Using Immunoliposomes as Carriers to Enhance the Therapeutic Effectiveness of Macamide N-3-Methoxybenzyl-Linoleamide
Abstract
1. Introduction
2. Methods and Materials
2.1. Materials
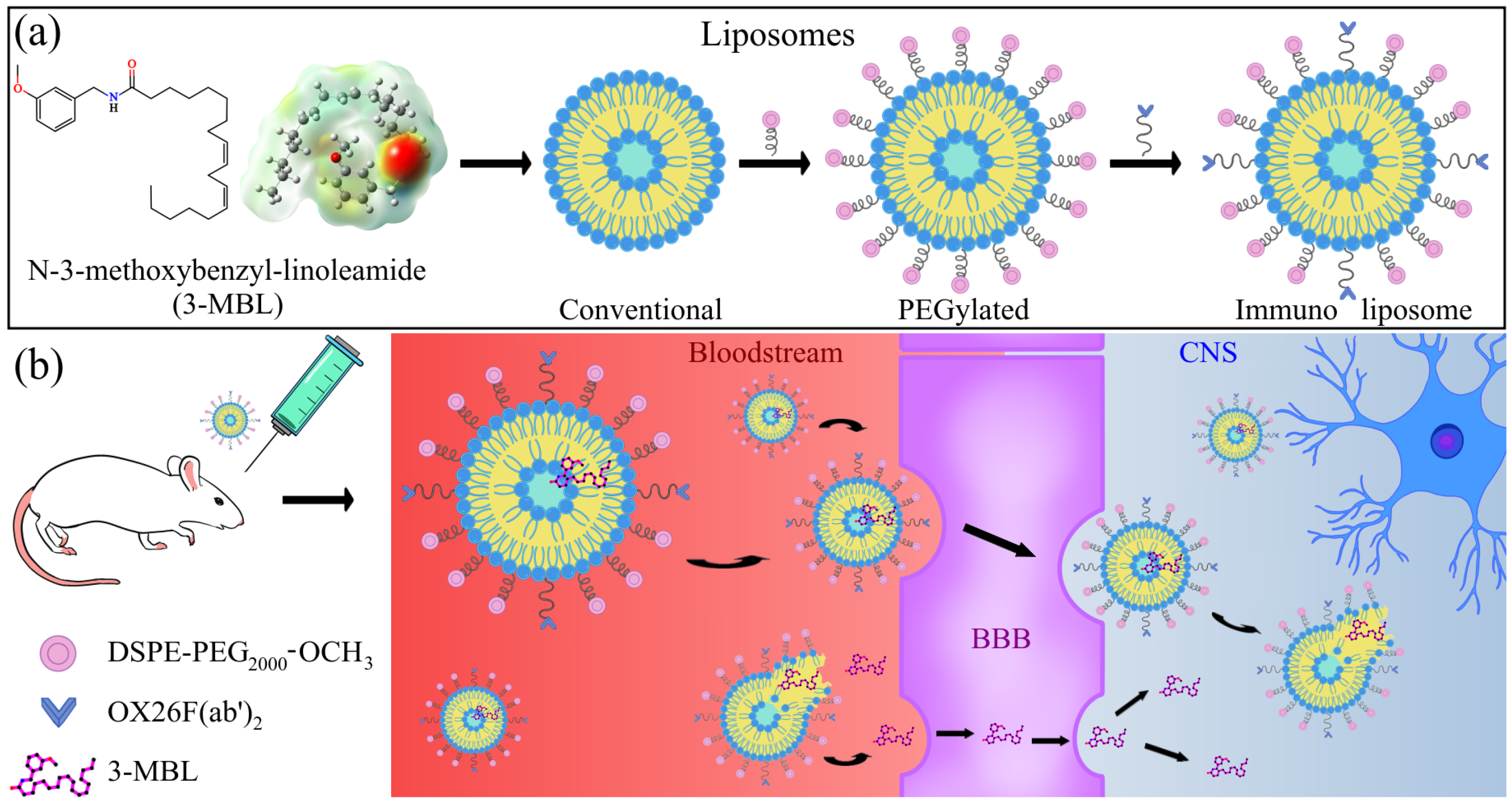
2.2. Preparation of N-3-Methoxybenzyl-linoleamide-Loaded Conventional Liposomes (CL) and N-3-Methoxybenzyl-linoleamide-Loaded PEGylated Liposomes (PL)
2.3. Preparation of OX26 F(ab′)2 Fragments
2.4. Preparation of N-3-Methoxybenzyl-linoleamide-Loaded PEGylated OX26 F(ab′)2 Immunoliposomes (IL)
2.5. Characterization of Liposomal Formulations
2.5.1. Size and Zeta Potential
2.5.2. Phospholipids Content
2.5.3. N-3-Methoxybenzyl-linoleamide Loading Efficiency (DLE)
2.5.4. Quantification of Antibody-Liposome Coupling Efficiency
2.6. Animals
2.7. Study Design
2.8. Experimental Procedure
2.9. Statistical Analysis
2.10. Structures of the Macamide 3-MBL
3. Results and Discussion
Characterization of Liposomal Formulations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steinmetz, J.D.; Seeher, K.M.; Schiess, N.; Nichols, E.; Cao, B.; Servili, C.; Cavallera, V.; Cousin, E.; Hagins, H.; Moberg, M.E.; et al. Global, regional, and national burden of disorders affecting the nervous system, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Neurol. 2024, 23, 344–381. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Epilepsy. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/epilepsy (accessed on 10 September 2024).
- Symonds, J.D.; Zuberi, S.M.; Stewart, K.; McLellan, A.; O ‘Regan, M.; MacLeod, S.; Jollands, A.; Joss, S.; Kirkpatrick, M.; Brunklaus, A.; et al. Incidence and phenotypes of childhood-onset genetic epilepsies: A prospective population-based national cohort. Brain 2019, 142, 2303–2318. [Google Scholar] [CrossRef]
- Cano, A.; Fonseca, E.; Ettcheto, M.; Sánchez-López, E.; de Rojas, I.; Alonso-Lana, S.; Morato, X.; Souto, E.B.; Toledo, M.; Boada, M.; et al. Epilepsy in neurodegenerative diseases: Related drugs and molecular pathways. Pharmaceuticals 2021, 14, 1057. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.Q. Epileptic mechanisms shared by Alzheimer’s disease: Viewed via the unique lens of genetic epilepsy. Int. J. Mol. Sci. 2021, 22, 7133. [Google Scholar] [CrossRef] [PubMed]
- Neri, S.; Mastroianni, G.; Gardella, E.; Aguglia, U.; Rubboli, G. Epilepsy in neurodegenerative diseases. Epileptic Disord. 2022, 24, 249–273. [Google Scholar] [CrossRef]
- Thijs, R.D.; Surges, R.; O’Brien, T.J.; Sander, J.W. Epilepsy in adults. Lancet 2019, 393, 689–701. [Google Scholar] [CrossRef]
- Moshé, S.L.; Perucca, E.; Ryvlin, P.; Tomson, T. Epilepsy: New advances. Lancet 2015, 385, 884–898. [Google Scholar] [CrossRef]
- Fisher, R.S.; Acevedo, C.; Arzimanoglou, A.; Bogacz, A.; Cross, J.H.; Elger, C.E.; Engel, J., Jr.; Forsgren, L.; French, J.A.; Glynn, M.; et al. ILAE official report: A practical clinical definition of epilepsy. Epilepsia 2014, 55, 475–482. [Google Scholar] [CrossRef]
- Oyrer, J.; Maljevic, S.; Scheffer, I.E.; Berkovic, S.F.; Petrou, S.; Reid, C.A.; Sexton, P.M. Ion channels in genetic epilepsy: From genes and mechanisms to disease-targeted therapies. Pharmacol. Rev. 2018, 70, 142–173. [Google Scholar] [CrossRef]
- Berg, A.T.; Berkovic, S.F.; Brodie, M.J.; Buchhalter, J.; Cross, J.H.; van Emde Boas, W.; Engel, J.; French, J.; Glauser, T.A.; Mathern, G.W.; et al. Revised Terminology and Concepts for Organization of Seizures and Epilepsies: Report of the ILAE Commission on Classification and Terminology, 2005–2009; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Yu, Y.; Nguyen, D.T.; Jiang, J. G protein-coupled receptors in acquired epilepsy: Druggability and translatability. Prog. Neurobiol. 2019, 183, 101682. [Google Scholar] [CrossRef]
- Whelan, C.D.; Altmann, A.; Botía, J.A.; Jahanshad, N.; Hibar, D.P.; Absil, J.; Alhusaini, S.; Alvim, M.K.; Auvinen, P.; Bartolini, E.; et al. Structural brain abnormalities in the common epilepsies assessed in a worldwide ENIGMA study. Brain 2018, 141, 391–408. [Google Scholar] [CrossRef] [PubMed]
- Galovic, M.; van Dooren, V.Q.; Postma, T.S.; Vos, S.B.; Caciagli, L.; Borzì, G.; Rosillo, J.C.; Vuong, K.A.; de Tisi, J.; Nachev, P.; et al. Progressive cortical thinning in patients with focal epilepsy. JAMA Neurol. 2019, 76, 1230–1239. [Google Scholar] [CrossRef]
- Sills, G.J.; Rogawski, M.A. Mechanisms of action of currently used antiseizure drugs. Neuropharmacology 2020, 168, 107966. [Google Scholar] [CrossRef] [PubMed]
- ukasiuk, K.; Lasoń, W. Emerging molecular targets for anti-epileptogenic and epilepsy modifying drugs. Int. J. Mol. Sci. 2023, 24, 2928. [Google Scholar] [CrossRef]
- Perucca, P.; Gilliam, F.G. Adverse effects of antiepileptic drugs. Lancet Neurol. 2012, 11, 792–802. [Google Scholar] [CrossRef]
- Anovadiya, A.P.; Sanmukhani, J.J.; Tripathi, C. Epilepsy: Novel therapeutic targets. J. Pharmacol. Pharmacother. 2012, 3, 112–117. [Google Scholar] [PubMed]
- Riva, A.; Golda, A.; Balagura, G.; Amadori, E.; Vari, M.S.; Piccolo, G.; Iacomino, M.; Lattanzi, S.; Salpietro, V.; Minetti, C.; et al. New trends and most promising therapeutic strategies for epilepsy treatment. Front. Neurol. 2021, 12, 753753. [Google Scholar] [CrossRef]
- Klein, P.; Kaminski, R.M.; Koepp, M.; Löscher, W. New epilepsy therapies in development. Nat. Rev. Drug Discov. 2024, 23, 682–708. [Google Scholar] [CrossRef]
- Ji, X.; Zeng, Y.; Wu, J. The CB2 receptor as a novel therapeutic target for epilepsy treatment. Int. J. Mol. Sci. 2021, 22, 8961. [Google Scholar] [CrossRef]
- Alasmari, M.; Bohlke, M.; Kelley, C.; Maher, T.; Pino-Figueroa, A. Inhibition of fatty acid amide hydrolase (FAAH) by macamides. Mol. Neurobiol. 2019, 56, 1770–1781. [Google Scholar] [CrossRef]
- Singh, N.; Barnych, B.; Morisseau, C.; Wagner, K.M.; Wan, D.; Takeshita, A.; Pham, H.; Xu, T.; Dandekar, A.; Liu, J.Y.; et al. N-Benzyl-linoleamide, a constituent of Lepidium meyenii (Maca), is an orally bioavailable soluble epoxide hydrolase inhibitor that alleviates inflammatory pain. J. Nat. Prod. 2020, 83, 3689–3697. [Google Scholar] [CrossRef]
- Taboada-Rosell, K.; Castro-García, F.; Medina-Saldivar, C.; Cruz-Visalaya, S.; Pacheco-Otalora, L. The novel FAAH inhibitor, MCH1, reduces the infarction area in the motor cortex-related region but does not affect the sensorimotor function or memory and spatial learning in rats exposed to transient middle cerebral artery occlusion. Brain Res. 2024, 1822, 148636. [Google Scholar] [CrossRef] [PubMed]
- Pino-Figueroa, A.; Nguyen, D.; Maher, T.J. Neuroprotective effects of Lepidium meyenii (Maca). Ann. N. Y. Acad. Sci. 2010, 1199, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Gugnani, K.S.; Vu, N.; Rondón-Ortiz, A.N.; Böhlke, M.; Maher, T.J.; Pino-Figueroa, A.J. Neuroprotective activity of macamides on manganese-induced mitochondrial disruption in U-87 MG glioblastoma cells. Toxicol. Appl. Pharmacol. 2018, 340, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Jin, W.; Cui, Y.; Ao, M.; Liu, H.; Xu, H.; Yu, L. Protective effects of macamides from Lepidium meyenii Walp. against corticosterone-induced neurotoxicity in PC12 cells. RSC Adv. 2019, 9, 23096–23108. [Google Scholar] [CrossRef]
- Vera-López, K.J.; Davila-Del-Carpio, G.; Nieto-Montesinos, R. Macamides as Potential Therapeutic Agents in Neurological Disorders. Neurol. Int. 2024, 16, 1611–1625. [Google Scholar] [CrossRef]
- Vera-López, K.J.; Aguilar-Pineda, J.A.; Moscoso-Palacios, R.M.; Davila-Del-Carpio, G.; Manrique-Murillo, J.L.; Gómez, B.; González-Melchor, M.; Nieto-Montesinos, R. Anticonvulsant Effects of Synthetic N-(3-Methoxybenzyl) oleamide and N-(3-Methoxybenzyl) linoleamide Macamides: An In Silico and In Vivo Study. Molecules 2025, 30, 333. [Google Scholar] [CrossRef]
- Wu, H.; Kelley, C.J.; Pino-Figueroa, A.; Vu, H.D.; Maher, T.J. Macamides and their synthetic analogs: Evaluation of in vitro FAAH inhibition. Bioorganic Med. Chem. 2013, 21, 5188–5197. [Google Scholar] [CrossRef]
- Almukadi, H.; Wu, H.; Böhlke, M.; Kelley, C.J.; Maher, T.J.; Pino-Figueroa, A. The macamide N-3-methoxybenzyl-linoleamide is a time-dependent fatty acid amide hydrolase (FAAH) inhibitor. Mol. Neurobiol. 2013, 48, 333–339. [Google Scholar] [CrossRef]
- Poustforoosh, A.; Nematollahi, M.H.; Hashemipour, H.; Pardakhty, A. Recent advances in Bio-conjugated nanocarriers for crossing the Blood-Brain Barrier in (pre-) clinical studies with an emphasis on vesicles. J. Control. Release 2022, 343, 777–797. [Google Scholar] [CrossRef]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; McCarty, D.M. Crossing the blood–brain-barrier with viral vectors. Curr. Opin. Virol. 2016, 21, 87–92. [Google Scholar] [CrossRef]
- Crommelin, D.J.; van Hoogevest, P.; Storm, G. The role of liposomes in clinical nanomedicine development. What now? Now what? J. Control. Release 2020, 318, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Terstappen, G.C.; Meyer, A.H.; Bell, R.D.; Zhang, W. Strategies for delivering therapeutics across the blood–brain barrier. Nat. Rev. Drug Discov. 2021, 20, 362–383. [Google Scholar] [CrossRef]
- Juhairiyah, F.; De Lange, E.C. Understanding drug delivery to the brain using liposome-based strategies: Studies that provide mechanistic insights are essential. AAPS J. 2021, 23, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.; Schlich, M.; Sinico, C.; Fadda, A.M. Liposomes as brain targeted delivery systems. In Nanomedicines for Brain Drug Delivery; Humana: New York, NY, USA, 2021; pp. 29–59. [Google Scholar]
- Raju, R.; Abuwatfa, W.H.; Pitt, W.G.; Husseini, G.A. Liposomes for the treatment of brain cancer—A review. Pharmaceuticals 2023, 16, 1056. [Google Scholar] [CrossRef]
- Gulati, M.; Grover, M.; Singh, S.; Singh, M. Lipophilic drug derivatives in liposomes. Int. J. Pharm. 1998, 165, 129–168. [Google Scholar] [CrossRef]
- Nieto Montesinos, R.; Beduneau, A.; Lamprecht, A.; Pellequer, Y. Liposomes coloaded with elacridar and tariquidar to modulate the P-glycoprotein at the blood–brain barrier. Mol. Pharm. 2015, 12, 3829–3838. [Google Scholar] [CrossRef]
- Schnyder, A.; Huwyler, J. Drug transport to brain with targeted liposomes. NeuroRx 2005, 2, 99–107. [Google Scholar] [CrossRef]
- Kreuter, J. Nanoparticulate systems for brain delivery of drugs. Adv. Drug Deliv. Rev. 2001, 47, 65–81. [Google Scholar] [CrossRef]
- Pang, Z.; Lu, W.; Gao, H.; Hu, K.; Chen, J.; Zhang, C.; Gao, X.; Jiang, X.; Zhu, C. Preparation and brain delivery property of biodegradable polymersomes conjugated with OX26. J. Control. Release 2008, 128, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Pellequer, Y.; Ollivon, M.; Barratt, G. Formulation of liposomes associated with recombinant interleukin-2: Effect on interleukin-2 activity. Biomed. Pharmacother. 2004, 58, 162–167. [Google Scholar] [CrossRef]
- Bogdanov, A.; Klibanov, A.; Torchilin, V. Protein immobilization on the surface of liposomes via carbodiimide activation in the presence of N-hydroxysulfosuccinimide. FEBS Lett. 1988, 231, 381–384. [Google Scholar] [CrossRef]
- Turski, W.A.; Cavalheiro, E.A.; Schwarz, M.; Czuczwar, S.J.; Kleinrok, Z.; Turski, L. Limbic seizures produced by pilocarpine in rats: Behavioural, electroencephalographic and neuropathological study. Behav. Brain Res. 1983, 9, 315–335. [Google Scholar] [CrossRef]
- Brophy, G.M.; Bell, R.; Claassen, J.; Alldredge, B.; Bleck, T.P.; Glauser, T.; LaRoche, S.M.; Riviello, J.J.; Shutter, L.; Sperling, M.R.; et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit. Care 2012, 17, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Racine, R.J. Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr. Clin. Neurophysiol. 1972, 32, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Moussally, J.; Cash, S.S.; Karnam, H.B.; Cole, A.J. Intravenous levetiracetam in the rat pilocarpine-induced status epilepticus model: Behavioral, physiological and histological studies. Neuropharmacology 2010, 58, 793–798. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Petersson, G.; Nakatsuji, H.; et al. Gaussian 16; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, Version 6.1; Semichem Inc.: Shawnee Mission, KS, USA, 2016.
- Spencer, A.P.; Torrado, M.; Custódio, B.; Silva-Reis, S.C.; Santos, S.D.; Leiro, V.; Pêgo, A.P. Breaking barriers: Bioinspired strategies for targeted neuronal delivery to the central nervous system. Pharmaceutics 2020, 12, 192. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, J.; Thomas, A.; Ou-Yang, D.; Muzykantov, V.R. The shape of things to come: Importance of design in nanotechnology for drug delivery. Ther. Deliv. 2012, 3, 181–194. [Google Scholar] [CrossRef]
- Liu, Y.; Bravo, K.M.C.; Liu, J. Targeted liposomal drug delivery: A nanoscience and biophysical perspective. Nanoscale Horizons 2021, 6, 78–94. [Google Scholar] [CrossRef]
- Pardeshi, C.; Rajput, P.; Belgamwar, V.; Tekade, A.; Patil, G.; Chaudhary, K.; Sonje, A. Solid lipid based nanocarriers: An overview/Nanonosači na bazi čvrstih lipida: Pregled. Acta Pharm. 2012, 62, 433–472. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Higashi, K.; Watabe, K.; Kobayashi, A.; Limwikrant, W.; Yamamoto, K.; Moribe, K. Effects of the PEG molecular weight of a PEG-lipid and cholesterol on PEG chain flexibility on liposome surfaces. Colloids Surfaces A Physicochem. Eng. Asp. 2015, 474, 63–70. [Google Scholar] [CrossRef]
- Chen, B.M.; Cheng, T.L.; Roffler, S.R. Polyethylene glycol immunogenicity: Theoretical, clinical, and practical aspects of anti-polyethylene glycol antibodies. ACS Nano 2021, 15, 14022–14048. [Google Scholar] [CrossRef] [PubMed]
- Béduneau, A.; Saulnier, P.; Hindré, F.; Clavreul, A.; Leroux, J.C.; Benoit, J.P. Design of targeted lipid nanocapsules by conjugation of whole antibodies and antibody Fab′fragments. Biomaterials 2007, 28, 4978–4990. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Smith, M.C.; Crist, R.M.; Clogston, J.D.; McNeil, S.E. Zeta potential: A case study of cationic, anionic, and neutral liposomes. Anal. Bioanal. Chem. 2017, 409, 5779–5787. [Google Scholar] [CrossRef]
- Lévesque, M.; Biagini, G.; de Curtis, M.; Gnatkovsky, V.; Pitsch, J.; Wang, S.; Avoli, M. The pilocarpine model of mesial temporal lobe epilepsy: Over one decade later, with more rodent species and new investigative approaches. Neurosci. Biobehav. Rev. 2021, 130, 274–291. [Google Scholar] [CrossRef]
- Klitgaard, H.; Matagne, A.; Vanneste-Goemaere, J.; Margineanu, D.G. Pilocarpine-induced epileptogenesis in the rat: Impact of initial duration of status epilepticus on electrophysiological and neuropathological alterations. Epilepsy Res. 2002, 51, 93–107. [Google Scholar] [CrossRef]
- Khan, A.; Aziz, S.; Anis, K.; Khan, M.Q.; Jamil, T.; Salman, M. Efficacy of Midazolam vs. Diazepam in the Treatment of Status Epilepticus. Pak. J. Health Sci. 2023, 4, 243–247. [Google Scholar] [CrossRef]
- Albright, P.S.; Bruni, J. Effects of carbamazepine and its epoxide metabolite on amygdala-kindled seizures in rats. Neurology 1984, 34, 1383. [Google Scholar] [CrossRef]
- Gierbolini, J.; Giarratano, M.; Benbadis, S.R. Carbamazepine-related antiepileptic drugs for the treatment of epilepsy—A comparative review. Expert Opin. Pharmacother. 2016, 17, 885–888. [Google Scholar] [CrossRef] [PubMed]
- Lattanzi, S.; Belcastro, V. What place do carbamazepine-related antiepileptic drugs have in the modern day treatment of epilepsy? Expert Opin. Pharmacother. 2020, 21, 1655–1658. [Google Scholar] [CrossRef] [PubMed]
- Gajbhiye, K.; Pawar, A.; Mahadik, K.; Gajbhiye, V. PEGylated nanocarriers: A promising tool for targeted delivery to the brain. Colloids Surfaces Biointerfaces 2020, 187, 110770. [Google Scholar] [CrossRef]
- Ying, X.; Wen, H.; Lu, W.L.; Du, J.; Guo, J.; Tian, W.; Men, Y.; Zhang, Y.; Li, R.J.; Yang, T.Y.; et al. Dual-targeting daunorubicin liposomes improve the therapeutic efficacy of brain glioma in animals. J. Control. Release 2010, 141, 183–192. [Google Scholar] [CrossRef]
- Gaillard, P.J.; Appeldoorn, C.C.; Dorland, R.; Van Kregten, J.; Manca, F.; Vugts, D.J.; Windhorst, B.; van Dongen, G.A.; de Vries, H.E.; Maussang, D.; et al. Pharmacokinetics, brain delivery, and efficacy in brain tumor-bearing mice of glutathione pegylated liposomal doxorubicin (2B3-101). PLoS ONE 2014, 9, e82331. [Google Scholar] [CrossRef]
- Taher, M.; Susanti, D.; Haris, M.S.; Rushdan, A.A.; Widodo, R.T.; Syukri, Y.; Khotib, J. PEGylated liposomes enhance the effect of cytotoxic drug: A review. Heliyon 2023, 9, e13823. [Google Scholar] [CrossRef]
- Muthu, M.S.; Kulkarni, S.A.; Xiong, J.; Feng, S.S. Vitamin E TPGS coated liposomes enhanced cellular uptake and cytotoxicity of docetaxel in brain cancer cells. Int. J. Pharm. 2011, 421, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Ekhator, C.; Qureshi, M.Q.; Zuberi, A.W.; Hussain, M.; Sangroula, N.; Yerra, S.; Devi, M.; Naseem, M.A.; Bellegarde, S.B.; Pendyala, P.R.; et al. Advances and opportunities in nanoparticle drug delivery for central nervous system disorders: A review of current advances. Cureus 2023, 15, e44302. [Google Scholar] [CrossRef]
- Kaasgaard, T.; Mouritsen, O.G.; Jørgensen, K. Screening effect of PEG on avidin binding to liposome surface receptors. Int. J. Pharm. 2001, 214, 63–65. [Google Scholar] [CrossRef]
- Du, Q.; Liu, Y.; Fan, M.; Wei, S.; Ismail, M.; Zheng, M. PEG length effect of peptide-functional liposome for blood brain barrier (BBB) penetration and brain targeting. J. Control. Release 2024, 372, 85–94. [Google Scholar] [CrossRef]
- Yue, P.J.; He, L.; Qiu, S.W.; Li, Y.; Liao, Y.J.; Li, X.P.; Xie, D.; Peng, Y. OX26/CTX-conjugated PEGylated liposome as a dual-targeting gene delivery system for brain glioma. Mol. Cancer 2014, 13, 191. [Google Scholar] [CrossRef] [PubMed]
- d’Avanzo, N.; Paolino, D.; Barone, A.; Ciriolo, L.; Mancuso, A.; Christiano, M.C.; Tolomeo, A.M.; Celia, C.; Deng, X.; Fresta, M. OX26-cojugated gangliosilated liposomes to improve the post-ischemic therapeutic effect of CDP-choline. Drug Deliv. Transl. Res. 2024, 14, 2771–2787. [Google Scholar] [CrossRef] [PubMed]
- Jefferies, W.A.; Brandon, M.R.; Hunt, S.V.; Williams, A.F.; Gatter, K.C.; Mason, D.Y. Transferrin receptor on endothelium of brain capillaries. Nature 1984, 312, 162–163. [Google Scholar] [CrossRef]
- Arguello, A.; Mahon, C.S.; Calvert, M.E.; Chan, D.; Dugas, J.C.; Pizzo, M.E.; Thomsen, E.R.; Chau, R.; Damo, L.A.; Duque, J.; et al. Molecular architecture determines brain delivery of a transferrin receptor–targeted lysosomal enzyme. J. Exp. Med. 2022, 219, e20211057. [Google Scholar] [CrossRef]
- van den Broek, S.L.; Shalgunov, V.; Herth, M.M. Transport of nanomedicines across the blood–brain barrier: Challenges and opportunities for imaging and therapy. Biomater. Adv. 2022, 141, 213125. [Google Scholar] [CrossRef] [PubMed]
- Bonvicini, G.; Syvänen, S.; Andersson, K.G.; Haaparanta-Solin, M.; Lopez-Picon, F.; Sehlin, D. ImmunoPET imaging of amyloid-beta in a rat model of Alzheimer’s disease with a bispecific, brain-penetrating fusion protein. Transl. Neurodegener. 2022, 11, 55. [Google Scholar] [CrossRef]
- Stocki, P.; Szary, J.; Rasmussen, C.L.; Demydchuk, M.; Northall, L.; Logan, D.B.; Gauhar, A.; Thei, L.; Moos, T.; Walsh, F.S.; et al. Blood-brain barrier transport using a high affinity, brain-selective VNAR antibody targeting transferrin receptor 1. FASEB J. 2021, 35, e21172. [Google Scholar] [CrossRef]
- Sun, L.; Mi, K.; Hou, Y.; Hui, T.; Zhang, L.; Tao, Y.; Liu, Z.; Huang, L. Pharmacokinetic and pharmacodynamic drug–drug interactions: Research methods and applications. Metabolites 2023, 13, 897. [Google Scholar] [CrossRef]
- Stielow, M.; Witczyńska, A.; Kubryń, N.; Fijałkowski, Ł.; Nowaczyk, J.; Nowaczyk, A. The bioavailability of drugs—The current state of knowledge. Molecules 2023, 28, 8038. [Google Scholar] [CrossRef]
- Deore, A.B.; Dhumane, J.R.; Wagh, R.; Sonawane, R. The stages of drug discovery and development process. Asian J. Pharm. Res. Dev. 2019, 7, 62–67. [Google Scholar] [CrossRef]
- Zou, H.; Banerjee, P.; Leung, S.S.Y.; Yan, X. Application of pharmacokinetic-pharmacodynamic modeling in drug delivery: Development and challenges. Front. Pharmacol. 2020, 11, 997. [Google Scholar] [CrossRef]
- Bjørnsdottir, I.; Støvring, B.; Søeborg, T.; Jacobsen, H.; Sternebring, O. Plasma polyethylene glycol (PEG) levels reach steady state following repeated treatment with N8-GP (Turoctocog Alfa Pegol; Esperoct®). Drugs R&D 2020, 20, 75–82. [Google Scholar]
- Raina, N.; Singh, A.K.; Islam, A. Biological implications of polyethylene glycol and PEGylation: Therapeutic approaches based on biophysical studies and protein structure-based drug design tools. In Innovations and Implementations of Computer Aided Drug Discovery Strategies in Rational Drug Design; Springer: Singapore, 2021; pp. 273–294. [Google Scholar]
- Gaballa, S.A.; Naguib, Y.; Mady, F.M.; Khaled, K.A. Polyethylene glycol: Properties, applications, and challenges. J. Adv. Biomed. Pharm. Sci. 2024, 7, 26–36. [Google Scholar] [CrossRef]
- Kozma, G.T.; Shimizu, T.; Ishida, T.; Szebeni, J. Anti-PEG antibodies: Properties, formation, testing and role in adverse immune reactions to PEGylated nano-biopharmaceuticals. Adv. Drug Deliv. Rev. 2020, 154, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Y.; Yuan, C.; Xu, X.; Zhou, W.; Huang, Y.; Lu, H.; Zheng, Y.; Luo, G.; Shang, J.; et al. Polyethylene glycol (PEG)-associated immune responses triggered by clinically relevant lipid nanoparticles in rats. NPJ Vaccines 2023, 8, 169. [Google Scholar] [CrossRef]
- Freire Haddad, H.; Burke, J.A.; Scott, E.A.; Ameer, G.A. Clinical relevance of pre-existing and treatment-induced anti-poly (ethylene glycol) antibodies. Regen. Eng. Transl. Med. 2022, 8, 32–42. [Google Scholar] [CrossRef]
- Elsadek, N.E.; Lila, A.S.A.; Ishida, T. Immunological responses to PEGylated proteins: Anti-PEG antibodies. In Polymer-Protein Conjugates; Elsevier: Amsterdam, The Netherlands, 2020; pp. 103–123. [Google Scholar]
- Ibrahim, M.; Ramadan, E.; Elsadek, N.E.; Emam, S.E.; Shimizu, T.; Ando, H.; Ishima, Y.; Elgarhy, O.H.; Sarhan, H.A.; Hussein, A.K.; et al. Polyethylene glycol (PEG): The nature, immunogenicity, and role in the hypersensitivity of PEGylated products. J. Control. Release 2022, 351, 215–230. [Google Scholar] [CrossRef]
- Ding, T.; Fu, J.; Yang, M.; Zhang, Z.; Ma, Y.; Wu, E.; Guo, Z.; Lin, S.; Wang, S.; Liu, X.; et al. Hydroxy polyethylene glycol: A solution to evade human pre-existing anti-PEG antibodies for efficient delivery. bioRxiv 2024, 1–31. [Google Scholar] [CrossRef]
- Fu, J.; Wu, E.; Li, G.; Wang, B.; Zhan, C. Anti-PEG antibodies: Current situation and countermeasures. Nano Today 2024, 55, 102163. [Google Scholar] [CrossRef]
- Sandeep, D.; AlSawaftah, N.M.; Husseini, G.A. Immunoliposomes: Synthesis, structure, and their potential as drug delivery carriers. Curr. Cancer Ther. Rev. 2020, 16, 306–319. [Google Scholar] [CrossRef]
- Kumari, M.; Chen, K.C.; Ke, F.Y.; Pan, P.L.; Putu, E.P.G.N.; Chen, W.Y.; Wu, H.C. Developing a delivery strategy for combined drug treatment with multi-targeting immunoliposomes. J. Drug Deliv. Sci. Technol. 2024, 101, 106283. [Google Scholar] [CrossRef]
- Cascione, M.; De Matteis, V.; Leporatti, S.; Rinaldi, R. The new frontiers in neurodegenerative diseases treatment: Liposomal-based strategies. Front. Bioeng. Biotechnol. 2020, 8, 566767. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Hu, B.; Hua, H.; Liu, C.; Cheng, Y.; Guo, Y.; Yao, W.; Qian, H. Macamides: A review of structures, isolation, therapeutics and prospects. Food Res. Int. 2020, 138, 109819. [Google Scholar] [CrossRef] [PubMed]
| Formulation | Composition | Molar Ratio | Mean Particle Diameter (nm) | PDI | Z-Potential (eV) |
|---|---|---|---|---|---|
| Conventional liposomes | DMPC | 100 | 79.69 ± 3.65 | 0.14 ± 0.02 | −1.74 ± 0.31 |
| PEGylated liposomes | DMPC:DSPE-PEG2000-OCH3 | 100:7.5 | 93.96 ± 4.00 | 0.16 ± 0.01 | −3.17 ± 0.69 |
| Immunoliposomes before | DMPC:DSPE-PEG2000-OCH3: | 100:6.25:1.25 | 105.40 ± 6.98 | 0.19 ± 0.03 | −15.32 ± 1.3 |
| OX26 F(ab′)2 coupling | DSPE-PEG5000- COOH | ||||
| Immunoliposomes after | DMPC:DSPE-PEG2000-OCH3: | 100:6.25:1.25 | 120.52 ± 9.46 | 0.23 ± 0.03 | −8.57 ± 0.80 |
| OX26 F(ab′)2 coupling | DSPE-PEG5000- COOH |
| Formulation | Composition | Molar Ratio | Phospholipids Content (%) | Loading Efficiency (%) |
|---|---|---|---|---|
| Conventional liposomes | DMPC | 100 | 92.46 ± 3.12 | 90.76 ± 1.40 |
| PEGylated liposomes | DMPC:DSPE-PEG2000-OCH3 | 100:7.5 | 93.24 ± 1.84 | 87.37 ± 2.43 |
| Immunoliposomes before | DMPC:DSPE-PEG2000-OCH3: | 100:6.25:1.25 | 91.51 ± 2.80 | 86.25 ± 0.70 |
| OX26 F(ab′)2 coupling | DSPE-PEG5000- COOH | |||
| Immunoliposomes after | DMPC:DSPE-PEG2000-OCH3: | 100:6.25:1.25 | 86.40 ± 4.35 | 79.42 ± 6.68 |
| OX26 F(ab′)2 coupling | DSPE-PEG5000- COOH |
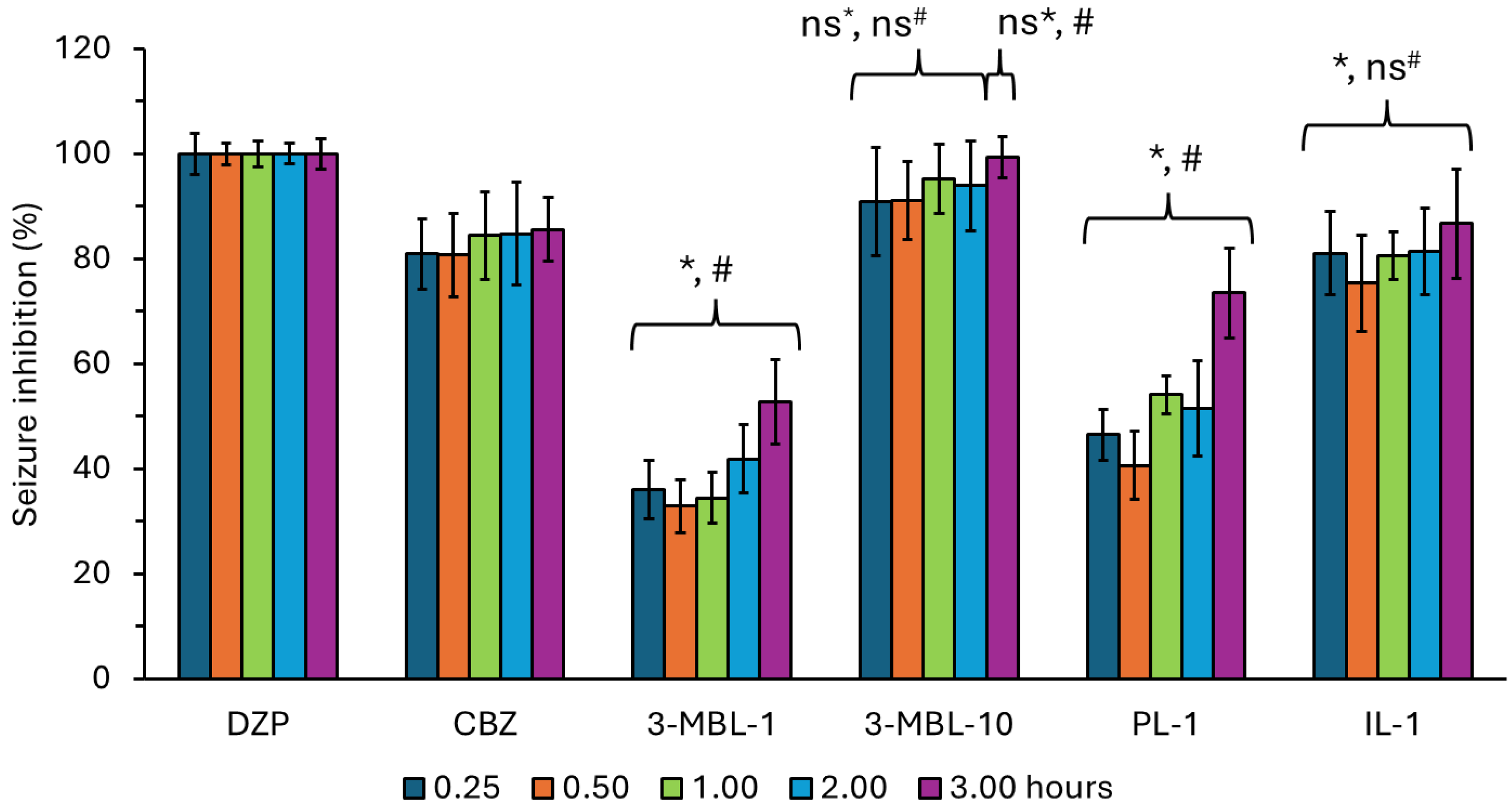
| Drug | Time Post Administration of Treatments (Hours) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.25 | 0.50 | 1.00 | 2.00 | 3.00 | 6.00 | |||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| DZP | 100.00 | 3.88 | 100.00 | 2.06 | 100.00 | 2.49 | 100.00 | 2.00 | 100.00 | 2.87 | 100.00 | 0.00 |
| CBZ | 80.91 | 6.64 | 80.73 | 7.96 | 84.43 | 8.33 | 84.75 | 9.76 | 85.53 | 6.09 | 87.40 | 2.41 |
| 3-MBL-1 | 36.10 | 5.57 | 32.87 | 5.04 | 34.45 | 4.83 | 41.86 | 6.55 | 52.72 | 7.94 | 69.79 | 2.42 |
| 3-MBL-10 | 90.93 | 10.31 | 91.16 | 7.44 | 95.23 | 6.59 | 93.89 | 8.59 | 99.28 | 3.89 | 97.47 | 6.21 |
| PL-1 | 46.48 | 4.84 | 40.57 | 6.49 | 54.12 | 3.58 | 51.50 | 9.07 | 75.53 | 8.57 | 76.34 | 5.98 |
| IL-1 | 81.05 | 8.00 | 75.32 | 9.18 | 80.55 | 4.57 | 81.40 | 8.30 | 86.66 | 10.42 | 97.47 | 6.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vera-López, K.J.; Aranzamendi-Zenteno, M.; Davila-Del-Carpio, G.; Nieto-Montesinos, R. Using Immunoliposomes as Carriers to Enhance the Therapeutic Effectiveness of Macamide N-3-Methoxybenzyl-Linoleamide. Neurol. Int. 2025, 17, 38. https://doi.org/10.3390/neurolint17030038
Vera-López KJ, Aranzamendi-Zenteno M, Davila-Del-Carpio G, Nieto-Montesinos R. Using Immunoliposomes as Carriers to Enhance the Therapeutic Effectiveness of Macamide N-3-Methoxybenzyl-Linoleamide. Neurology International. 2025; 17(3):38. https://doi.org/10.3390/neurolint17030038
Chicago/Turabian StyleVera-López, Karin J., María Aranzamendi-Zenteno, Gonzalo Davila-Del-Carpio, and Rita Nieto-Montesinos. 2025. "Using Immunoliposomes as Carriers to Enhance the Therapeutic Effectiveness of Macamide N-3-Methoxybenzyl-Linoleamide" Neurology International 17, no. 3: 38. https://doi.org/10.3390/neurolint17030038
APA StyleVera-López, K. J., Aranzamendi-Zenteno, M., Davila-Del-Carpio, G., & Nieto-Montesinos, R. (2025). Using Immunoliposomes as Carriers to Enhance the Therapeutic Effectiveness of Macamide N-3-Methoxybenzyl-Linoleamide. Neurology International, 17(3), 38. https://doi.org/10.3390/neurolint17030038







