Haloperidol-Induced Catalepsy and Its Correlations with Acetylcholinesterase Activity in Different Brain Structures of Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Animals and Drug
2.3. Effects of Hal on Commercial Enzyme AChE
2.4. AChE Activity Distribution in Different Tissues of the Brain and Inhibitory Effects of Hal
2.5. Catalepsy Test and AChE Activity in Striatum, Hippocampus, Septo-Hippocampal System
2.6. Statistical Analyses
3. Results
3.1. Inhibitory Effects of Hal on the Commercial Enzyme AChE from Electrophorus electricus
3.2. Distribution of AChE Activity in Several Mouse Brain Regions
3.3. Inhibitory Effects of Hal on AChE Activity In Vitro in Homogenate Obtained from Striatum, Hippocampus, and Septo-Hippocampal System
3.4. Effects of Hal on Catalepsy
3.5. Effects of Hal on AChE Activity In Vivo in Striatum, Hippocampus, and Septo-Hippocampal System
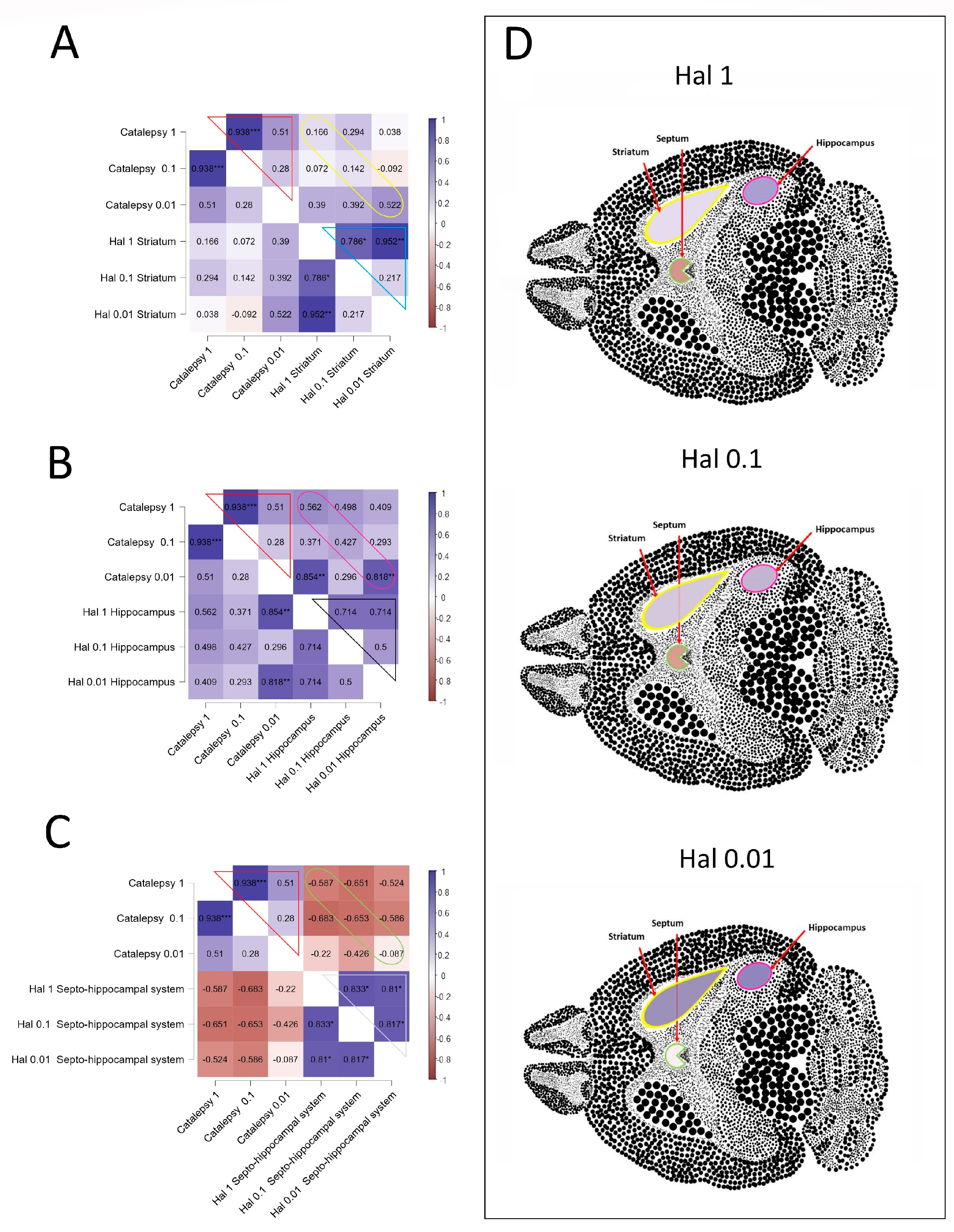
3.6. Correlations Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ballard, C.; Kales, H.C.; Lyketsos, C.; Aarsland, D.; Creese, B.; Mills, R.; Williams, H.; Sweet, R.A. Psychosis in Alzheimer’s Disease. Curr. Neurol. Neurosci. Rep. 2020, 20, 57. [Google Scholar] [CrossRef] [PubMed]
- Podsiedlik, M.; Markowicz-Piasecka, M.; Sikora, J. The Influence of Selected Antipsychotic Drugs on Biochemical Aspects of Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 4621. [Google Scholar] [CrossRef]
- Remington, G.; Hahn, M.K.; Agarwal, S.M.; Chintoh, A.; Agid, O. Schizophrenia: Antipsychotics and drug development. Behav. Brain Res. 2021, 414, 113507. [Google Scholar] [CrossRef] [PubMed]
- Grinchii, D.; Dremencov, E. Mechanism of Action of Atypical Antipsychotic Drugs in Mood Disorders. Int. J. Mol. Sci. 2020, 21, 1–15. [Google Scholar] [CrossRef]
- Prieto, S.G.; Silva, J.C.S.; de Lima, M.O.; Almeida, M.C.; Echeverry, M.B. Cross-tolerance between nitric oxide synthase inhibition and atypical antipsychotics modify nicotinamide-adenine-dinucleotide phosphate-diaphorase activity in mouse lateral striatum. Behav. Pharmacol. 2019, 30, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.W. A history of antipsychotic drug development. Compr. Psychiatry 1999, 40, 407–414. [Google Scholar] [CrossRef]
- Lacasse, H.; Perreault, M.M.; Williamson, D.R. Systematic review of antipsychotics for the treatment of hospital-associated delirium in medically or surgically ill patients. Ann. Pharmacother. 2006, 40, 1966–1973. [Google Scholar] [CrossRef]
- Ozbolt, L.B.; Paniagua, M.A.; Kaiser, R.M. Atypical antipsychotics for the treatment of delirious elders. J. Am. Med. Dir. Assoc. 2008, 9, 18–28. [Google Scholar] [CrossRef]
- Porcelli, S.; Crisafulli, C.; Calabrò, M.; Serretti, A.; Rujescu, D. Possible biomarkers modulating haloperidol efficacy and/or tolerability. Pharmacogenomics 2016, 17, 507–529. [Google Scholar] [CrossRef]
- De la Casa, L.G.; Cintado, M.A.; González-Tirado, G.; Cárcel, L. Conditioned catalepsy vs. Increase in locomotor activity induced by haloperidol. Neurosci. Lett. 2023, 802. [Google Scholar] [CrossRef]
- Waku, I.; Magalhães, M.S.; Alves, C.O.; de Oliveira, A.R. Haloperidol-induced catalepsy as an animal model for parkinsonism: A systematic review of experimental studies. Eur. J. Neurosci. 2021, 53, 3743–3767. [Google Scholar] [CrossRef] [PubMed]
- Kharkwal, G.; Brami-Cherrier, K.; Lizardi-Ortiz, J.E.; Nelson, A.B.; Ramos, M.; Del Barrio, D.; Sulzer, D.; Kreitzer, A.C.; Borrelli, E. Parkinsonism Driven by Antipsychotics Originates from Dopaminergic Control of Striatal Cholinergic Interneurons. Neuron 2016, 91, 67–78. [Google Scholar] [CrossRef]
- Prasasty, V.; Radifar, M.; Istyastono, E. Natural Peptides in Drug Discovery Targeting Acetylcholinesterase. Molecules 2018, 23, 2344. [Google Scholar] [CrossRef] [PubMed]
- Soreq, H.; Seidman, S. Acetylcholinesterase--new roles for an old actor. Nat. Rev. Neurosci. 2001, 2, 294–302. [Google Scholar] [CrossRef]
- Wevers, A. Localisation of pre- and postsynaptic cholinergic markers in the human brain. Behav. Brain Res. 2011, 221, 341–355. [Google Scholar] [CrossRef]
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef]
- Obara, K.; Fujii, A.; Arie, C.; Harada, N.; Yamaki, F.; Matsuo, K.; Yoshio, T.; Tanaka, Y. Inhibition of Recombinant Human Acetylcholinesterase Activity by Antipsychotics. Pharmacology 2019, 104, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Bolden, C.; Cusack, B.; Richelson, E. Antagonism by antimuscarinic and neuroleptic compounds at the five cloned human muscarinic cholinergic receptors expressed in Chinese hamster ovary cells. J. Pharmacol. Exp. Ther. 1992, 260, 576–580. [Google Scholar]
- Tenjin, T.; Miyamoto, S.; Ninomiya, Y.; Kitajima, R.; Ogino, S.; Miyake, N.; Yamaguchi, N. Profile of blonanserin for the treatment of schizophrenia. Neuropsychiatr. Dis. Treat. 2013, 9, 587–594. [Google Scholar] [CrossRef]
- Bymaster, F.P.; Calligaro, D.O.; Falcone, J.F.; Marsh, R.D.; Moore, N.A.; Tye, N.C.; Seeman, P.; Wong, D.T. Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology 1996, 14, 87–96. [Google Scholar] [CrossRef]
- Richelson, E.; Souder, T. Binding of antipsychotic drugs to human brain receptors focus on newer generation compounds. Life Sci. 2000, 68, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, V.C.; Gomes, J.L.; Zanini, D.; Abdalla, F.H.; da Costa, P.; Gonçalves, J.F.; Duarte, M.M.; Moretto, M.B.; Morsch, V.M.; Schetinger, M.R. Evaluation of acetylcholinesterase and adenosine deaminase activities in brain and erythrocytes and proinflammatory cytokine levels in rats submitted to neonatal hypoxia-ischemia model. Mol. Cell Biochem. 2013, 378, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Padilla, S.; Lassiter, T.L.; Hunter, D. Biochemical Measurement of Cholinesterase Activity. Neurodegener. Methods Protoc. 2003, 22, 237–246. [Google Scholar]
- Sebens, J.B.; Koch, T.; Ter Horst, G.J.; Korf, J. Olanzapine-induced Fos expression in the rat forebrain; cross-tolerance with haloperidol and clozapine. Eur. J. Pharmacol. 1998, 353, 13–21. [Google Scholar] [CrossRef]
- Bardin, L.; Kleven, M.S.; Barret-Grévoz, C.; Depoortère, R.; Newman-Tancredi, A. Antipsychotic-like vs cataleptogenic actions in mice of novel antipsychotics having D2 antagonist and 5-HT1A agonist properties. Neuropsychopharmacology 2006, 31, 1869–1879. [Google Scholar] [CrossRef]
- Echeverry, M.B.; Salgado, M.L.; Ferreira, F.R.; da-Silva, C.A.; Del Bel, E.A. Intracerebroventricular administration of nitric oxide-sensitive guanylyl cyclase inhibitors induces catalepsy in mice. Psychopharmacology 2007, 194, 271–278. [Google Scholar] [CrossRef]
- Del Bel, E.A.; Guimarães, F.S. Sub-chronic inhibition of nitric-oxide synthesis modifies haloperidol-induced catalepsy and the number of NADPH-diaphorase neurons in mice. Psychopharmacology 2000, 147, 356–361. [Google Scholar] [CrossRef]
- Prieto, S.G.; Almeida, M.C.; Silva, J.C.S.; Del-Bel, E.; Echeverry, M.B. Extrapyramidal Side Effects with Chronic Atypical Antipsychotic Can Be Predicted by Labeling Pattern of FosB and phosphoThr34-DARPP-32 in Nucleus Accumbens. Biomedicines 2023, 11, 2677. [Google Scholar] [CrossRef]
- Arai, M. Parkinsonism onset in a patient concurrently using tiapride and donepezil. Intern. Med. 2000, 39, 863. [Google Scholar] [CrossRef]
- Magnuson, T.M.; Keller, B.K.; Burke, W.J. Extrapyramidal side effects in a patient treated with risperidone plus donepezil. Am. J. Psychiatry 1998, 155, 1458–1459. [Google Scholar] [CrossRef] [PubMed]
- Bourke, D.; Druckenbrod, R.W. Possible association between donepezil and worsening Parkinson’s disease. Ann. Pharmacother. 1998, 32, 610–611. [Google Scholar] [CrossRef] [PubMed]
- Klemm, W.R. Cholinergic-dopaminergic interactions in experimental catalepsy. Psychopharmacology 1983, 81, 24–27. [Google Scholar] [CrossRef]
- Mahadik, S.P.; Laev, H.; Korenovsky, A.; Karpiak, S.E. Haloperidol alters rat CNS cholinergic system: Enzymatic and morphological analyses. Biol. Psychiatry 1988, 24, 199–217. [Google Scholar] [CrossRef]
- Wu, D.; Yu, N.; Gao, Y.; Xiong, R.; Liu, L.; Lei, H.; Jin, S.; Liu, J.; Liu, Y.; Xie, J.; et al. Targeting a vulnerable septum-hippocampus cholinergic circuit in a critical time window ameliorates tau-impaired memory consolidation. Mol. Neurodegener. 2023, 18, 23. [Google Scholar] [CrossRef]
- Grisaru, D.; Sternfeld, M.; Eldor, A.; Glick, D.; Soreq, H. Structural roles of acetylcholinesterase variants in biology and pathology. Eur. J. Biochem. 1999, 264, 672–686. [Google Scholar] [CrossRef] [PubMed]
- McNaughton, N.; Ruan, M.; Woodnorth, M.A. Restoring theta-like rhythmicity in rats restores initial learning in the Morris water maze. Hippocampus 2006, 16, 1102–1110. [Google Scholar] [CrossRef]
- Cai, Y.; Ford, C.P. Dopamine Cells Differentially Regulate Striatal Cholinergic Transmission across Regions through Corelease of Dopamine and Glutamate. Cell Rep. 2018, 25, 3148–3157.e3. [Google Scholar] [CrossRef]
- Sethy, V.H. Effects of chronic treatment with neuroleptics on striatal acetylcholine concentration. J. Neurochem. 1976, 27, 325–326. [Google Scholar] [CrossRef]
- Seibt, K.J.; da Luz Oliveira, R.; Rico, E.P.; Dias, R.D.; Bogo, M.R.; Bonan, C.D. Typical and atypical antipsychotics alter acetylcholinesterase activity and ACHE expression in zebrafish (Danio rerio) brain. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2009, 150, 10–15. [Google Scholar] [CrossRef]
- Klemm, W.R. Evidence for a cholinergic role in haloperidol-induced catalepsy. Psychopharmacology 1985, 85, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Sklan, E.H.; Berson, A.; Birikh, K.R.; Gutnick, A.; Shahar, O.; Shoham, S.; Soreq, H. Acetylcholinesterase modulates stress-induced motor responses through catalytic and noncatalytic properties. Biol. Psychiatry 2006, 60, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Kaygisiz, B.; Aydin, S.; Yildirim, E.; Musmul, A.; Erol, K.; Kilic, F.S. The effects of galangin in prepulse inhibition test and experimental schizophrenia models. Acta Neuropsychiatr. 2022, 34, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic. Clin. Pharm. 2016, 7, 27. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, B.R.d.; Lima, J.M.F.A.; Echeverry, M.B.; Alberto-Silva, C. Haloperidol-Induced Catalepsy and Its Correlations with Acetylcholinesterase Activity in Different Brain Structures of Mice. Neurol. Int. 2024, 16, 1731-1741. https://doi.org/10.3390/neurolint16060125
Silva BRd, Lima JMFA, Echeverry MB, Alberto-Silva C. Haloperidol-Induced Catalepsy and Its Correlations with Acetylcholinesterase Activity in Different Brain Structures of Mice. Neurology International. 2024; 16(6):1731-1741. https://doi.org/10.3390/neurolint16060125
Chicago/Turabian StyleSilva, Brenda Rufino da, Joyce Maria Ferreira Alexandre Lima, Marcela Bermudez Echeverry, and Carlos Alberto-Silva. 2024. "Haloperidol-Induced Catalepsy and Its Correlations with Acetylcholinesterase Activity in Different Brain Structures of Mice" Neurology International 16, no. 6: 1731-1741. https://doi.org/10.3390/neurolint16060125
APA StyleSilva, B. R. d., Lima, J. M. F. A., Echeverry, M. B., & Alberto-Silva, C. (2024). Haloperidol-Induced Catalepsy and Its Correlations with Acetylcholinesterase Activity in Different Brain Structures of Mice. Neurology International, 16(6), 1731-1741. https://doi.org/10.3390/neurolint16060125






