, 110–118. [Google Scholar] [CrossRef]
- Park, S.; Kang, H.-J.; Jeon, J.-H.; Kim, M.-J.; Lee, I.-K. Recent advances in the pathogenesis of microvascular complications in diabetes. Arch. Pharmacal. Res. 2019, 42, 252–262. [Google Scholar] [CrossRef]
- Pathak, R.; Sachan, N.; Chandra, P. Mechanistic approach towards diabetic neuropathy screening techniques and future challenges: A review. Biomed. Pharmacother. 2022, 150, 113025. [Google Scholar] [CrossRef]
- Ang, L.; Jaiswal, M.; Martin, C.; Pop-Busui, R. Glucose control and diabetic neuropathy: Lessons from recent large clinical trials. Curr. Diabetes Rep. 2014, 14, 528. [Google Scholar] [CrossRef] [PubMed]
- Baum, P.; Toyka, K.V.; Blüher, M.; Kosacka, J.; Nowicki, M. Inflammatory Mechanisms in the Pathophysiology of Diabetic Peripheral Neuropathy (DN)—New Aspects. Int. J. Mol. Sci. 2021, 22, 10835. [Google Scholar] [CrossRef]
- Hall, B.E.; Macdonald, E.; Cassidy, M.; Yun, S.; Sapio, M.R.; Ray, P.; Doty, M.; Nara, P.; Burton, M.D.; Shiers, S.; et al. Transcriptomic analysis of human sensory neurons in painful diabetic neuropathy reveals inflammation and neuronal loss. Sci. Rep. 2022, 12, 4729. [Google Scholar] [CrossRef]
- Pang, L.; Lian, X.; Liu, H.; Zhang, Y.; Li, Q.; Cai, Y.; Ma, H.; Yu, X. Understanding Diabetic Neuropathy: Focus on Oxidative Stress. Oxid. Med. Cell. Longev. 2020, 2020, 9524635. [Google Scholar] [CrossRef] [PubMed]
- Albers, J.W.; Pop-Busui, R. Diabetic neuropathy: Mechanisms, emerging treatments, and subtypes. Curr. Neurol. Neurosci. Rep. 2014, 14, 473. [Google Scholar] [CrossRef]
- Stevens, M.J.; Obrosova, I.; Cao, X.; Van Huysen, C.; Greene, D.A. Effects of DL-alpha-lipoic acid on peripheral nerve conduction, blood flow, energy metabolism, and oxidative stress in experimental diabetic neuropathy. Diabetes 2000, 49, 1006–1015. [Google Scholar] [CrossRef] [PubMed]
- Hung, A.L.; Lim, M.; Doshi, T.L. Targeting cytokines for treatment of neuropathic pain. Scand. J. Pain 2017, 17, 287–293. [Google Scholar] [CrossRef]
- Sloan, G.; Shillo, P.; Selvarajah, D.; Wu, J.; Wilkinson, I.D.; Tracey, I.; Anand, P.; Tesfaye, S. A new look at painful diabetic neuropathy. Diabetes Res. Clin. Pract. 2018, 144, 177–191. [Google Scholar] [CrossRef]
- Staehelin, J.T. The pathogenesis of painful diabetic neuropathy and clinical presentation. Diabetes Res. Clin. Pract. 2023, 206 (Suppl. 1), 110753. [Google Scholar] [CrossRef]
- Du, X.; Hao, H.; Yang, Y.; Huang, S.; Wang, C.; Gigout, S.; Ramli, R.; Li, X.; Jaworska, E.; Edwards, I.; et al. Local GABAergic signaling within sensory ganglia controls peripheral nociceptive transmission. J. Clin. Investig. 2017, 127, 1741–1756. [Google Scholar] [CrossRef] [PubMed]
- Miljanich, G.P. Ziconotide: Neuronal Calcium Channel Blocker for Treating Severe Chronic Pain. Curr. Med. Chem. 2004, 11, 3029–3040. [Google Scholar] [CrossRef] [PubMed]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and Molecular Mechanisms of Pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef]
- Ma, J.; Shi, M.; Zhang, X.; Liu, X.; Chen, J.; Zhang, R.; Wang, X.; Zhang, H. GLP-1R agonists ameliorate peripheral nerve dysfunction and inflammation via p38 MAPK/NF-κB signaling pathways in streptozotocin-induced diabetic rats. Int. J. Mol. Med. 2018, 41, 2977–2985. [Google Scholar] [CrossRef] [PubMed]
- Han, J.W.; Choi, D.; Lee, M.Y.; Huh, Y.H.; Yoon, Y.-S. Bone Marrow-Derived Mesenchymal Stem Cells Improve Diabetic Neuropathy by Direct Modulation of Both Angiogenesis and Myelination in Peripheral Nerves. Cell Transplant. 2016, 25, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, A.F.; Vannier-Santos, M.A.; De Silva, G.S.D.; Silva, D.N.; Juiz, P.J.L.; Nonaka, C.K.V.; Dos Santos, R.R.; Soares, M.B.P.; Villarreal, C.F. Bone marrow-derived mesenchymal stem/stromal cells reverse the sensorial diabetic neuropathy via modulation of spinal neuroinflammatory cascades. J. Neuroinflamm. 2018, 15, 189. [Google Scholar] [CrossRef]
- He, D.; Xu, Y.; Xiong, X.; Yin, C.; Lei, S.; Cheng, X. The bone marrow-derived mesenchymal stem cells (BMSCs) alleviate diabetic peripheral neuropathy induced by STZ via activating GSK-3β/β-catenin signaling pathway. Environ. Toxicol. Pharmacol. 2020, 79, 103432. [Google Scholar] [CrossRef]
- Kim, B.J.; Jin, H.K.; Bae, J.-S. Bone Marrow-Derived Mesenchymal Stem Cells Improve the Functioning of Neurotrophic Factors in a Mouse Model of Diabetic Neuropathy. Lab. Anim. Res. 2011, 27, 171–176. [Google Scholar] [CrossRef]
- Waterman, R.S.; Morgenweck, J.; Nossaman, B.D.; Scandurro, A.E.; Scandurro, S.A.; Betancourt, A.M. Anti-Inflammatory Mesenchymal Stem Cells(MSC2)Attenuate Symptoms of Painful Diabetic Peripheral Neuropathy. Stem Cells Transl. Med. 2012, 1, 557–565. [Google Scholar] [CrossRef]
- Abdelrahman, S.A.; Samak, M.A.; Shalaby, S.M. Fluoxetine pretreatment enhances neurogenic, angiogenic and immunomodulatory effects of MSCs on experimentally induced diabetic neuropathy. Cell Tissue Res. 2018, 374, 83–97. [Google Scholar] [CrossRef]
- Shahani, P.; Mahadevan, A.; Mondal, K.; Waghmare, G.; Datta, I. Repeat intramuscular transplantation of human dental pulp stromal cells is more effective in sustaining Schwann cell survival and myelination for functional recovery after onset of diabetic neuropathy. Cytotherapy 2023, 25, 1200–1211. [Google Scholar] [CrossRef] [PubMed]
- Omi, M.; Hata, M.; Nakamura, N.; Miyabe, M.; Kobayashi, Y.; Kamiya, H.; Nakamura, J.; Ozawa, S.; Tanaka, Y.; Takebe, J.; et al. Transplantation of dental pulp stem cells suppressed inflammation in sciatic nerves by promoting macrophage polarization towards anti-inflammation phenotypes and ameliorated diabetic polyneuropathy. J. Diabetes Investig. 2016, 7, 485–496. [Google Scholar] [CrossRef]
- Hata, M.; Omi, M.; Kobayashi, Y.; Nakamura, N.; Tosaki, T.; Miyabe, M.; Kojima, N.; Kubo, K.; Ozawa, S.; Maeda, H.; et al. Transplantation of cultured dental pulp stem cells into the skeletal muscles ameliorated diabetic polyneuropathy: Therapeutic plausibility of freshly isolated and cryopreserved dental pulp stem cells. Stem Cell Res. Ther. 2015, 6, 162. [Google Scholar] [CrossRef]
- Yigitturk, G.; Erbas, O.; Yavasoglu, N.U.K.; Acikgoz, E.; Buhur, A.; Gokhan, A.; Gurel, C.; Gunduz, C.; Yavasoglu, A. The neuro-restorative effect of adipose-derived mesenchymal stem cell transplantation on a mouse model of diabetic neuropathy. Neurol. Res. 2022, 44, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Oses, C.; Olivares, B.; Ezquer, M.; Acosta, C.; Bosch, P.; Donoso, M.; Léniz, P.; Ezquer, F. Preconditioning of adipose tissue-derived mesenchymal stem cells with deferoxamine increases the production of pro-angiogenic, neuroprotective and anti-inflammatory factors: Potential application in the treatment of diabetic neuropathy. PLoS ONE 2017, 12, e0178011. [Google Scholar] [CrossRef]
- Zhang, S.; Shi, B. Erythropoietin Modification Enhances the Protection of Mesenchymal Stem Cells on Diabetic Rat-Derived Schwann Cells: Implications for Diabetic Neuropathy. BioMed Res. Int. 2017, 2017, 6352858. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yang, Y.; Zhang, Y.; Hao, G.; Liu, T.; Wang, L.; Yang, T.; Wang, Q.; Zhang, G.; Wei, J.; et al. Placental mesenchymal stem cells of fetal and maternal origins demonstrate different therapeutic potentials. Stem Cell Res. Ther. 2014, 5, 48. [Google Scholar] [CrossRef]
- Pan, S.; Hada, S.S.; Liu, Y.; Hu, C.; Zhou, M.; Zheng, S.; Xu, M.; Shi, C.; Yin, S.; Xie, X. Human Placenta-Derived Mesenchymal Stem Cells Ameliorate Diabetic Neuropathy via Wnt Signaling Pathway. Stem Cells Int. 2022, 2022, 6897056. [Google Scholar] [CrossRef]
- Yang, L.-F.; He, J.-D.; Jiang, W.-Q.; Wang, X.-D.; Yang, X.-C.; Liang, Z.; Zhou, Y.-K. Interferon-gamma treatment of human umbilical cord mesenchymal stem cells can significantly reduce damage associated with diabetic peripheral neuropathy in mice. Curr. Stem Cell Res. Ther. 2023, 19, 1129–1141. [Google Scholar] [CrossRef]
- Wang, C.; Chi, J.; Che, K.; Ma, X.; Qiu, M.; Wang, Z.; Wang, Y. The combined effect of mesenchymal stem cells and resveratrol on type 1 diabetic neuropathy. Exp. Ther. Med 2019, 17, 3555–3563. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Liang, H.; Cai, Y.; Li, X.; Yan, L.; Zhou, L.; Shan, L.; Wang, H. Human umbilical cord-derived mesenchymal stem cells not only ameliorate blood glucose but also protect vascular endothelium from diabetic damage through a paracrine mechanism mediated by MAPK/ERK signaling. Stem Cell Res. Ther. 2022, 13, 258. [Google Scholar] [CrossRef] [PubMed]
- Lindoso, R.S.; Collino, F.; Vieyra, A. Extracellular vesicles as regulators of tumor fate: Crosstalk among cancer stem cells, tumor cells and mesenchymal stem cells. Stem Cell Investig. 2017, 4, 75. [Google Scholar] [CrossRef]
- Fan, B.; Chopp, M.; Zhang, Z.G.; Liu, X.S. Treatment of diabetic peripheral neuropathy with engineered mesenchymal stromal cell-derived exosomes enriched with microRNA-146a provide amplified therapeutic efficacy. Exp. Neurol. 2021, 341, 113694. [Google Scholar] [CrossRef]
- Røikjer, J.; Mørch, C.D.; Ejskjaer, N. Diabetic Peripheral Neuropathy: Diagnosis and Treatment. Curr. Drug Saf. 2021, 16, 2–16. [Google Scholar] [CrossRef]
- Alam, U.; Sloan, G.; Tesfaye, S. Treating Pain in Diabetic Neuropathy: Current and Developmental Drugs. Drugs 2020, 80, 363–384. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wu, L.; Deng, H.; Chen, Y.; Zhou, H.; Liu, M.; Wang, S.; Zheng, L.; Zhu, L.; Lv, X. Anti-inflammatory protein TSG-6 secreted by bone marrow mesenchymal stem cells attenuates neuropathic pain by inhibiting the TLR2/MyD88/NF-κB signaling pathway in spinal microglia. J. Neuroinflamm. 2020, 17, 154. [Google Scholar] [CrossRef]
- Miyano, K.; Ikehata, M.; Ohshima, K.; Yoshida, Y.; Nose, Y.; Yoshihara, S.-I.; Oki, K.; Shiraishi, S.; Uzu, M.; Nonaka, M.; et al. Intravenous administration of human mesenchymal stem cells derived from adipose tissue and umbilical cord improves neuropathic pain via suppression of neuronal damage and anti-inflammatory actions in rats. PLoS ONE 2022, 17, e0262892. [Google Scholar] [CrossRef]
- Chiang, C.-Y.; Liu, S.-A.; Sheu, M.-L.; Chen, F.-C.; Chen, C.-J.; Su, H.-L.; Pan, H.-C. Feasibility of human amniotic fluid derived stem cells in alleviation of neuropathic pain in chronic constrictive injury nerve model. PLoS ONE 2016, 11, e0159482. [Google Scholar] [CrossRef] [PubMed]
- Brini, A.T.; Amodeo, G.; Ferreira, L.M.; Milani, A.; Niada, S.; Moschetti, G.; Franchi, S.; Borsani, E.; Rodella, L.F.; Panerai, A.E.; et al. Therapeutic effect of human adipose-derived stem cells and their secretome in experimental diabetic pain. Sci. Rep. 2017, 7, 9904. [Google Scholar] [CrossRef]
- Cao, X.; Han, Z.-B.; Zhao, H.; Liu, Q. Transplantation of mesenchymal stem cells recruits trophic macrophages to induce pancreatic beta cell regeneration in diabetic mice. Int. J. Biochem. Cell Biol. 2014, 53, 372–379. [Google Scholar] [CrossRef]
- Maacha, S.; Sidahmed, H.; Jacob, S.; Gentilcore, G.; Calzone, R.; Grivel, J.-C.; Cugno, C. Paracrine mechanisms of mesenchymal stromal cells in angiogenesis. Stem Cells Int. 2020, 2020, 4356359. [Google Scholar] [CrossRef] [PubMed]
- Sacerdote, P.; Niada, S.; Franchi, S.; Arrigoni, E.; Rossi, A.; Yenagi, V.; De Girolamo, L.; Panerai, A.E.; Brini, A.T. Systemic administration of human adipose-derived stem cells reverts nociceptive hypersensitivity in an experimental model of neuropathy. Stem Cells Dev. 2013, 22, 1252–1263. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, C.; Contador, D.; Díaz, D.; Cárcamo, C.; Santapau, D.; Lobos-Gonzalez, L.; Acosta, C.; Campero, M.; Carpio, D.; Gabriele, C.; et al. Human adipose-derived mesenchymal stem cell-conditioned medium ameliorates polyneuropathy and foot ulceration in diabetic BKS db/db mice. Stem Cell Res. Ther. 2020, 11, 168. [Google Scholar] [CrossRef] [PubMed]
- Drobiova, H.; Sindhu, S.; Ahmad, R.; Haddad, D.; Al-Mulla, F.; Al Madhoun, A. Wharton’s jelly mesenchymal stem cells: A concise review of their secretome and prospective clinical applications. Front. Cell Dev. Biol. 2023, 11, 1211217. [Google Scholar] [CrossRef]
- Li, J.; Deng, G.; Wang, H.; Yang, M.; Yang, R.; Li, X.; Zhang, X.; Yuan, H. Interleukin-1β pre-treated bone marrow stromal cells alleviate neuropathic pain through CCL7-mediated inhibition of microglial activation in the spinal cord. Sci. Rep. 2017, 7, 42260. [Google Scholar] [CrossRef]
- Yerofeyeva, A.V.; Pinchuk, S.V.; Rjabceva, S.N.; Molchanova, A.Y. The role of cannabinoid CB1 receptors in the antinociceptive and reparative actions of mesenchymal stem cells in rats with peripheral neuropathic pain. Ibrain. Ibrain 2023, 9, 245–257. [Google Scholar] [CrossRef]
- Yamamoto, W.; Mikami, T.; Iwamura, H. Involvement of central cannabinoid CB2 receptor in reducing mechanical allodynia in a mouse model of neuropathic pain. Eur. J. Pharmacol. 2008, 583, 56–61. [Google Scholar] [CrossRef]
- Ruhl, T.; Karthaus, N.; Kim, B.-S.; Beier, J.P. The endocannabinoid receptors CB1 and CB2 affect the regenerative potential of adipose tissue MSCs. Exp. Cell Res. 2020, 389, 111881. [Google Scholar] [CrossRef]
- Racz, I.; Nadal, X.; Alferink, J.; Baños, J.E.; Rehnelt, J.; Martín, M.; Pintado, B.; Gutierrez-Adan, A.; Sanguino, E.; Manzanares, J.; et al. Crucial role of CB(2) cannabinoid receptor in the regulation of central immune responses during neuropathic pain. J. Neurosci. 2008, 28, 12125–12135. [Google Scholar] [CrossRef]
- Adelipour, M.; Lubman, D.M.; Kim, J. Potential applications of mesenchymal stem cells and their derived exosomes in regenerative medicine. Expert Opin. Biol. Ther. 2023, 23, 491–507. [Google Scholar] [CrossRef]
- Hade, M.D.; Suire, C.N.; Suo, Z. Mesenchymal stem cell-derived exosomes: Applications in regenerative medicine. Cells 2021, 10, 1959. [Google Scholar] [CrossRef] [PubMed]
- Shiue, S.-J.; Rau, R.-H.; Shiue, H.-S.; Hung, Y.-W.; Li, Z.-X.; Yang, K.D.; Cheng, J.-K. Mesenchymal stem cell exosomes as a cell–free therapy for nerve injury–induced pain in rats. Pain 2018, 160, 210–223. [Google Scholar] [CrossRef]
- Neuropathic Pain in Adults: Pharmacological Management in Non-Specialist Settings; NICE Clinical Guidelines, No. 173; National Institute for Health and Care Excellence (NICE): London, UK, 22 September 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK552848/ (accessed on 19 June 2024).
- Lapin, B.R.; Pantalone, K.M.; Milinovich, A.; Morrison, S.; Schuster, A.; Boulos, F.; Johnson, K.; Thakore, N.J. Pain in Patients with Type 2 Diabetes-Related Polyneuropathy Is Associated with Vascular Events and Mortality. J. Clin. Endocrinol. Metab. 2020, 105, 3005–3014. [Google Scholar] [CrossRef]
- Castro-Manrreza, M.E.; Montesinos, J.J. Immunoregulation by mesenchymal stem cells: Biological aspects and clinical applications. Clin. Dev. Immunol. 2015, 2015, 394917. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Zhang, X.; Li, X. Exosomes derived from mesenchymal stem cells. Int. J. Mol. Sci. 2014, 15, 4142–4157. [Google Scholar] [CrossRef]
- Nery, A.A.; Nascimento, I.C.; Glaser, T.; Bassaneze, V.; Krieger, J.E.; Ulrich, H. Human mesenchymal stem cells: From immunophenotyping by flow cytometry to clinical applications. Cytom. Part A J. Int. Soc. Anal. Cytol. 2013, 83, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.K.; Shih, H.-H.; Parveen, F.; Lenzen, D.; Ito, E.; Chan, T.-F.; Ke, L.-Y. Identifying the Therapeutic Significance of Mesenchymal Stem Cells. Cells 2020, 9, 1145. [Google Scholar] [CrossRef]
- Izadi, M.; Nejad, A.S.H.; Moazenchi, M.; Masoumi, S.; Rabbani, A.; Kompani, F.; Asl, A.A.H.; Kakroodi, F.A.; Jaroughi, N.; Meybodi, M.A.M.; et al. Mesenchymal stem cell transplantation in newly diagnosed type-1 diabetes patients: A phase I/II randomized placebo-controlled clinical trial. Stem Cell Res. Ther. 2022, 13, 264. [Google Scholar] [CrossRef]
- Ryu, V.; Garretson, J.T.; Liu, Y.; Vaughan, C.H.; Bartness, T.J. Brown adipose tissue has sympathetic-sensory feedback circuits. J. Neurosci. 2015, 35, 2181–2190. [Google Scholar] [CrossRef]
- Kingham, P.J.; Kolar, M.K.; Novikova, L.N.; Wiberg, M. Stimulating the neurotrophic and angiogenic properties of human adipose-derived stem cells enhances nerve repair. Stem Cells Dev 2014, 23, 741–754. [Google Scholar] [CrossRef]
- Hua, T.; Yang, M.; Song, H.; Kong, E.; Deng, M.; Li, Y.; Li, J.; Liu, Z.; Fu, H.; Wang, Y.; et al. Huc-MSCs-derived exosomes attenuate inflammatory pain by regulating microglia pyroptosis and autophagy via the miR-146a-5p/TRAF6 axis. J. Nanobiotechnol. 2022, 20, 324. [Google Scholar] [CrossRef]
- Du, S.; Zeugolis, D.I.; O’brien, T. Scaffold-based delivery of mesenchymal stromal cells to diabetic wounds. Stem Cell Res. Ther. 2022, 13, 426. [Google Scholar] [CrossRef] [PubMed]
- Nikfarjam, S.; Rezaie, J.; Zolbanin, N.M.; Jafari, R. Mesenchymal stem cell derived-exosomes: A modern approach in translational medicine. J. Transl. Med. 2020, 18, 449. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Wu, Y.; Xu, Y.; Li, G.; Li, Z.; Liu, T. Mesenchymal stem cell-derived exosomes in cancer therapy resistance: Recent advances and therapeutic potential. Mol. Cancer 2022, 21, 179. [Google Scholar] [CrossRef] [PubMed]
- Kioskli, K.; Scott, W.; Winkley, K.; Kylakos, S.; McCracken, L.M. Psychosocial factors in painful diabetic neuropathy: A systematic review of treatment trials and survey studies. Pain Med. 2019, 20, 1756–1773. [Google Scholar] [CrossRef] [PubMed]
- Jovic, D.; Yu, Y.; Wang, D.; Wang, K.; Li, H.; Xu, F.; Liu, C.; Liu, J.; Luo, Y. A Brief Overview of Global Trends in MSC-Based Cell Therapy. Stem Cell Rev. Rep. 2022, 18, 1525–1545. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montagnoli, T.L.; Santos, A.D.; Sudo, S.Z.; Gubert, F.; Vasques, J.F.; Mendez-Otero, R.; de Sá, M.P.L.; Zapata-Sudo, G. Perspectives on Stem Cell Therapy in Diabetic Neuropathic Pain. Neurol. Int. 2024, 16, 933-944. https://doi.org/10.3390/neurolint16050070
Montagnoli TL, Santos AD, Sudo SZ, Gubert F, Vasques JF, Mendez-Otero R, de Sá MPL, Zapata-Sudo G. Perspectives on Stem Cell Therapy in Diabetic Neuropathic Pain. Neurology International. 2024; 16(5):933-944. https://doi.org/10.3390/neurolint16050070
Chicago/Turabian StyleMontagnoli, Tadeu Lima, Aimeé Diogenes Santos, Susumu Zapata Sudo, Fernanda Gubert, Juliana Ferreira Vasques, Rosalia Mendez-Otero, Mauro Paes Leme de Sá, and Gisele Zapata-Sudo. 2024. "Perspectives on Stem Cell Therapy in Diabetic Neuropathic Pain" Neurology International 16, no. 5: 933-944. https://doi.org/10.3390/neurolint16050070
APA StyleMontagnoli, T. L., Santos, A. D., Sudo, S. Z., Gubert, F., Vasques, J. F., Mendez-Otero, R., de Sá, M. P. L., & Zapata-Sudo, G. (2024). Perspectives on Stem Cell Therapy in Diabetic Neuropathic Pain. Neurology International, 16(5), 933-944. https://doi.org/10.3390/neurolint16050070






