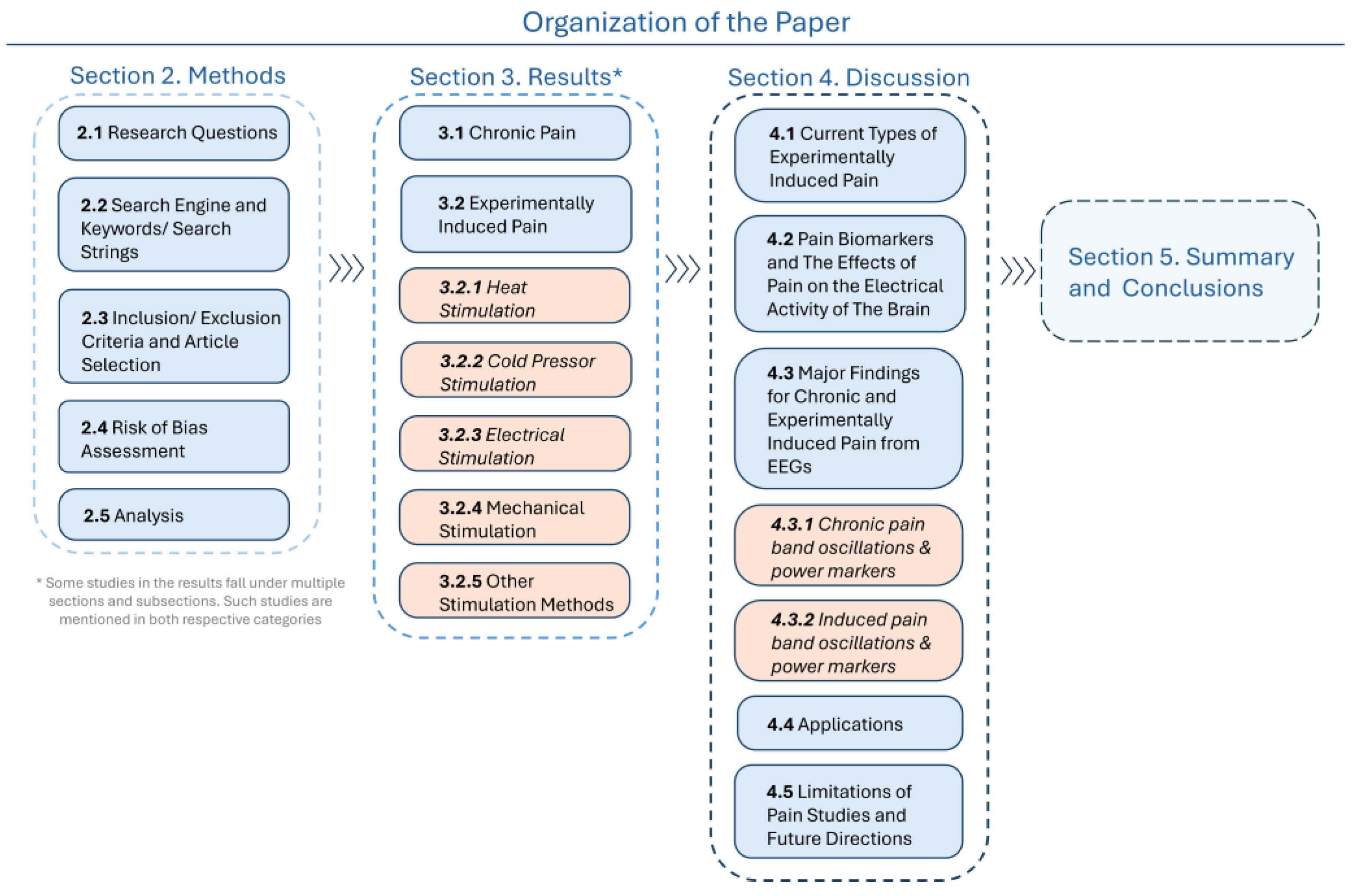
Pain and the Brain: A Systematic Review of Methods, EEG Biomarkers, Limitations, and Future Directions
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Questions
- In humans, what experimental EEG approaches are being used to study pain states compared to pain-free control states, and what neurophysiological insights do they provide?
- In individuals experiencing chronic pain, what electrical brain signal responses differ from those undergoing experimentally induced pain, as measured by neurophysiological techniques?
- In studies investigating human pain states, what methodological limitations affect the reliability and interpretation of the findings?
2.2. Search Engine and Keywords/Search Strings
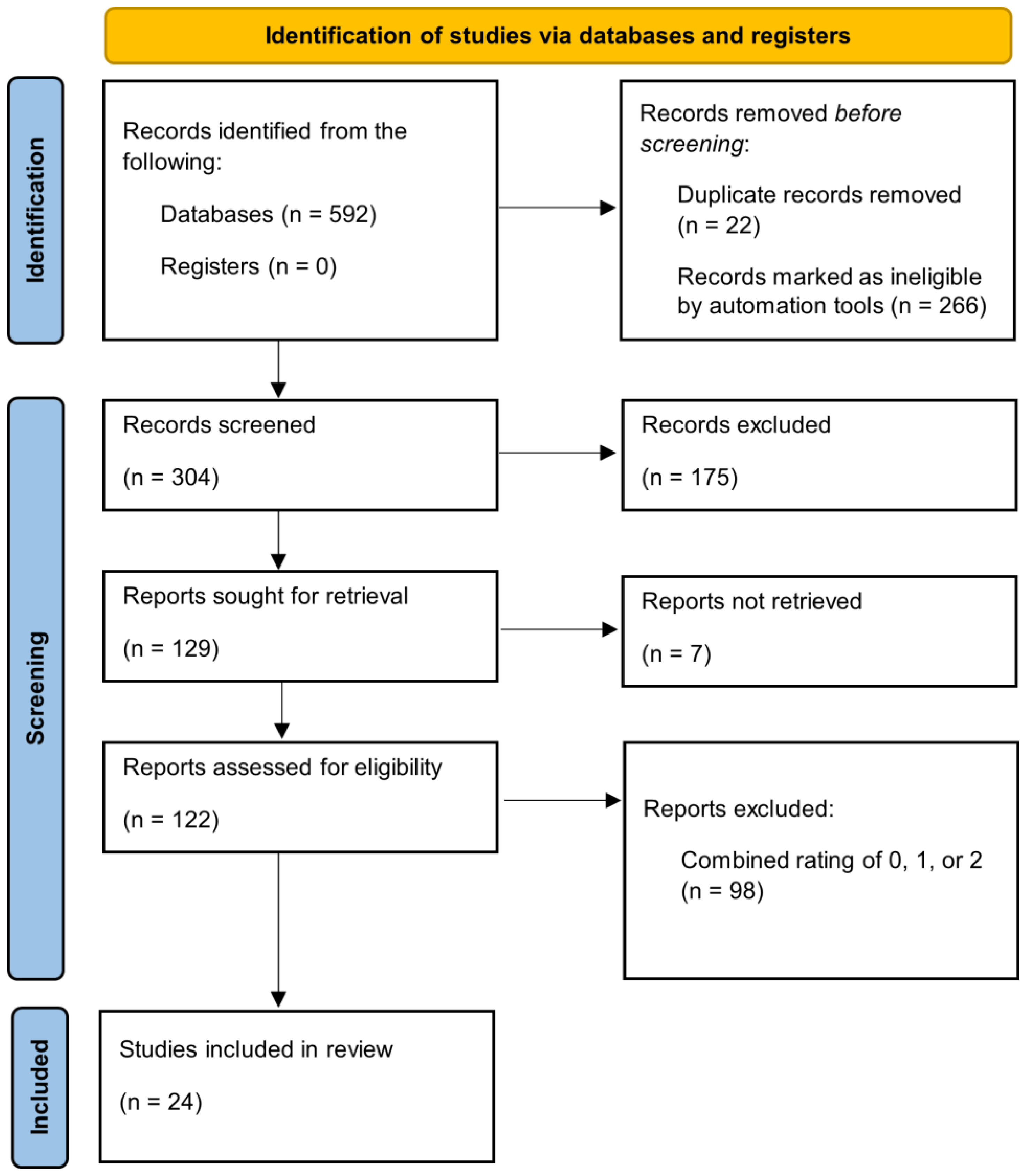
2.3. Inclusion/Exclusion Criteria and Article Selection
2.4. Risk of Bias Assessment
2.5. Analysis
3. Results
3.1. Chronic Pain
3.2. Experimentally Induced Pain
3.2.1. Heat Stimulation
3.2.2. Cold Pressor Stimulation
3.2.3. Electrical Stimulation
3.2.4. Mechanical Stimulation
3.2.5. Other Stimulation Methods
4. Discussion
4.1. Current Types of Experimentally Induced Pain
4.2. Pain Biomarkers and the Effects of Pain on the Electrical Activity of the Brain
4.3. Major Findings for Chronic and Experimentally Induced Pain from EEGs
4.3.1. Chronic Pain Band Oscillations and Power Markers
4.3.2. Induced Pain Band Oscillations and Power Markers
4.4. Applications
4.5. Limitations of Pain Studies and Future Directions
5. Summary and Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
Appendix A
| Source | Criteria for Subject Selection | Use of Any Medication | Quality Criteria from Newcastle–Ottawa Scale | Total Stars (Up to 10 Stars) | ||
|---|---|---|---|---|---|---|
| Selection (Up to 4 Stars) | Comparability Between Groups (Up to 2 Stars) | Exposure (Up to 4 Stars) | ||||
| Barbosa SP et al. (2024) [77] | PDQ Pre-existing dataset from Zolezzi et al., (2023) [83] | Partially Yes (n = 18 centrally acting drugs, cannabidiol derivatives, nonsteroidal anti-inflammatory (NSAID) drugs) No (n = 12) | 4 | 2 | 3 | 9 |
| Ding K et al. (2024) [78] | Study-specific inclusion/exclusion criteria (chronic pain versus healthy control) | No | 4 | 2 | 3 | 9 |
| Camargo et al. (2024) [79] | Study-specific inclusion/exclusion criteria (chronic pain and stimulated pain) | No substance abuse within past six months and use of opioids in large doses (more than 30 mgofoxycodone/hydrocodone or 7.5 mg of hydromorphone or equivalent) | 2 | 1 | 3 | 6 |
| Chowdhury NS et al. (2023) [75] | Inclusion criteria: PREDICT study participants Study-specific exclusion criteria | No | 4 | 2 | 3 | 9 |
| Vanneste S, De Ridder D. (2023) [59] | Study-specific inclusion/exclusion criteria | Yes. Opioids and antiepileptic drugs | 2 | 2 | 2 | 6 |
| Kenefati G et al. (2023) [74] | Study-specific inclusion/exclusion criteria | No benzodiazepine | 3 | 2 | 3 | 8 |
| Wang H et al. (2023) [65] | Study-specific inclusion/exclusion criteria (healthy control and noxious contact heat stimuli) | Not informed | 4 | 2 | 3 | 9 |
| Simis M, Pacheco-Barrios K et al. (2023) [72] | Study-specific inclusion/exclusion criteria (only chronic knee OA pain patients); No healthy control group | Yes, but not specified | 2 | 2 | 2 | 6 |
| Simis M et al. (2022a) [60] | Study-specific inclusion/exclusion criteria (only chronic knee OA pain patients) No healthy control group | Yes, but not specified | 2 | 2 | 2 | 6 |
| Simis M et al. (2022b) [81] | Study-specific inclusion/exclusion criteria (chronic pain only) | Yes, neuroactive medication | 2 | 2 | 2 | 6 |
| Heitmann H et al. (2022) [61] | Study-specific inclusion/exclusion criteria (only chronic pain patients) No healthy control group | Yes, except for regular benzodiazepine medication | 2 | 2 | 2 | 6 |
| Ocay DD et al. (2022) [62] | Study-specific inclusion/exclusion criteria (chronic pain patients versus healthy control group) | Not informed | 3 | 2 | 3 | 8 |
| Peier F. (2022) [68] | Study-specific inclusion/exclusion criteria (healthy highly trained athletes versus healthy non-trained non-athletes) CPT | No | 4 | 2 | 3 | 9 |
| Nuñez-Ibero M et al. (2021) [67] | Study-specific inclusion/exclusion criteria (heat pain threshold (HPT)) Control group. | No | 4 | 2 | 3 | 9 |
| Chouchou F, Perchet C, Garcia-Larrea L. (2021) [69] | Study-specific inclusion/exclusion criteria. (stimulated pain by CPT versus stereotyped grimaces mimicking those evoked by pain) | No, except for contraceptive pills | 4 | 2 | 3 | 9 |
| Rustamov N et al. (2021) [70] | Study-specific inclusion/exclusion criteria (stimulated pain by CPT versus baseline) | No analgesics, including non-steroidal anti-inflammatory drugs (NSAIDs) or acetaminophen, for at least five drug half-lives prior | 4 | 2 | 3 | 9 |
| van den Berg B et al. (2021) [73] | Study-specific inclusion/exclusion criteria (failed back surgery patients versus healthy control group) | Yes, not specified | 4 | 2 | 3 | 9 |
| Furman AJ, Prokhorenko M et al. (2020) [64] | Study-specific inclusion/exclusion criteria (stimulated pain by phasic heat pain and capsaicin heat pain models) | No | 4 | 2 | 3 | 9 |
| Uygur-Kucukseymen E et al. (2020) [82] | Study-specific inclusion/exclusion criteria (chronic pain) | No opiate use in large doses | 2 | 2 | 2 | 6 |
| Furman AJ, Thapa T et al. (2019) [76] | Study-specific inclusion/exclusion criteria (stimulated pain by repeated intramuscular injection of nerve growth factor (NGF)) | No | 4 | 2 | 2 | 8 |
| Beck B et al. (2019) [66] | Study-specific inclusion/exclusion criteria (stimulated pain) | No | 4 | 2 | 3 | 9 |
| Case M (2018) [80] | Study-specific inclusion/exclusion criteria (chronic pain versus healthy control) | Yes, not specified | 4 | 2 | 4 | 10 |
| Levitt J et al. (2017) [71] | Study-specific inclusion/exclusion criteria (stimulated pain) | No | 4 | 2 | 3 | 9 |
| De Vries M et al. (2013) [63] | Study-specific inclusion/exclusion criteria Chronic pain versus healthy controls | Yes, analgesics, including opioids, and centrally acting medication No alcohol | 4 | 2 | 3 | 9 |
References
- U.S. Pain Foundation. Research Pain to Manage Pain. 2023. Available online: https://uspainfoundation.org/news/research-pain-to-manage-pain/ (accessed on 4 January 2024).
- Goldberg, D.S.; McGee, S.J. Pain as a global public health priority. BMC Public Health 2011, 11, 770. [Google Scholar]
- Corewell Health. Types of Pain. Available online: https://www.beaumont.org/services/pain-management-services/types-of-pain (accessed on 4 January 2024).
- Cleveland Clinic. Chronic Pain. Available online: https://my.clevelandclinic.org/health/diseases/4798-chronic-pain (accessed on 4 January 2024).
- American Cancer Society. Pain in People with Cancer. Available online: https://www.cancer.org/cancer/managing-cancer/side-effects/pain/cancer-pain/pain-in-people-with-cancer.html (accessed on 4 January 2024).
- Neil, M.J.; Macrae, W.A. Post surgical pain-the transition from acute to chronic pain. Rev. Pain 2009, 3, 6–9. [Google Scholar] [PubMed]
- The Anatomy and Physiology of Pain. In Pain and Disability: Clinical, Behavioral, and Public Policy Perspectives; Committee on Pain, Disability, and Chronic Illness Behavior, Osterweis, M., Kleinman, A., Mechanic, D., Eds.; National Academies Press: Washington, DC, USA, 1987; Volume 7. Available online: https://www.ncbi.nlm.nih.gov/books/NBK219252/ (accessed on 4 January 2024).
- Ibrahim, F.; Ma, M.; Scott, D.L.; Scott, I.C. Defining the relationship between pain intensity and disease activity in patients with rheumatoid arthritis: A secondary analysis of six studies. Arthritis Res. Ther. 2022, 24, 218. [Google Scholar]
- National Institute of Environmental Health Sciences. Biomarkers. Available online: https://www.niehs.nih.gov/health/topics/science/biomarkers#:~:text=A%20biomarker%20(short%20for%20biological,warning%20systems%20for%20your%20health (accessed on 5 January 2024).
- International Association for The Study of Pain. Terminology. Available online: https://www.iasp-pain.org/resources/terminology/ (accessed on 18 November 2024).
- Treede, R.D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. A classification of chronic pain for ICD-11. Pain 2015, 56, 1003–1007. [Google Scholar]
- National Institutes of Health. Acute to Chronic pain Signatures Program. Available online: https://heal.nih.gov/research/clinical-research/pain-signatures#:~:text=Between%2028%20percent%20and%2061,types%20of%20injuries%20or%20surgery (accessed on 15 January 2024).
- Measuring Pain and Dysfunction. In Pain and Disability: Clinical, Behavioral, and Public Policy Perspectives; Committee on Pain, Disability, and Chronic Illness Behavior, Osterweis, M., Kleinman, A., Mechanic, D., Eds.; National Academies Press: Washington, DC, USA, 1987; Volume 11. Available online: https://www.ncbi.nlm.nih.gov/books/NBK219245/ (accessed on 4 January 2024).
- Lundeberg, S.; Lundeberg, T. Pain in infants and children-Physiological background and clinical aspects. Acupunct. Relat. Ther. 2013, 1, 46–49. [Google Scholar] [CrossRef]
- Johns Hopkins Medicine. Chronic Pain. Available online: https://www.hopkinsmedicine.org/health/conditions-and-diseases/chronic-pain (accessed on 16 January 2024).
- Substance Abuse and Mental Health Services Administration. Managing Chronic Pain in Adults with or in Recovery from Substance Use Disorders; Treatment Improvement Protocol (TIP) Series 54. HHS Publication No. (SMA) 12-4671; Substance Abuse and Mental Health Services Administration: Rockville, MD, USA, 2011. [Google Scholar]
- Michael, M.M.; MacDonald, C.J. Analysis of opioid efficacy, tolerance, addiction and dependence from cell culture to human. Br. J. Pharmacol. 2011, 164, 1322–1334. [Google Scholar]
- Knotkova, H.; Hamani, C.; Sivanesan, E.; Le Beuffe, M.F.E.; Moon, J.Y.; Cohen, S.P.; Huntoon, M.A. Neuromodulation for chronic pain. Lancet 2021, 397, 2111–2400. [Google Scholar] [CrossRef]
- Hofstoetter, U.S.; Freundl, B.; Danner, S.M.; Krenn, M.J.; Mayr, W.; Binder, H.; Minassian, K. Transcutaneous spinal cord stimulation induces temporary attenuation of spasticity in individuals with spinal cord injury. J. Neurotrauma 2020, 37, 481–493. [Google Scholar]
- Asefi Rad, A.; Wippert, P.M. Insights into pain distraction and the impact of pain catastrophizing on pain perception during different types of distraction tasks. Front. Pain Res. 2024, 5, 1266974. [Google Scholar] [CrossRef]
- Dağcan, N.; Özden, D.; Gürol Arslan, G. Pain perception of patients in intensive care unit after cardiac surgery: A qualitative study using Roy’s Adaptation Model. Nurs. Crit. Care 2024, 29, 512–520. [Google Scholar] [CrossRef]
- Sánchez-López, M.I.; Lluesma-Vidal, M.; Ruiz-Zaldibar, C.; Tomás-Saura, I.; Martínez-Fleta, M.I.; Gutiérrez-Alonso, G.; García-Garcés, L. The effect of virtual reality versus standard-of-care treatment on pain perception during paediatric vaccination: A randomised controlled trial. J. Clin. Nurs. 2025, 34, 1045–1062. [Google Scholar] [CrossRef] [PubMed]
- Ogez, D.; Landry, M.; Caron-Trahan, R.; Jusseaux, A.E.; Aubin, M.; Véronneau, J.; Fournier, V.; Godin, N.; Idrissi, M.; Rainville, P.; et al. Make me more comfortable: Effects of a hypnosis session on pain perception in chronic pain patients. Front. Psychol. 2024, 15, 1362208. [Google Scholar] [CrossRef] [PubMed]
- Tamvaki, E.; Giannakopoulou, M.; Bozas, E.; Zachpoulou, D. Use of Biomarkers to Objectively Evaluate Pain in Critically Ill Children: A Scoping Review. Crit. Care Nurse 2024, 44, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Favretti, M.; Iannuccelli, C.; Di Franco, M. Pain biomarkers in fibromyalgia syndrome: Current understanding and future directions. Int. J. Mol. Sci. 2023, 24, 10443. [Google Scholar] [CrossRef]
- Diaz, M.M.; Caylor, J.; Strigo, I.; Lerman, I.; Henry, B.; Lopez, E.; Wallace, M.S.; Ellis, R.J.; Simmons, A.N.; Keltner, J.R. Toward composite pain biomarkers of neuropathic pain—Focus on peripheral neuropathic pain. Front. Pain Res. 2022, 3, 869215. [Google Scholar] [CrossRef]
- Talbot, K.; Madden, V.J.; Jones, S.L.; Moseley, G.L. The sensory and affective components of pain: Are they differentially modifiable dimensions or inseparable aspects of a unitary experience? A systematic review. Br. J. Anaesth. 2019, 123, e263–e272. [Google Scholar] [CrossRef]
- De Leon-Casasola, O.A. Multimodal approaches to the management of neuropathic pain: The role of topical analgesia. J. Pain Symptom Manage 2007, 33, 356–364. [Google Scholar] [CrossRef]
- Gram, M.; Graversen, C.; Olesen, S.S.; Drewes, A.M. Dynamic spectral indices of the electroencephalogram provide new insights into tonic pain. Clin. Neurophysiol. 2015, 126, 763–771. [Google Scholar] [CrossRef]
- Schmidt, S.; Naranjo, J.R.; Brenneisen, C.; Gundlach, J.; Schultz, C.; Kaube, H.; Hinterberger, T.; Jeanmonod, D. Pain ratings, psychological functioning and quantitative EEG in a controlled study of chronic back pain patients. PLoS ONE 2012, 7, e31138. [Google Scholar]
- Wang, J.; Li, X.; Lu, C.; Voss, L.J.; Barnard, J.P.; Sleigh, J.W. Characteristics of evoked potential multiple EEG recordings in patients with chronic pain by means of parallel factor analysis. Comput. Math. Methods Med. 2012, 2012, 279560. [Google Scholar]
- May, E.S.; Ávila, C.G.; Dinh, S.T.; Heitmann, H.; Hohn, V.D.; Nickel, M.M.; Tiemann, L.; Tölle, T.R.; Ploner, M. Dynamics of brain function in patients with chronic pain assessed by microstate analysis of resting-state electroencephalography. Pain 2021, 162, 2894–2908. [Google Scholar] [PubMed]
- Egsgaard, L.L.; Wang, L.; Arendt-Nielsen, L. Volunteers with high versus low alpha EEG have different pain–EEG relationship: A human experimental study. Exp. Brain Res. 2009, 193, 361–369. [Google Scholar] [PubMed]
- Nezam, T.; Boostani, R.; Abootalebi, V.; Rastegar, K. A novel classification strategy to distinguish five levels of pain using the EEG signal features. IEEE Trans. Affect. Comput. 2018, 12, 131–140. [Google Scholar]
- Modares-Haghighi, P.; Boostani, R.; Nami, M.; Sanei, S. Quantification of pain severity using EEG-based functional connectivity. Biomed. Signal Process Control 2021, 69, 102840. [Google Scholar]
- Afrasiabi, S.; Boostani, R.; Masnadi-Shirazi, M.A.; Nezam, T. An EEG based hierarchical classification strategy to differentiate five intensities of pain. Expert Syst. Appl. 2021, 180, 115010. [Google Scholar]
- Neogi, T.; Felson, D.; Niu, J.; Nevitt, M.; Lewis, C.E.; Aliabadi, P.; Sack, B.; Torner, J.; Bradley, L.; Zhang, Y. Association between radiographic features of knee osteoarthritis and pain: Results from two cohort studies. BMJ 2009, 339, b2884. [Google Scholar]
- Neogi, T. The epidemiology and impact of pain in osteoarthritis. Osteoarthr. Cartil. 2013, 21, 1145–1153. [Google Scholar]
- Wilcox, C.E.; Mayer, A.R.; Teshiba, T.M.; Ling, J.; Smith, B.W.; Wilcox, G.L.; Mullins, P.G. The subjective experience of pain: An FMRI study of percept-related models and functional connectivity. Pain Med. 2015, 16, 2121–2133. [Google Scholar]
- Kim, G.; Chung, H.; Jang, H.Y.; Park, J.; Cho, Y.; Lee, Y. The relationship between extremity injury severity and pain intensity according to age. J. Korean Soc. Emerg. Med. 2017, 28, 602–609. [Google Scholar]
- Kim, J.; Lyon, D.; Weave, M.T.; Keenan, G.; Chen, X. The role of psychological distress in the relationship between the severity of pressure injury and pain intensity in hospitalized adults. J. Adv. Nurs. 2019, 75, 1219–1228. [Google Scholar]
- Plant, K.; Goebel, A.; Nair, J.; Moots, R.; Chadwick, L.; Goodson, N. Characteristics of pain and their relationship to disease activity in UK patients with Behçet’s syndrome: A prospective cohort study. Br. J. Pain 2023, 17, 560–568. [Google Scholar] [PubMed]
- Chorlian, D.B.; Porjesz, B.; Cohen, H.L. Measuring electrical activity of the brain: ERP mapping in alcohol research. Alcohol Health Res, World 1995, 19, 315–320. [Google Scholar] [PubMed]
- Barkana, B.B.; Ozkan, Y.; Badara, J.A. Analysis of working memory from EEG signals under different emotional states. Biomed. Signal Process Control 2022, 71, 103249. [Google Scholar]
- RadiologyInfo.org for Patients. Functional MRI (fMRI). Available online: https://www.radiologyinfo.org/en/info/fmribrain#:~:text=Functional%20magnetic%20resonance%20imaging%20(fMRI,or%20to%20guide%20brain%20treatment (accessed on 19 January 2024).
- Andreone, B.J.; Lacoste, B.; Gu, C. Neuronal and vascular interactions. Annu. Rev. Neurosci. 2015, 38, 25–46. [Google Scholar]
- Burle, B.; Spieser, L.; Roger, C.; Casini, L.; Hasbroucq, T.; Vidal, F. Spatial and temporal resolutions of EEG: Is it really black and white? A scalp current density view. Int. J. Psychophysiol. 2015, 97, 210–220. [Google Scholar]
- Barrow Neurological Institute. High Density EEG (HD EEG). Available online: https://www.barrowneuro.org/treatment/high-density-eeg-hd-eeg/#:~:text=Increased%20Electrode%20Density:%20High%2Ddensity,likely%20originating%20in%20the%20brain (accessed on 1 March 2025).
- Janiukstyte, V.; Owen, T.W.; Chaudhary, U.J.; Diehl, B.; Lemieux, L.; Duncan, J.S.; de Tisi, J.; Wang, Y.; Taylor, P.N. Normative brain mapping using scalp EEG and potential clinical application. Sci. Rep. 2023, 13, 13442. [Google Scholar]
- Beres, A.M. Time is of the essence: A review of electroencephalography (EEG) and event-related brain potentials (ERPs) in language research. Appl. Psychophysiol. Biofeedback 2017, 42, 247–255. [Google Scholar]
- Sohrabpour, A.; Lu, Y.; Kankirawatana, P.; Blount, J.; Kim, H.; He, B. Effect of EEG electrode number on epileptic source localization in pediatric patients. Clin. Neurophysiol. 2015, 126, 472–480. [Google Scholar]
- Epilepsy Action. EEG Tests for Epilepsy. Available online: https://www.epilepsy.org.uk/info/diagnosis/eeg-electroencephalogram#:~:text=before%20the%20test.-,What%20information%20does%20an%20EEG%20test%20give?,mean%20that%20you%20have%20epilepsy (accessed on 2 March 2025).
- Richardson, W.S.; Wilson, M.C.; Nishikawa, J.; Hayward, R.S. The well-built clinical question: A key to evidence-based decisions. ACP J. Club 1995, 123, A12–A13. [Google Scholar]
- Mussigmann, T.; Bardel, B.; Lefaucheur, J.P. Resting-state electroencephalography (EEG) biomarkers of chronic neuropathic pain. A systematic review. NeuroImage 2022, 258, 119351. [Google Scholar]
- Pinheiro, E.S.; Queirós, F.C.; Montoya, P.; Santos, C.L.; Nascimento, M.A. Electroencephalographic patterns in chronic Pain: A systematic review of literature. PLoS ONE 2016, 11, e0149085. [Google Scholar]
- Roy, R.; de la Vega, R.; Jensen, M.P.; Miró, J. Neurofeedback for pain management: A systematic review. Front. Neurosci. 2020, 14, 671. [Google Scholar] [CrossRef] [PubMed]
- Burns, E.; Chipchase, L.S.; Schabrun, S.M. Primary sensory and motor cortex function in response to acute muscle pain: A systematic review and meta-analysis. Eur. J. Pain 2016, 20, 1203–1213. [Google Scholar] [PubMed]
- The Ottawa Hospital Research Institute; Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 27 February 2025).
- Vanneste, S.; De Ridder, D. Burst DR spinal cord stimulation rebalances pain input and pain suppression in the brain in chronic neuropathic pain. Brain Stimul. 2023, 16, 1186–1195. [Google Scholar]
- Simis, M.; Imamura, M.; Pacheco-Barrios, K.; Marduy, A.; de Melo, P.S.; Mendes, A.J.; Teixeira, P.E.P.; Battistella, L.; Fregni, F. EEG theta and beta bands as brain oscillations for different knee osteoarthritis phenotypes according to disease severity. Sci. Rep. 2022, 12, 1480. [Google Scholar]
- Heitmann, H.; Gil Ávila, C.; Nickel, M.M.; Ta Dinh, S.; May, E.S.; Tiemann, L.; Hohn, V.D.; Tölle, T.R.; Ploner, M. Longitudinal resting-state electroencephalography in patients with chronic pain undergoing interdisciplinary multimodal pain therapy. Pain 2022, 163, e997–e1005. [Google Scholar]
- Ocay, D.D.; Teel, E.F.; Luo, O.D.; Savignac, C.; Mahdid, Y.; Blain-Moraes, S.; Ferland, C.E. Electroencephalographic characteristics of children and adolescents with chronic musculoskeletal pain. Pain Rep. 2022, 7, e1054. [Google Scholar]
- De Vries, M.; Wilder-Smith, O.H.; Jongsma, M.L.; van den Broeke, E.N.; Arns, M.; van Goor, H.; van Rijn, C.M. Altered resting state EEG in chronic pancreatitis patients: Toward a marker for chronic pain. J. Pain Res. 2013, 6, 815–824. [Google Scholar]
- Furman, A.J.; Prokhorenko, M.; Keaser, M.L.; Zhang, J.; Chen, S.; Mazaheri, A.; Seminowicz, D.A. Sensorimotor peak alpha frequency is a reliable biomarker of pain sensitivity. Cereb. Cortex 2020, 30, 6069–6082. [Google Scholar]
- Wang, H.; Guo, Y.; Tu, Y.; Peng, W.; Lu, X.; Bi, Y.; Iannetti, G.D.; Hu, L. Neural processes responsible for the translation of sustained nociceptive inputs into subjective pain experience. Cereb. Cortex 2022, 33, 634–650. [Google Scholar]
- Beck, B.; Cook, S.; Iannetti, G.; Haggard, P. Neural markers of nociceptive input and pain intensity coding: A signal detection approach. 2019; preprint. [Google Scholar] [CrossRef]
- Nuñez-Ibero, M.; Camino-Pontes, B.; Diez, I.; Erramuzpe, A.; Martinez-Gutierrez, E.; Stramaglia, S.; Alvarez-Cienfuegos, J.O.; Cortes, J.M. A controlled thermoalgesic stimulation device for exploring novel pain perception biomarkers. IEEE J. Biomed. Health Inf. 2021, 25, 2948–2957. [Google Scholar] [CrossRef]
- Peier, F. Effects of Long-Lasting and Intensive Physical Exercise Regime on Experimental Pain Response and Sensitization, and Their EEG GABAergic Correlate. Master’s Thesis, University of Fribourg, Fribourg, Switzerland, 2022. [Google Scholar]
- Chouchou, F.; Perchet, C.; Garcia-Larrea, L. EEG changes reflecting pain: Is alpha suppression better than gamma enhancement? Neurophysiol. Clin. 2021, 51, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Rustamov, N.; Sharma, L.; Chiang, S.N.; Burk, C.; Haroutounian, S.; Leuthardt, E.C. Spatial and frequency-specific electrophysiological signatures of tonic pain recovery in humans. Neuroscience 2021, 465, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Levitt, J.; Choo, H.J.; Smith, K.A.; LeBlanc, B.W.; Saab, C.Y. Electroencephalographic frontal synchrony and caudal asynchrony during painful hand immersion in cold water. Brain Res. Bull. 2017, 130, 75–80. [Google Scholar] [CrossRef]
- Simis, M.; Pacheco-Barrios, K.; Vasquez-Avila, K.; Rebello-Sanchez, I.; Parente, J.; Castelo-Branco, L.; Marduy, A.; de Melo, P.S.; Imamura, M.; Battistella, L.; et al. Functional and neural correlates associated with conditioned pain modulation in patients with chronic knee osteoarthritis pain: A cross-sectional study. Life 2023, 13, 1697. [Google Scholar] [CrossRef]
- van den Berg, B.; Berfelo, T.; Verhoeven, E.M.; Krabbenbos, P.; Buitenweg, J.R. Combining psychophysical and EEG biomarkers for improved observation of altered nociceptive processing in failed back surgery syndrome. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Virtual, 1–5 November 2021; pp. 174–177. [Google Scholar]
- Kenefati, G.; Rockholt, M.M.; Ok, D.; McCartin, M.; Zhang, Q.; Sun, G.; Maslinski, J.; Wang, A.; Chen, B.; Voigt, E.P.; et al. Changes in alpha, theta, and gamma oscillations in distinct cortical areas are associated with altered acute pain responses in chronic low back pain patients. Front. Neurosci. 2023, 17, 1278183. [Google Scholar] [CrossRef]
- Chowdhury, N.S.; Skippen, P.; Si, E.; Chiang, A.K.; Millard, S.K.; Furman, A.J.; Chen, S.; Schabrun, S.M.; Seminowicz, D.A. The reliability of two prospective cortical biomarkers for pain: EEG peak alpha frequency and TMS corticomotor excitability. J. Neurosci. Methods 2023, 385, 109766. [Google Scholar] [CrossRef]
- Furman, A.J.; Thapa, T.; Summers, S.J.; Cavaleri, R.; Fogarty, J.S.; Steiner, G.Z.; Schabrun, S.M.; Seminowicz, D.A. Cerebral peak alpha frequency reflects average pain severity in a human model of sustained, musculoskeletal pain. J. Neurophysiol. 2019, 122, 1784–1793. [Google Scholar] [CrossRef]
- Barbosa, S.P.; Junqueira, Y.N.; Akamatsu, M.A.; Marques, L.M.; Teixeira, A.; Lobo, M.; Mahmoud, M.H.; Omer, W.E.; Pacheco-Barrios, K.; Fregni, F. Resting-state electroencephalography delta and theta bands as compensatory oscillations in chronic neuropathic pain: A secondary data analysis. Brain Netw. Modul. 2024, 3, 52–60. [Google Scholar] [CrossRef]
- Ding, K.; Arginteanu, T.; White, M.A.; Lovell, L.; Thakor, N.V.; Doshi, T. Electroencephalographic power ratio and peak frequency difference associate with central sensitization in chronic pain. J. Neural. Eng. 2024, 21, 066035. [Google Scholar] [CrossRef]
- Camargo, L.; Pacheco-Barrios, K.; Marques, L.M.; Caumo, W.; Fregni, F. Adaptive and Compensatory Neural Signatures in Fibromyalgia: An Analysis of Resting-State and Stimulus-Evoked EEG Oscillations. Biomedicines 2024, 12, 1428. [Google Scholar] [CrossRef] [PubMed]
- Case, M. Functional Multimodal Imaging of Sickle Cell Disease Patients to Understand How Chronic Pain Affects Neural Dynamics of Patients. Ph.D. Thesis, University of Minnesota, Minneapolis, MN, USA, 2018. [Google Scholar]
- Simis, M.; Pacheco-Barrios, K.; Uygur-Kucukseymen, E.; Castelo-Branco, L.; Battistella, L.R.; Fregni, F. Specific electroencephalographic signatures for pain and descending pain inhibitory system in spinal cord injury. Pain Med. 2022, 23, 955–964. [Google Scholar] [PubMed]
- Uygur-Kucukseymen, E.; Castelo-Branco, L.; Pacheco-Barrios, K.; Luna-Cuadros, M.A.; Cardenas-Rojas, A.; Giannoni-Luza, S.; Zeng, H.; Gianlorenco, A.C.; Gnoatto-Medeiros, M.; Shaikh, E.S.; et al. Decreased neural inhibitory state in fibromyalgia pain: A cross-sectional study. Neurophysiol. Clin. 2020, 50, 279–288. [Google Scholar] [PubMed]
- Zolezzi, D.M.; Naal-Ruiz, N.E.; Alonso-Valerdi, L.M.; Ibarra-Zarate, D.I. Chronic neuropathic pain: EEG data in eyes open and eyes closed with painDETECT and brief pain inventory reports. Data Brief 2023, 48, 109060. [Google Scholar]
- Castelo-Branco, L.; Kucukseymen, E.U.; Duarte, D.; El-Hagrassy, M.M.; Pinto, C.B.; Gunduz, M.E.; Cardenas-Rojas, A.; Pacheco-Barrios, K.; Yang, Y.; Gonzalez-Mego, P.; et al. Optimised transcranial direct current stimulation (tDCS) for fibromyalgia—Targeting the endogenous pain control system: A randomised, double-blind, factorial clinical trial protocol. BMJ Open 2019, 9, e032710. [Google Scholar]
- Latremoliere, A.; Woolf, C.J. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J. Pain 2009, 10, 895–926. [Google Scholar]
- van Driel, M.E.; Huygen, F.J.; Rijsdijk, M. Quantitative sensory testing: A practical guide and clinical applications. BJA Educ. 2024, 24, 326–334. [Google Scholar]
- LeBlanc, B.W.; Lii, T.R.; Silverman, A.E.; Alleyne, R.T.; Saab, C.Y. Cortical theta is increased while thalamocortical coherence is decreased in rat models of acute and chronic pain. Pain 2014, 155, 773–782. [Google Scholar]
- Edens, J.L.; Gil, K.M. Experimental induction of pain: Utility in the study of clinical pain. Behav. Ther. 1995, 26, 197–216. [Google Scholar]
- Brooks, J.; Tracey, I. Review: From nociception to pain perception: Imaging the spinal and supraspinal pathways. J. Anat. 2005, 207, 19–33. [Google Scholar]
- Atherton, D.D.; Facer, P.; Roberts, K.M.; Misra, V.P.; A Chizh, B.; Bountra, C.; Anand, P. Use of the novel Contact Heat Evoked Potential Stimulator (CHEPS) for the assessment of small fibre neuropathy: Correlations with skin flare responses and intra-epidermal nerve fibre counts. BMC Neurol. 2007, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Lejeune, N.; Petrossova, E.; Frahm, K.S.; Mouraux, A. High-speed heating of the skin using a contact thermode elicits brain responses comparable to CO2 laser-evoked potentials. Clin. Neurophysiol. 2023, 146, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fanninger, S.; Plener, P.L.; Fischer, M.J.; Kothgassner, O.D.; Goreis, A. Water temperature during the cold pressor test: A scoping review. Physiol. Behav. 2023, 271, 114354. [Google Scholar] [CrossRef]
- Lamotte, G.; Boes, C.J.; Low, P.A.; Coon, E.A. The expanding role of the cold pressor test: A brief history. Clin. Auton. Res. 2021, 31, 153–155. [Google Scholar]
- Mitchell, L.A.; MacDonald, R.A.; Brodie, E.E. Temperature and the cold pressor test. J. Pain 2004, 5, 233–237. [Google Scholar]
- Belmonte, C.; Brock, J.A.; Viana, F. Converting cold into pain. Exp. Brain Res. 2009, 196, 13–30. [Google Scholar] [CrossRef]
- Johnson, M. Transcutaneous electrical nerve stimulation: Mechanisms, clinical application and evidence. Rev. Pain 2007, 1, 7–11. [Google Scholar] [CrossRef]
- Watson, T. Transcutaneous Electrical Nerve Stimulation (TENS). Available online: https://web.archive.org/web/20170809062344id_/https://www.wwmsi.com/documents/WWMSI_Transcutaneous_Electrical_Nerve_Stimulation_Tim_Watson.pdf (accessed on 15 May 2024).
- Brenner, B.; Ericson, T.; Kohan, L. Advances in non-invasive neuromodulation. Curr. Pain Headache Rep. 2022, 26, 709–717. [Google Scholar] [CrossRef]
- Sluka, K.A.; Walsh, D. Transcutaneous electrical nerve stimulation: Basic science mechanisms and clinical effectiveness. J. Pain 2003, 4, 109–121. [Google Scholar]
- Hird, E.J.; Jones, A.K.P.; Talmi, D.; El-Deredy, W. A comparison between the neural correlates of laser and electric pain stimulation and their modulation by expectation. J. Neurosci. Methods 2018, 293, 117–127. [Google Scholar]
- Tran, T.D.; Hoshiyama, M.; Inui, K.; Kakigi, R. Electrical-induced pain diminishes somatosensory evoked magnetic cortical fields. Clin. Neurophysiol. 2003, 114, 1704–1714. [Google Scholar] [PubMed]
- Nie, H.; Graven-Nielsen, T.; Arendt-Nielsen, L. Spatial and temporal summation of pain evoked by mechanical pressure stimulation. Eur. J. Pain 2009, 13, 592–599. [Google Scholar] [PubMed]
- Lee, I.-S.; Wallraven, C.; Kong, J.; Chang, D.-S.; Lee, H.; Park, H.-J.; Chae, Y. When pain is not only pain: Inserting needles into the body evokes distinct reward-related brain responses in the context of a treatment. Physiol. Behav. 2015, 140, 148–155. [Google Scholar] [PubMed]
- Iheanacho, F.; Vellipuram, A.R. Physiology, Mechanoreceptors; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Hill, R.Z.; Bautista, D.M. Getting in touch with mechanical pain mechanisms. Trends Neurosci. 2020, 43, 311–325. [Google Scholar] [CrossRef]
- Mechanoreceptors. In Encyclopedia of Neuroscience; Squire, L.R., Ed.; Academic Press: Cambridge, MA, USA, 2009; pp. 689–695. [Google Scholar]
- Świeboda, P.; Filip, R.; Prystupa, A.; Drozd, M. Assessment of pain: Types, mechanism and treatment. Pain 2013, 2, 2–7. [Google Scholar]
- Mizumura, K.; Murase, S. Role of nerve growth factor in pain. In Pain Control; Schaible, H.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 57–77. [Google Scholar]
- Andresen, T.; Nilsson, M.; Nielsen, A.K.; Lassen, D.; Arendt-Nielsen, L.; Drewes, A.M. Intradermal injection with nerve growth factor: A reproducible model to induce experimental allodynia and hyperalgesia. Pain Pract. 2016, 16, 12–23. [Google Scholar]
- Barker, P.A.; Mantyh, P.; Arendt-Nielsen, L.; Viktrup, L.; Tive, L. Nerve growth factor signaling and its contribution to pain. J. Pain Res. 2020, 13, 1223–1241. [Google Scholar]
- Fingelkurts, A.A.; Fingelkurts, A.A.; Kähkönen, S. Functional connectivity in the brain-Is it an elusive concept? Neurosci. Biobehav. Rev. 2005, 28, 827–836. [Google Scholar] [CrossRef]
- Friston, K.J.; Frith, C.D.; Liddle, P.F.; Frackowiak, R.S. Functional connectivity: The principal-component analysis of large (PET) data sets. J. Cereb. Blood Flow Metab. 1993, 13, 5–14. [Google Scholar]
- van den Heuvel, M.P.; Hulshoff Pol, H.E. Exploring the brain network: A review on resting-state fMRI functional connectivity. Eur. Neuropsychopharmacol. 2010, 20, 519–534. [Google Scholar] [CrossRef]
- Aru, J.; Aru, J.; Priesemann, V.; Wibral, M.; Lana, L.; Pipa, G.; Singer, W.; Vicente, R. Untangling cross-frequency coupling in neuroscience. Curr. Opin. Neurobiol. 2015, 31, 51–61. [Google Scholar] [PubMed]
- Bijsterbosch, J.D.; Woolrich, M.W.; Glasser, M.F.; Robinson, E.C.; Beckmann, C.F.; Van Essen, D.C.; Harrison, S.J.; Smith, S.M. The relationship between spatial configuration and functional connectivity of brain regions. eLife 2018, 7, e32992. [Google Scholar] [CrossRef] [PubMed]
- Ploner, M.; Lee, M.C.; Wiech, K.; Bingel, U.; Tracey, I. Prestimulus functional connectivity determines pain perception in humans. Proc. Natl. Acad. Sci. USA 2010, 107, 355–360. [Google Scholar] [PubMed]
- González-Roldán, A.M.; Terrasa, J.L.; Sitges, C.; van der Meulen, M.; Anton, F.; Montoya, P. Age-related changes in pain perception are associated with altered functional connectivity during resting state. Front. Aging Neurosci. 2020, 12, 116. [Google Scholar]
- Bolwerk, A.; Seifert, F.; Maihöfner, C. Altered resting-state functional connectivity in complex regional pain syndrome. J. Pain 2013, 14, 1107–1115. [Google Scholar]
- Feng, L.; Li, H.; Cui, H.; Xie, X.; Xu, S.; Hu, Y. Low back pain assessment based on alpha oscillation changes in spontaneous electroencephalogram (EEG). Neural Plast. 2021, 2021, 8537437. [Google Scholar]
- May, E.S.; Butz, M.; Kahlbrock, N.; Hoogenboom, N.; Brenner, M.; Schnitzler, A. Pre-and post-stimulus alpha activity shows differential modulation with spatial attention during the processing of pain. Neuroimage 2012, 62, 1965–1974. [Google Scholar]
- Zhang, Z.G.; Hu, L.; Hung, Y.S.; Mouraux, A.; Iannetti, G.D. Gamma-band oscillations in the primary somatosensory cortex—A direct and obligatory correlate of subjective pain intensity. J. Neurosci. 2012, 32, 7429–7438. [Google Scholar]
- Li, L.; Liu, X.; Cai, C.; Yang, Y.; Li, D.; Xiao, L.; Xiong, D.; Hu, L.; Qiu, Y. Changes of gamma-band oscillatory activity to tonic muscle pain. Neurosci. Lett. 2016, 627, 126. [Google Scholar]
- May, E.S.; Nickel, M.M.; Dinh, S.T.; Tiemann, L.; Heitmann, H.; Voth, I.; Tölle, T.R.; Gross, J.; Ploner, M. Prefrontal gamma oscillations reflect ongoing pain intensity in chronic back pain patients. Hum. Brain Mapp. 2019, 40, 293–305. [Google Scholar]
- Gross, J.; Schnitzler, A.; Timmermann, L.; Ploner, M. Gamma oscillations in human primary somatosensory cortex reflect pain perception. PLoS Biol. 2007, 5, e133. [Google Scholar]
- Schulz, E.; May, E.S.; Postorino, M.; Tiemann, L.; Nickel, M.M.; Witkovsky, V.; Schmidt, P.; Gross, J.; Ploner, M. Prefrontal gamma oscillations encode tonic pain in humans. Cereb. Cortex 2015, 25, 4407–4414. [Google Scholar] [PubMed]
- Lyu, Y.; Zidda, F.; Radev, S.T.; Liu, H.; Guo, X.; Tong, S.; Flor, H.; Andoh, J. Gamma band oscillations reflect sensory and affective dimensions of pain. Front. Neurol. 2022, 12, 695187. [Google Scholar]
- Xiao, R.; Shida-Tokeshi, J.; Vanderbilt, D.L.; Smith, B.A. Electroencephalography power and coherence changes with age and motor skill development across the first half year of life. PLoS ONE 2018, 13, e0190276. [Google Scholar]
- Saby, J.N.; Marshall, P.J. The utility of EEG band power analysis in the study of infancy and early childhood. Dev. Neuropsychol. 2012, 37, 253–273. [Google Scholar]
- Jensen, O.; Colgin, L.L. Cross-frequency coupling between neuronal oscillations. Trends Cogn. Sci. 2007, 11, 267–269. [Google Scholar]
- Cohen, M.X. Assessing transient cross-frequency coupling in EEG data. J. Neurosci. Methods 2008, 168, 494–499. [Google Scholar] [CrossRef]
- Kim, J.A.; Bosma, R.L.; Hemington, K.S.; Rogachov, A.; Osborne, N.R.; Cheng, J.C.; Oh, J.; Dunkley, B.T.; Davis, K.D. Cross-network coupling of neural oscillations in the dynamic pain connectome reflects chronic neuropathic pain in multiple sclerosis. NeuroImage Clin. 2020, 26, 102230. [Google Scholar]
- Vanneste, S.; Ost, J.; Van Havenbergh, T.; De Ridder, D. Resting state electrical brain activity and connectivity in fibromyalgia. PLoS ONE 2017, 12, e0178516. [Google Scholar]
- Graversen, C.; Brock, C.; Drewes, A.M.; Farina, D. Biomarkers for visceral hypersensitivity identified by classification of electroencephalographic frequency alterations. J. Neural. Eng. 2011, 8, 056014. [Google Scholar]
- PainQx, Objective Pain Assessment: PainQx, 106 W 32nd St, 2nd Floor New York, NY 10001. Available online: https://painqx.com/ (accessed on 2 March 2025).
- Medtronic, Inceptiv™ SCS system, 710 Medtronic Parkway, Minneapolis, MN 55432-5640 USA. Available online: https://www.medtronic.com/en-us/l/c/neurological/inceptiv-pain-relief.html?cmpid=Vanity_URL_MIX_painrelief_202210_US_EN_NM_Inceptiv_FY23.html (accessed on 2 March 2025).
- Abbott. Learn How Neurostimulation Works. Available online: https://www.neuromodulation.abbott/us/en/chronic-pain/how-neurostimulation-treats.html (accessed on 2 March 2025).
- Button, K.S.; Ioannidis, J.P.; Mokrysz, C.; Nosek, B.A.; Flint, J.; Robinson, E.S.; Munafò, M.R. Power failure: Why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 2013, 14, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Torday, J.S.; Baluška, F. Why control an experiment? From empiricism, via consciousness, toward implicate order. EMBO Rep. 2019, 20, e49110. [Google Scholar] [PubMed]
- Kato, K.; Sullivan, P.F.; Evengård, B.; Pedersen, N.L. Chronic widespread pain and its comorbidities: A population-based study. AMA Arch. Intern. Med. 2006, 166, 1649–1654. [Google Scholar] [PubMed]
- Zanzotto, F.M.; Croce, D. Comparing EEG/ERP-like and fMRI-like techniques for reading machine thoughts. In International Conference on Brain Informatics; Springer: Berlin/Heidelberg, Germany, 2010; pp. 133–144. [Google Scholar]


| Author (Year) | Participant Profile [# of Participants in Analysis] | EEG Equipment | EEG Acquisition Method [Sampling Frequency] | Additional Data Acquisition | Stimulation Method | Pain Scoring Method |
|---|---|---|---|---|---|---|
| Barbosa SP et al. (2024) [77] |
|
|
| N/A or not mentioned in study | N/A or not mentioned in study |
|
| Beck B et al. (2019) [66] |
|
|
|
|
| N/A or not mentioned in study |
| Camargo L et al. (2024) [79] |
|
|
|
|
|
|
| Chouchou F, Perchet C, Garcia-Larrea L. (2021) [69] |
|
|
| N/A or not mentioned in study |
|
|
| Chowdhury NS et al. (2023) [75] |
|
|
|
|
|
|
| De Vries M et al. (2013) [63] |
|
|
|
| N/A or not mentioned in study | N/A or not mentioned in study |
| Ding K et al. (2024) [78] |
|
|
|
|
|
|
| Furman AJ, Thapa T et al. (2019) [76] |
|
|
|
|
|
|
| Furman AJ, Prokhorenko M et al. (2020) [64] |
|
|
|
|
|
|
| Heitmann H et al. (2022) [61] |
|
|
|
|
|
|
| Kenefati G et al. (2023) [74] |
|
|
|
|
|
|
| Levitt J et al. (2017) [71] |
|
|
| N/A or not mentioned in study |
|
|
| Case M. (EEG–fMRI) (2018) [80] |
|
|
|
| N/A or not mentioned in study |
|
| Case M. (Resting–state EEG) (2018) [80] |
|
|
|
| N/A or not mentioned in study |
|
| Nuñez-Ibero M et al. (2021) [67] |
|
|
|
|
| N/A or not mentioned in study |
| Ocay DD et al. (2022) [62] |
|
|
|
|
|
|
| G*Peier F. (2022) [68] |
|
|
|
|
|
|
| Rustamov N et al. (2021) [70] |
|
|
| N/A or not mentioned in study |
|
|
| Simis M, Imamura M et al. (2022a) [60] |
|
|
|
|
|
|
| Simis M et al. (2022b) [81] |
|
|
|
|
|
|
| Simis M, Pacheco-Barrios K et al. (2023) [72] |
|
|
|
|
|
|
| Uygur-Kucukseymen E et al. (2020) [82] |
|
|
|
|
|
|
| van den Berg B et al. (2021) [73] |
|
|
| N/A or not mentioned in study |
| N/A or not mentioned in study |
| Vanneste S, De Ridder D. (2023) [59] |
|
|
|
|
|
|
| Wang H et al. (2023) [65] |
|
|
|
|
|
|
| Author (Year) | Study Features | Statistical Methods | Analytical Methods | Key Findings | Limitations |
|---|---|---|---|---|---|
| Barbosa SP et al. (2024) [77] |
|
|
|
|
|
| Beck B et al. (2019) [66] |
|
|
|
|
|
| Camargo L et al. (2024) [79] |
|
|
|
|
|
| Chouchou F, Perchet C, Garcia-Larrea L. (2021) [69] |
|
|
|
|
|
| Chowdhury NS et al. (2023) [75] |
|
|
|
|
|
| De Vries M et al. (2013) [63] |
|
|
|
|
|
| Ding K et al. (2024) [78] |
|
|
|
|
|
| Furman AJ, Thapa T et al. (2019) [76] |
|
|
|
|
|
| Furman AJ, Prokhorenko M et al. (2020) [64] |
|
|
|
|
|
| Heitmann H et al. (2022) [61] |
|
|
|
|
|
| Kenefati G et al. (2023) [74] |
|
|
|
|
|
| Levitt J et al. (2017) [71] |
|
|
|
|
|
| Case M. (EEG–fMRI) (2018) [80] |
|
|
|
|
|
| Case M. (Resting–state EEG) (2018) [80] |
|
|
|
|
|
| Nuñez-Ibero M et al. (2021) [67] |
|
|
|
|
|
| Ocay DD et al. (2022) [62] |
|
|
|
|
|
| Peier F. (2022) [68] |
|
|
|
|
|
| Rustamov N et al. (2021) [70] |
|
|
|
|
|
| Simis M, Imamura M et al. (2022a) [60] |
|
|
|
|
|
| Simis M et al. (2022b) [81] |
|
|
|
|
|
| Simis M, Pacheco-Barrios K et al. (2023) [72] |
|
|
|
|
|
| Uygur-Kucukseymen E et al. (2020) [82] |
|
|
|
|
|
| van den Berg B et al. (2021) [73] |
|
|
|
|
|
| Vanneste S, De Ridder D. (2023) [59] |
|
|
|
|
|
| Wang H et al. (2023) [65] |
|
|
|
|
|
| Feature | Chronic Pain | Experimentally Induced Pain |
|---|---|---|
| Peak Alpha Frequency (PAF) |
|
|
| Prefrontal Control |
|
|
| Emotional Networks |
|
|
| Default Mode Network (DMN) |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, B.; Barkana, B.D. Pain and the Brain: A Systematic Review of Methods, EEG Biomarkers, Limitations, and Future Directions. Neurol. Int. 2025, 17, 46. https://doi.org/10.3390/neurolint17040046
Ahmad B, Barkana BD. Pain and the Brain: A Systematic Review of Methods, EEG Biomarkers, Limitations, and Future Directions. Neurology International. 2025; 17(4):46. https://doi.org/10.3390/neurolint17040046
Chicago/Turabian StyleAhmad, Bayan, and Buket D. Barkana. 2025. "Pain and the Brain: A Systematic Review of Methods, EEG Biomarkers, Limitations, and Future Directions" Neurology International 17, no. 4: 46. https://doi.org/10.3390/neurolint17040046
APA StyleAhmad, B., & Barkana, B. D. (2025). Pain and the Brain: A Systematic Review of Methods, EEG Biomarkers, Limitations, and Future Directions. Neurology International, 17(4), 46. https://doi.org/10.3390/neurolint17040046






