Point-of-Care Electroencephalography in Acute Neurological Care: A Narrative Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
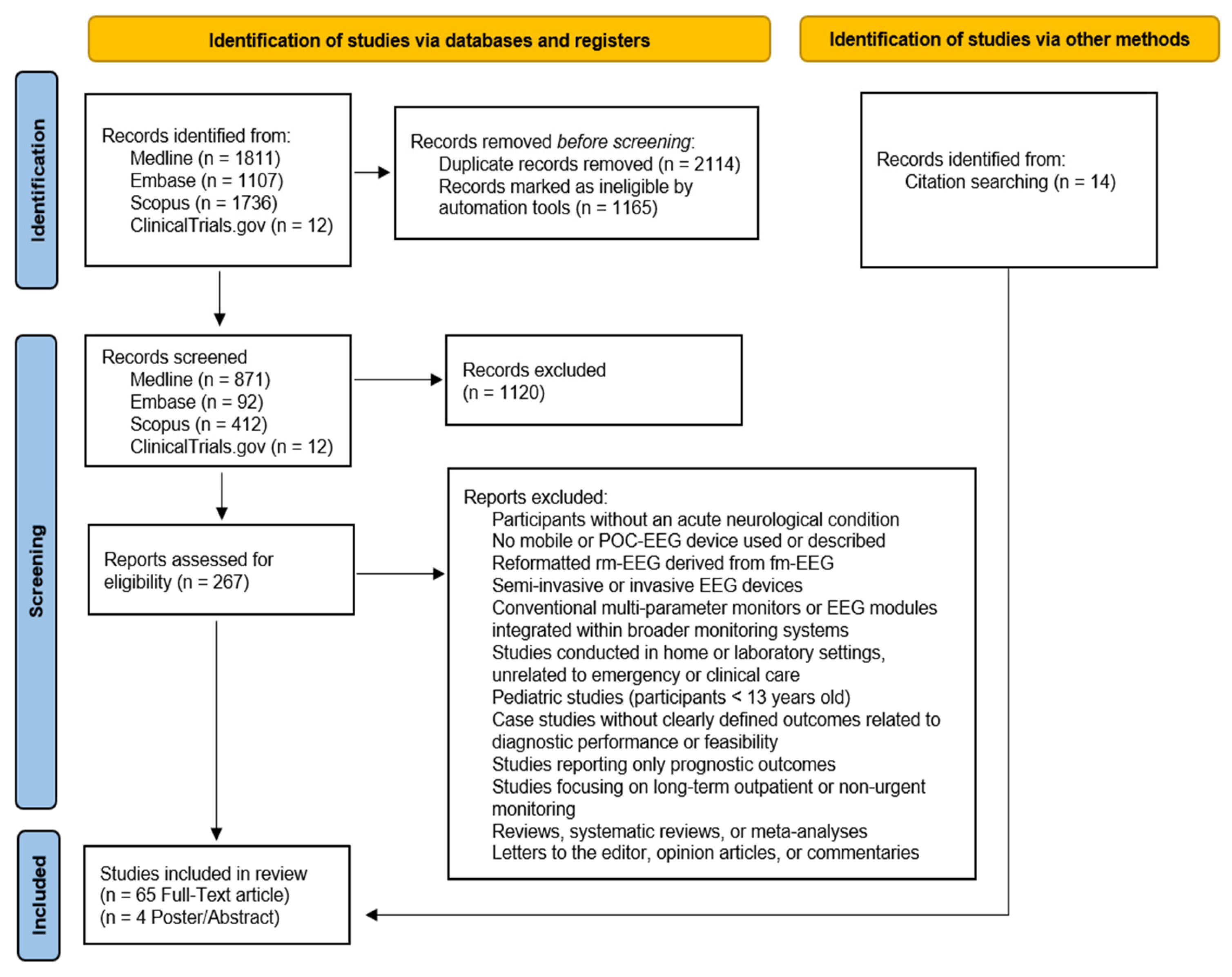
2.3. Search Process
2.4. Data Extraction, Synthesis, Analysis, and Quality Appraisal
3. Results
3.1. POC-EEG Systems in the Assessment of NCSE
3.1.1. Evaluating Diagnostic Accuracy and Clinical Implications of POC-EEG Systems for NCSE Detection
3.1.2. Evaluating Feasibility of POC-EEG Systems for NCSE Detection
3.2. POC-EEG Systems in the Assessment of TBI
3.2.1. Evaluating Diagnostic Accuracy and Clinical Implications of POC-EEG Systems for TBI Evaluation
3.2.2. Evaluating Feasibility of POC-EEG Systems for TBI Evaluation
3.3. POC-EEG Systems in the Detection and Management of Strokes
3.3.1. Evaluating the Diagnostic Accuracy and Clinical Implications of POC-EEG Systems in Stroke Assessment
3.3.2. Evaluating Feasibility of POC-EEG Systems in Stroke Assessment
3.4. POC-EEG Systems in Delirium Detection
3.4.1. Evaluating the Diagnostic Accuracy and Clinical Implications of POC-EEG Systems for Delirium Identification
3.4.2. Evaluating the Feasibility of POC-EEG Systems for Delirium Identification
4. Discussion
4.1. Diagnostic Accuracy, Feasibility, and Clinical Implications of POC-EEG Systems in the Assessment of NCSE
4.2. Diagnostic Accuracy, Feasibility, and Clinical Implications of POC-EEG Systems in the Assessment of TBIs
4.3. Diagnostic Accuracy, Feasibility, and Clinical Implications of POC-EEG Systems in the Assessment of Strokes
4.4. Diagnostic Accuracy, Feasibility, and Clinical Implications of POC-EEG Systems in the Assessment of Delirium
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AIS | Acute ischemic stroke |
| AMS | Altered mental status |
| ASM | Anti-seizure medication |
| BAI | Brain Abnormality Index |
| BD | Brain death |
| BFI | Brain function index |
| BIS | Bispectral index |
| BSEEG | Bispectral EEG |
| BSI | Brain symmetry index |
| CCAT | Critical Care Air Transport |
| CCHR | Canadian CT Head Rule |
| CI | Concussion Index |
| CSs | Continuous slow waves |
| CT+ | Computed Tomography-positive |
| CT− | Computed Tomography-negative |
| DA | Drugs and alcohol |
| DAR | Delta/alpha ratio |
| DBATR | (Delta + Theta)/(Alpha + Beta) Ratio |
| DECIDE | Does Use of Rapid-Response EEG Impact Clinical Decision-Making |
| DRG | Diagnosis-related group |
| DSD | Delirium superimposed on dementia |
| DTI | Diffusion tensor imaging |
| EA | Epileptic activity |
| EDs | Emergency departments |
| ESE | Electrographic SE |
| ESz | Electrographic seizure |
| GA | Genetic algorithm |
| GCS | Glasgow Coma Scale |
| GPD | Generalized PD |
| HEP | Highly epileptiform patterns |
| ICU | Intensive care unit |
| IED | Interictal epileptiform discharge |
| LASSO | Least Absolute Shrinkage and Selection Operator |
| LPD | Lateralized periodic discharges |
| LVO | Large vessel occlusion |
| LVO-a | Anterior LVO |
| MTBI-DS | mTBI discriminant score |
| NCS | Non-convulsive seizure |
| NCSE | Non-convulsive status epilepticus |
| NEXUS | National Emergency X-Radiography Utilization Study |
| NOC | New Orleans Criteria |
| NPV | Negative predictive value |
| Non-EA | Non-epileptic activity |
| PABI | Post-anoxic brain injury |
| PD | Periodic discharges |
| POC-EEG | Point-of-care electroencephalography |
| PPV | Positive predictive value |
| QI | Quality improvement |
| RA | Rhythmic activity |
| RDA | Rhythmic delta activity |
| RTP | Return-to-play |
| SAFER-EEG | Seizure Assessment and Forecasting with Efficient Rapid-EEG |
| SBII | Structural Brain Injury Index |
| SE | Status epilepticus |
| SIC | Structural Injury Classifier |
| SRC | Sports-related concussion |
| SVM | Support Vector Machine |
| SW | Spikes and waves |
| SWLDA | Stepwise Linear Discriminant Analysis |
| SzB | Seizure burden |
| TAR | Theta–alpha ratio |
| TBI | Traumatic brain injury |
| UCH-L1 | Ubiquitin C-terminal hydrolase L1 |
| ViT | Vision Transformer |
| cEEG | Continuous EEG |
| conv-EEG | Conventional EEG |
| c-conv-EEG | Continuous conventional EEG |
| eBFI | Enhanced BFI |
| fm-EEG | Full-montage EEG |
| mTBI | Mild TBI |
| pESE | Possible ESE |
| pdBSI | Pairwise-derived BSI |
| qEEG | Quantitative EEG |
| rEEG | Routine EEG |
| rm-EEG | Reduced-montage EEG |
| rr-EEG | Rapid-response EEG |
| rsBSI | Revised BSI |
References
- Sharma, S.; Nunes, M.; Alkhachroum, A. Adult Critical Care Electroencephalography Monitoring for Seizures: A Narrative Review. Front. Neurol. 2022, 13, 951286. [Google Scholar] [CrossRef]
- Davey, Z.; Gupta, P.B.; Li, D.R.; Nayak, R.U.; Govindarajan, P. Rapid Response EEG: Current State and Future Directions. Curr. Neurol. Neurosci. Rep. 2022, 22, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Simma, L.; Romano, F.; Schmidt, S.; Ramantani, G.; Bölsterli, B.K. Integrating Neuromonitoring in Pediatric Emergency Medicine: Exploring Two Options for Point-of-Care Electroencephalogram (pocEEG) via Patient Monitors—A Technical Note. J. Pers. Med. 2023, 13, 1411. [Google Scholar] [CrossRef] [PubMed]
- Moctezuma, L.A.; Molinas, M. EEG Channel-Selection Method for Epileptic-Seizure Classification Based on Multi-Objective Optimization. Front. Neurosci. 2020, 14, 593. [Google Scholar] [CrossRef]
- Eberhard, E.; Beckerman, S.R. Rapid-Response Electroencephalography in Seizure Diagnosis and Patient Care: Lessons From a Community Hospital. J. Neurosci. Nurs. 2023, 55, 157–163. [Google Scholar] [CrossRef]
- Kozak, R.; Gururangan, K.; Dorriz, P.J.; Kaplan, M. Point-of-Care Electroencephalography Enables Rapid Evaluation and Management of Non-convulsive Seizures and Status Epilepticus in the Emergency Department. J. Am. Coll. Emerg. Physicians Open 2023, 4, e13004. [Google Scholar] [CrossRef]
- Wright, N.M.K.; Madill, E.S.; Isenberg, D.; Gururangan, K.; McClellen, H.; Snell, S.; Jacobson, M.P.; Gentile, N.T.; Govindarajan, P. Evaluating the Utility of Rapid Response EEG in Emergency Care. Emerg. Med. J. 2021, 38, 923–926. [Google Scholar] [CrossRef]
- Madill, E.S.; Gururangan, K.; Krishnamohan, P. Improved Access to Rapid Electroencephalography at a Community Hospital Reduces Inter-hospital Transfers for Suspected Non-convulsive Seizures. Epileptic Disord. 2022, 24, 507–516. [Google Scholar] [CrossRef]
- Vespa, P.M.; Olson, D.M.; John, S.; Hobbs, K.S.; Gururangan, K.; Nie, K.; Desai, M.J.; Markert, M.; Parvizi, J.; Bleck, T.P.; et al. Evaluating the Clinical Impact of Rapid Response Electroencephalography: The DECIDE Multicenter Prospective Observational Clinical Study*. Crit. Care Med. 2020, 48, 1249–1257. [Google Scholar] [CrossRef]
- Laccheo, I.; Sonmezturk, H.; Bhatt, A.B.; Tomycz, L.; Shi, Y.; Ringel, M.; DiCarlo, G.; Harris, D.; Barwise, J.; Abou-Khalil, B.; et al. Non-Convulsive Status Epilepticus and Non-Convulsive Seizures in Neurological ICU Patients. Neurocrit. Care 2015, 22, 202–211. [Google Scholar] [CrossRef]
- Young, G.B.; Jordan, K.G. Do Nonconvulsive Seizures Damage the Brain?—Yes. Arch. Neurol. 1998, 55, 117–119. [Google Scholar] [CrossRef] [PubMed]
- Abend, N.S.; Arndt, D.H.; Carpenter, J.L.; Chapman, K.E.; Cornett, K.M.; Gallentine, W.B.; Giza, C.C.; Goldstein, J.L.; Hahn, C.D.; Lerner, J.T.; et al. Electrographic Seizures in Pediatric ICU Patients: Cohort Study of Risk Factors and Mortality. Neurology 2013, 81, 383. [Google Scholar] [CrossRef] [PubMed]
- Parikh, H.; Hoffman, K.; Sun, H.; Zafar, S.F.; Ge, W.; Jing, J.; Liu, L.; Sun, J.; Struck, A.; Volfovsky, A.; et al. Effects of Epileptiform Activity on Discharge Outcome in Critically Ill Patients in the USA: A Retrospective Cross-Sectional Study. Lancet Digit. Health 2023, 5, e495–e502. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, P.; Menon, D.K.; Citerio, G.; Vespa, P.; Bader, M.K.; Brophy, G.M.; Diringer, M.N.; Stocchetti, N.; Videtta, W.; Armonda, R.; et al. Consensus Summary Statement of the International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care: A Statement for Healthcare Professionals from the Neurocritical Care Society and the European Society of Intensive Care Medicine. Neurocrit. Care 2014, 21, 1–26. [Google Scholar] [CrossRef]
- Gavvala, J.; Abend, N.; LaRoche, S.; Hahn, C.; Herman, S.T.; Claassen, J.; Macken, M.; Schuele, S.; Gerard, E. Critical Care EEG Monitoring Research Consortium (CCEMRC) Continuous EEG Monitoring: A Survey of Neurophysiologists and Neuro-intensivists. Epilepsia 2014, 55, 1864–1871. [Google Scholar] [CrossRef]
- Beniczky, S.; Schomer, D.L. Electroencephalography: Basic Biophysical and Technological Aspects Important for Clinical Applications. Epileptic Disord. 2020, 22, 697–715. [Google Scholar] [CrossRef]
- MacDarby, L.; Healy, M.; McHugh, J.C. EEG Availability in the Intensive Care Setting: A Multicentre Study. Neurocrit. Care 2021, 34, 287–290. [Google Scholar] [CrossRef]
- Abend, N.S.; Topjian, A.A.; Williams, S. How Much Does It Cost to Identify a Critically Ill Child Experiencing Electrographic Seizures? J. Clin. Neurophysiol. 2015, 32, 257–264. [Google Scholar] [CrossRef]
- Ma, B.B.; Johnson, E.L.; Ritzl, E.K. Sensitivity of a Reduced EEG Montage for Seizure Detection in the Neurocritical Care Setting. J. Clin. Neurophysiol. 2018, 35, 256–262. [Google Scholar] [CrossRef]
- Kolls, B.J.; Husain, A.M. Assessment of Hairline EEG as a Screening Tool for Nonconvulsive Status Epilepticus. Epilepsia 2007, 48, 959–965. [Google Scholar] [CrossRef]
- Tanner, A.E.J.; Särkelä, M.O.K.; Virtanen, J.; Viertiö-Oja, H.E.; Sharpe, M.D.; Norton, L.; Davies-Schinkel, C.; Young, G.B. Application of Subhairline EEG Montage in Intensive Care Unit: Comparison With Full Montage. J. Clin. Neurophysiol. 2014, 31, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Gururangan, K. Diagnostic Utility of Reduced Electroencephalography. J. Clin. Neurophysiol. 2018, 35, 356. [Google Scholar] [CrossRef] [PubMed]
- Herta, J.; Koren, J.; Fürbass, F.; Hartmann, M.; Gruber, A.; Baumgartner, C. Reduced Electrode Arrays for the Automated Detection of Rhythmic and Periodic Patterns in the Intensive Care Unit: Frequently Tried, Frequently Failed? Clin. Neurophysiol. 2017, 128, 1524–1531. [Google Scholar] [CrossRef]
- Westover, M.B.; Gururangan, K.; Markert, M.S.; Blond, B.N.; Lai, S.; Benard, S.; Bickel, S.; Hirsch, L.J.; Parvizi, J. Diagnostic Value of Electroencephalography with Ten Electrodes in Critically Ill Patients. Neurocrit. Care 2020, 33, 479–490. [Google Scholar] [CrossRef]
- Tjepkema-Cloostermans, M.C.; Hofmeijer, J.; Hom, H.W.; Bosch, F.H.; Van Putten, M.J.A.M. Predicting Outcome in Post-anoxic Coma: Are Ten EEG Electrodes Enough? J. Clin. Neurophysiol. 2017, 34, 207–212. [Google Scholar] [CrossRef]
- Gururangan, K.; Razavi, B.; Parvizi, J. Diagnostic Utility of Eight-Channel EEG for Detecting Generalized or Hemispheric Seizures and Rhythmic Periodic Patterns. Clin. Neurophysiol. Pract. 2018, 3, 65–73. [Google Scholar] [CrossRef]
- LaMonte, M.P. Ceribell EEG Shortens Seizure Diagnosis and Workforce Time and Is Useful for COVID Isolation. Epilepsia Open 2021, 6, 331–338. [Google Scholar] [CrossRef]
- Hanley, D.F.; Chabot, R.; Mould, W.A.; Morgan, T.; Naunheim, R.; Sheth, K.N.; Chiang, W.; Prichep, L.S. Use of Brain Electrical Activity for the Identification of Hematomas in Mild Traumatic Brain Injury. J. Neurotrauma 2013, 30, 2051–2056. [Google Scholar] [CrossRef]
- Rittenberger, J.C.; Weissman, A.; Baldwin, M.; Flickinger, K.; Repine, M.J.; Guyette, F.X.; Doshi, A.A.; Dezfulian, C.; Callaway, C.W.; Elmer, J. Preliminary Experience with Point-of-Care EEG in Post-Cardiac Arrest Patients. Resuscitation 2019, 135, 98–102. [Google Scholar] [CrossRef]
- Mahmood, S.; El-Menyar, A.; Shabana, A.; Mahmood, I.; Asim, M.; Abdelrahman, H.; Al-Thani, H. Bispectral Index as a Predictor of Unsalvageable Traumatic Brain Injury. Brain Inj. 2017, 31, 1382–1386. [Google Scholar] [CrossRef]
- Parvizi, J.; Gururangan, K.; Razavi, B.; Chafe, C. Detecting Silent Seizures by Their Sound. Epilepsia 2018, 59, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Yazbeck, M.; Sra, P.; Parvizi, J. Rapid Response Electroencephalography for Urgent Evaluation of Patients in Community Hospital Intensive Care Practice. J. Neurosci. Nurs. 2019, 51, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Kamousi, B.; Karunakaran, S.; Gururangan, K.; Markert, M.; Decker, B.; Khankhanian, P.; Mainardi, L.; Quinn, J.; Woo, R.; Parvizi, J. Monitoring the Burden of Seizures and Highly Epileptiform Patterns in Critical Care with a Novel Machine Learning Method. Neurocrit. Care 2021, 34, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Dorriz, P.; Gururangan, K.; Kozak, R.; Kaplan, M. 583: Real-world performance of a seizure burden algorithm (clarity) in a community hospital. Crit. Care Med. 2024, 52, S265. [Google Scholar] [CrossRef]
- Kamousi, B.; Gupta, A.; Karunakaran, S.; Marjaninejad, A.; Woo, R.; Parvizi, J. 580: Improvements in a machine-learning algorithm for detecting status epilepticus. Crit. Care Med. 2024, 52, S263. [Google Scholar] [CrossRef]
- Ward, J.; Green, A.; Cole, R.; Zarbiv, S.; Dumond, S.; Clough, J.; Rincon, F. Implementation and Impact of a Point of Care Electroencephalography Platform in a Community Hospital: A Cohort Study. Front. Digit. Health 2023, 5, 1035442. [Google Scholar] [CrossRef]
- Hobbs, K.; Krishnamohan, P.; Legault, C.; Goodman, S.; Parvizi, J.; Gururangan, K.; Mlynash, M. Rapid Bedside Evaluation of Seizures in the ICU by Listening to the Sound of Brainwaves: A Prospective Observational Clinical Trial of Ceribell’s Brain Stethoscope Function. Neurocrit. Care 2018, 29, 302–312. [Google Scholar] [CrossRef]
- Rossini, P.M.; Cole, J.; Paulus, W.; Ziemann, U.; Chen, R. 1924–2024: First Centennial of EEG. Clin. Neurophysiol. 2025, 170, 132–135. [Google Scholar] [CrossRef]
- Faul, M.; Wald, M.M.; Xu, L.; Coronado, V.G. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations, and Deaths, 2002–2006; CDC: Centers for Disease Control and Prevention: Atlanta, GA, USA, 2010. [Google Scholar]
- Sariaslan, A.; Sharp, D.J.; D’Onofrio, B.M.; Larsson, H.; Fazel, S. Long-Term Outcomes Associated with Traumatic Brain Injury in Childhood and Adolescence: A Nationwide Swedish Cohort Study of a Wide Range of Medical and Social Outcomes. PLoS Med. 2016, 13, e1002103. [Google Scholar] [CrossRef]
- Elbin, R.J.; Sufrinko, A.; Schatz, P.; French, J.; Henry, L.; Burkhart, S.; Collins, M.W.; Kontos, A.P. Removal from Play After Concussion and Recovery Time. Pediatrics 2016, 138, e20160910. [Google Scholar] [CrossRef]
- Guskiewicz, K.M.; McCrea, M.; Marshall, S.W.; Cantu, R.C.; Randolph, C.; Barr, W.; Onate, J.A.; Kelly, J.P. Cumulative Effects Associated with Recurrent Concussion in Collegiate Football Players: The NCAA Concussion Study. JAMA 2003, 290, 2549–2555. [Google Scholar] [CrossRef] [PubMed]
- Anderson, V.; Godfrey, C.; Rosenfeld, J.V.; Catroppa, C. 10 Years Outcome from Childhood Traumatic Brain Injury. Int. J. Dev. Neurosci. 2012, 30, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Scholten, A.C.; Haagsma, J.A.; Cnossen, M.C.; Olff, M.; van Beeck, E.F.; Polinder, S. Prevalence of and Risk Factors for Anxiety and Depressive Disorders after Traumatic Brain Injury: A Systematic Review. J. Neurotrauma 2016, 33, 1969–1994. [Google Scholar] [CrossRef] [PubMed]
- Cole, W.R.; Arrieux, J.P.; Schwab, K.; Ivins, B.J.; Qashu, F.M.; Lewis, S.C. Test–Retest Reliability of Four Computerized Neurocognitive Assessment Tools in an Active Duty Military Population. Arch. Clin. Neuropsychol. 2013, 28, 732–742. [Google Scholar] [CrossRef]
- Tebano, M.T.; Cameroni, M.; Gallozzi, G.; Loizzo, A.; Palazzino, G.; Pezzini, G.; Ricci, G.F. EEG Spectral Analysis after Minor Head Injury in Man. Electroencephalogr. Clin. Neurophysiol. 1988, 70, 185–189. [Google Scholar] [CrossRef]
- Huff, J.S.; Naunheim, R.; Ghosh Dastidar, S.; Bazarian, J.; Michelson, E.A. Referrals for CT Scans in Mild TBI Patients Can Be Aided by the Use of a Brain Electrical Activity Biomarker. Am. J. Emerg. Med. 2017, 35, 1777–1779. [Google Scholar] [CrossRef]
- Hanley, D.; Prichep, L.S.; Badjatia, N.; Bazarian, J.; Chiacchierini, R.; Curley, K.C.; Garrett, J.; Jones, E.; Naunheim, R.; O’Neil, B.; et al. A Brain Electrical Activity Electroencephalographic-Based Biomarker of Functional Impairment in Traumatic Brain Injury: A Multi-Site Validation Trial. J. Neurotrauma 2018, 35, 41–47. [Google Scholar] [CrossRef]
- Hanley, D.; Prichep, L.S.; Bazarian, J.; Huff, J.S.; Naunheim, R.; Garrett, J.; Jones, E.B.; Wright, D.W.; O’Neill, J.; Badjatia, N.; et al. Emergency Department Triage of Traumatic Head Injury Using a Brain Electrical Activity Biomarker: A Multisite Prospective Observational Validation Trial. Acad. Emerg. Med. 2017, 24, 617–627. [Google Scholar] [CrossRef]
- Prichep, L.S.; Jacquin, A.; Filipenko, J.; Dastidar, S.G.; Zabele, S.; Vodencarevic, A.; Rothman, N.S. Classification of Traumatic Brain Injury Severity Using Informed Data Reduction in a Series of Binary Classifier Algorithms. IEEE Trans. Neural Syst. Rehabil. Eng. 2012, 20, 806–822. [Google Scholar] [CrossRef]
- Rapp, P.E.; Keyser, D.O.; Albano, A.; Hernandez, R.; Gibson, D.B.; Zambon, R.A.; Hairston, W.D.; Hughes, J.D.; Krystal, A.; Nichols, A.S. Traumatic Brain Injury Detection Using Electrophysiological Methods. Front. Hum. Neurosci. 2015, 9, 11. [Google Scholar] [CrossRef]
- Thatcher, R.W.; North, D.M.; Curtin, R.T.; Walker, R.A.; Biver, C.J.; Gomez, J.F.; Salazar, A.M. An EEG Severity Index of Traumatic Brain Injury. J. Neuropsychiatry Clin. Neurosci. 2001, 13, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Thatcher, R.W.; Biver, C.; McAlaster, R.; Camacho, M.; Salazar, A. Biophysical Linkage between MRI and EEG Amplitude in Closed Head Injury. NeuroImage 1998, 7, 352–367. [Google Scholar] [CrossRef] [PubMed]
- Wilde, E.A.; Goodrich-Hunsaker, N.J.; Ware, A.L.; Taylor, B.A.; Biekman, B.D.; Hunter, J.V.; Newman-Norlund, R.; Scarneo, S.; Casa, D.J.; Levin, H.S. Diffusion Tensor Imaging Indicators of White Matter Injury Are Correlated with a Multimodal Electroencephalography-Based Biomarker in Slow Recovering, Concussed Collegiate Athletes. J. Neurotrauma 2020, 37, 2093–2101. [Google Scholar] [CrossRef]
- Brooks, M.A.; Bazarian, J.J.; Prichep, L.S.; Dastidar, S.G.; Talavage, T.M.; Barr, W. The Use of an Electrophysiological Brain Function Index in the Evaluation of Concussed Athletes. J. Head Trauma Rehabil. 2018, 33, 1–6. [Google Scholar] [CrossRef]
- Prichep, L.S.; McCrea, M.; Barr, W.; Powell, M.; Chabot, R.J. Time Course of Clinical and Electrophysiological Recovery After Sport-Related Concussion. J. Head Trauma Rehabil. 2013, 28, 266–273. [Google Scholar]
- O’Neil, B.; Prichep, L.S.; Naunheim, R.; Chabot, R. Quantitative Brain Electrical Activity in the Initial Screening of Mild Traumatic Brain Injuries. West. J. Emerg. Med. 2012, 13, 394. [Google Scholar]
- Mulder, M.J.H.L.; Jansen, I.G.H.; Goldhoorn, R.-J.B.; Venema, E.; Chalos, V.; Compagne, K.C.J.; Roozenbeek, B.; Lingsma, H.F.; Schonewille, W.J.; van den Wijngaard, I.R.; et al. Time to Endovascular Treatment and Outcome in Acute Ischemic Stroke: MR CLEAN Registry Results. Circulation 2018, 138, 232–240. [Google Scholar] [CrossRef]
- Menon, B.K.; Sajobi, T.T.; Zhang, Y.; Rempel, J.L.; Shuaib, A.; Thornton, J.; Williams, D.; Roy, D.; Poppe, A.Y.; Jovin, T.G.; et al. Analysis of Workflow and Time to Treatment on Thrombectomy Outcome in the Endovascular Treatment for Small Core and Proximal Occlusion Ischemic Stroke (ESCAPE) Randomized, Controlled Trial. Circulation 2016, 133, 2279–2286. [Google Scholar] [CrossRef]
- Saver, J.L.; Goyal, M.; van der Lugt, A.; Menon, B.K.; Majoie, C.B.L.M.; Dippel, D.W.; Campbell, B.C.; Nogueira, R.G.; Demchuk, A.M.; Tomasello, A.; et al. Time to Treatment with Endovascular Thrombectomy and Outcomes from Ischemic Stroke: A Meta-Analysis. JAMA 2016, 316, 1279–1289. [Google Scholar] [CrossRef]
- Turc, G.; Maïer, B.; Naggara, O.; Seners, P.; Isabel, C.; Tisserand, M.; Raynouard, I.; Edjlali, M.; Calvet, D.; Baron, J.C.; et al. Clinical Scales Do Not Reliably Identify Acute Ischemic Stroke Patients with Large-Artery Occlusion. Stroke 2016, 47, 1466–1472. [Google Scholar] [CrossRef]
- Anadani, M.; Almallouhi, E.; Wahlquist, A.E.; Debenham, E.; Holmstedt, C.A. The Accuracy of Large Vessel Occlusion Recognition Scales in Telestroke Setting. Telemed. J. E-Health 2019, 25, 1071. [Google Scholar] [CrossRef] [PubMed]
- Antipova, D.; Eadie, L.; Macaden, A.; Wilson, P. Diagnostic Accuracy of Clinical Tools for Assessment of Acute Stroke: A Systematic Review. BMC Emerg. Med. 2019, 19, 49. [Google Scholar]
- Sharbrough, F.W.; Messick, J.M.; Sundt, T.M. Correlation of Continuous Electroencephalograms with Cerebral Blood Flow Measurements During Carotid Endarterectomy. Stroke 1973, 4, 674–683. [Google Scholar] [CrossRef] [PubMed]
- van Putten, M.J.A.M.; Hofmeijer, J. EEG Monitoring in Cerebral Ischemia: Basic Concepts and Clinical Applications. J. Clin. Neurophysiol. 2016, 33, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Srinivasan, R.; Burke Quinlan, E.; Solodkin, A.; Small, S.L.; Cramer, S.C. Utility of EEG Measures of Brain Function in Patients with Acute Stroke. J. Neurophysiol. 2016, 115, 2399–2405. [Google Scholar] [CrossRef]
- Shreve, L.; Kaur, A.; Vo, C.; Wu, J.; Cassidy, J.M.; Nguyen, A.; Zhou, R.J.; Tran, T.B.; Yang, D.Z.; Medizade, A.I.; et al. Electroencephalography Measures Are Useful for Identifying Large Acute Ischemic Stroke in the Emergency Department. J. Stroke Cerebrovasc. Dis. 2019, 28, 2280–2286. [Google Scholar] [CrossRef]
- Erani, F.; Zolotova, N.; Vanderschelden, B.; Khoshab, N.; Sarian, H.; Nazarzai, L.; Wu, J.; Chakravarthy, B.; Hoonpongsim-anont, W.; Yu, W.; et al. Electroencephalography Might Improve Diagnosis of Acute Stroke and Large Vessel Occlusion. Stroke 2020, 51, 3361–3365. [Google Scholar] [CrossRef]
- Finnigan, S.; Wong, A.; Read, S. Defining Abnormal Slow EEG Activity in Acute Ischaemic Stroke: Delta/Alpha Ratio as an Optimal QEEG Index. Clin. Neurophysiol. 2016, 127, 1452–1459. [Google Scholar] [CrossRef]
- Foreman, B.; Claassen, J. Quantitative EEG for the Detection of Brain Ischemia. Crit. Care 2012, 16, 216. [Google Scholar] [CrossRef]
- Finnigan, S.; Van Putten, M.J.A.M. EEG in Ischaemic Stroke: Quantitative EEG Can Uniquely Inform (Sub-)Acute Prognoses and Clinical Management. Clin. Neurophysiol. 2013, 124, 10–19. [Google Scholar] [CrossRef]
- van Putten, M.J.A.M.; Tavy, D.L.J. Continuous Quantitative EEG Monitoring in Hemispheric Stroke Patients Using the Brain Symmetry Index. Stroke 2004, 35, 2489–2492. [Google Scholar] [CrossRef] [PubMed]
- Van Kaam, R.C.; van Putten, M.J.A.M.; Vermeer, S.E.; Hofmeijer, J. Contralesional Brain Activity in Acute Ischemic Stroke. Cerebrovasc. Dis. 2018, 45, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Gottlibe, M.; Rosen, O.; Weller, B.; Mahagney, A.; Omar, N.; Khuri, A.; Srugo, I.; Genizi, J. Stroke Identification Using a Portable EEG Device—A Pilot Study. Neurophysiol. Clin. 2020, 50, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Van Meenen, L.C.C.; Van Stigt, M.N.; Marquering, H.A.; Majoie, C.B.L.M.; Roos, Y.B.W.E.M.; Koelman, J.H.T.M.; Potters, W.V.; Coutinho, J.M. Detection of Large Vessel Occlusion Stroke with Electroencephalography in the Emergency Room: First Results of the ELECTRA-STROKE Study. J. Neurol. 2022, 269, 2030–2038. [Google Scholar] [CrossRef]
- Shahrestani, S.; Wishart, D.; Han, S.M.J.; Strickland, B.A.; Bakhsheshian, J.; Mack, W.J.; Toga, A.W.; Sanossian, N.; Tai, Y.-C.; Zada, G. A Systematic Review of Next-Generation Point-of-Care Stroke Diagnostic Technologies. Neurosurg. Focus 2021, 51, E11. [Google Scholar] [CrossRef]
- Michelson, E.A.; Hanley, D.; Chabot, R.; Prichep, L.S. Identification of Acute Stroke Using Quantified Brain Electrical Activity. Acad. Emerg. Med. 2015, 22, 67–72. [Google Scholar] [CrossRef]
- Wilkinson, C.M.; Burrell, J.I.; Kuziek, J.W.P.; Thirunavukkarasu, S.; Buck, B.H.; Mathewson, K.E. Predicting Stroke Severity with a 3-Min Recording from the Muse Portable EEG System for Rapid Diagnosis of Stroke. Sci. Rep. 2020, 10, 18465. [Google Scholar] [CrossRef]
- Van Stigt, M.N.; Groenendijk, E.A.; Van Meenen, L.C.C.; Van De Munckhof, A.A.G.A.; Theunissen, M.; Franschman, G.; Smeekes, M.D.; Van Grondelle, J.A.F.; Geuzebroek, G.; Siegers, A.; et al. Prehospital Detection of Large Vessel Occlusion Stroke with EEG: Results of the ELECTRA-STROKE Study. Neurology 2023, 101, e2522–e2532. [Google Scholar] [CrossRef]
- Inouye, S.K.; Westendorp, R.G.J.; Saczynski, J.S. Delirium in Elderly People. Lancet 2014, 383, 911–922. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, Y.; Jin, J. Poor Outcomes of Delirium in the Intensive Care Units Are Amplified by Increasing Age: A Retrospective Cohort Study. World J. Emerg. Med. 2021, 12, 117. [Google Scholar] [CrossRef]
- Khan, B.A.; Perkins, A.J.; Prasad, N.K.; Shekhar, A.; Campbell, N.L.; Gao, S.; Wang, S.; Khan, S.H.; Marcantonio, E.R.; Twigg, H.L., III; et al. Biomarkers of Delirium Duration and Delirium Severity in the ICU. Crit. Care Med. 2020, 48, 353. [Google Scholar] [CrossRef] [PubMed]
- Andrews, P.S.; Wang, S.; Perkins, A.J.; Gao, S.; Khan, S.; Lindroth, H.; Boustani, M.; Khan, B. Relationship Between Intensive Care Unit Delirium Severity and 2-Year Mortality and Health Care Utilization. Am. J. Crit. Care 2020, 29, 311–317. [Google Scholar] [CrossRef] [PubMed]
- DiLibero, J.; O’Donoghue, S.C.; DeSanto-Madeya, S.; Felix, J.; Ninobla, A.; Woods, A. An Innovative Approach to Improving the Accuracy of Delirium Assessments Using the Confusion Assessment Method for the Intensive Care Unit. Dimens. Crit. Care Nurs. 2016, 35, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, A.; Kobayashi, S.; Matsui, K.; Akaho, R.; Nishimura, K. The Accuracy of Delirium Assessment by Cardiologists Treating Heart Failure Inpatients: A Single Center Retrospective Survey. Biopsychosoc. Med. 2020, 14, 15. [Google Scholar] [CrossRef]
- Mulkey, M.; Albanese, T.; Kim, S.; Huang, H.; Yang, B. Delirium Detection Using GAMMA Wave and Machine Learning: A Pilot Study. Res. Nurs. Health 2022, 45, 652–663. [Google Scholar] [CrossRef]
- Tanabe, S.; Mohanty, R.; Lindroth, H.; Casey, C.; Ballweg, T.; Farahbakhsh, Z.; Krause, B.; Prabhakaran, V.; Banks, M.I.; Sanders, R.D. Cohort Study into the Neural Correlates of Postoperative Delirium: The Role of Connectivity and Slow-Wave Activity. Br. J. Anaesth. 2020, 125, 55–66. [Google Scholar] [CrossRef]
- Shinozaki, G.; Bormann, N.L.; Chan, A.C.; Zarei, K.; Sparr, N.A.; Klisares, M.J.; Jellison, S.S.; Heinzman, J.T.; Dahlstrom, E.B.; Duncan, G.N.; et al. Identification of Patients with High Mortality Risk and Prediction of Outcomes in Delirium by Bispectral EEG. J. Clin. Psychiatry 2019, 80, 19m12749. [Google Scholar] [CrossRef]
- Lee, S.; Yuki, K.; Chan, A.; Cromwell, J.; Shinozaki, G. The Point-of-Care EEG for Delirium Detection in the Emergency Department. Am. J. Emerg. Med. 2019, 37, 995–996. [Google Scholar] [CrossRef]
- Shinozaki, G.; Chan, A.C.; Sparr, N.A.; Zarei, K.; Gaul, L.N.; Heinzman, J.T.; Robles, J.; Yuki, K.; Chronis, T.J.; Ando, T.; et al. Delirium Detection by a Novel Bispectral Electroencephalography Device in General Hospital. Psychiatry Clin. Neurosci. 2018, 72, 856–863. [Google Scholar] [CrossRef]
- Ferrari, R. Writing Narrative Style Literature Reviews. Med. Writ. 2015, 24, 230–235. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Brenner, J.M.; Kent, P.; Wojcik, S.M.; Grant, W. Rapid Diagnosis of Nonconvulsive Status Epilepticus Using Reduced-Lead Electroencephalography. West J. Emerg. Med. 2015, 16, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Muraja-Murro, A.; Mervaala, E.; Westeren-Punnonen, S.; Lepola, P.; Töyräs, J.; Myllymaa, S.; Julkunen, P.; Kantanen, A.-M.; Kälviäinen, R.; Myllymaa, K. Forehead EEG Electrode Set versus Full-Head Scalp EEG in 100 Patients with Altered Mental State. Epilepsy Behav. 2015, 49, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Egawa, S.; Hifumi, T.; Nakamoto, H.; Kuroda, Y.; Kubota, Y. Diagnostic Reliability of Headset-Type Continuous Video EEG Monitoring for Detection of ICU Patterns and NCSE in Patients with Altered Mental Status with Unknown Etiology. Neurocrit. Care 2020, 32, 217–225. [Google Scholar] [CrossRef]
- Caricato, A.; Della Marca, G.; Ioannoni, E.; Silva, S.; Benzi Markushi, T.; Stival, E.; Biasucci, D.G.; Montano, N.; Gelormini, C.; Melchionda, I. Continuous EEG Monitoring by a New Simplified Wireless Headset in Intensive Care Unit. BMC Anesthesiol. 2020, 20, 298. [Google Scholar] [CrossRef]
- Meyer, M.; Fuest, S.; Krain, D.; Juenemann, M.; Braun, T.; Thal, S.C.; Schramm, P. Evaluation of a New Wireless Technique for Continuous Electroencephalography Monitoring in Neurological Intensive Care Patients. J. Clin. Monit. Comput. 2021, 35, 765–770. [Google Scholar] [CrossRef]
- Welte, T.M.; Janner, F.; Lindner, S.; Gollwitzer, S.; Stritzelberger, J.; Lang, J.D.; Reindl, C.; Sprügel, M.I.; Olmes, D.; Schwab, S.; et al. Evaluation of Simplified Wireless EEG Recordings in the Neurological Emergency Room. PLoS ONE 2024, 19, e0310223. [Google Scholar] [CrossRef]
- Kamousi, B.; Grant, A.M.; Bachelder, B.; Yi, J.; Hajinoroozi, M.; Woo, R. Comparing the Quality of Signals Recorded with a Rapid Response EEG and Conventional Clinical EEG Systems. Clin. Neurophysiol. Pract. 2019, 4, 69–75. [Google Scholar] [CrossRef]
- Chen, W.; Toprani, S.; Werbaneth, K.; Falco-Walter, J. Status Epilepticus and Other EEG Findings in Patients with COVID-19: A Case Series. Seizure 2020, 81, 198–200. [Google Scholar] [CrossRef]
- Kalkach-Aparicio, M.; Fatima, S.; Selte, A.; Sheikh, I.S.; Cormier, J.; Gallagher, K.; Avagyan, G.; Cespedes, J.; Krishnamurthy, P.V.; Elazim, A.A.; et al. Seizure Assessment and Forecasting with Efficient Rapid-EEG. Neurology 2024, 103, e209621. [Google Scholar] [CrossRef] [PubMed]
- Kurup, D.; Gururangan, K.; Desai, M.J.; Markert, M.S.; Eliashiv, D.S.; Vespa, P.M.; Parvizi, J. Comparing Seizures Captured by Rapid Response EEG and Conventional EEG Recordings in a Multicenter Clinical Study. Front. Neurol. 2022, 13, 915385. [Google Scholar] [CrossRef]
- Villamar, M.F.; Ayub, N.; Koenig, S.J. Automated Seizure Detection in Patients with Cardiac Arrest: A Retrospective Review of Ceribell™ Rapid-EEG Recordings. Neurocrit. Care 2023, 39, 505–513. [Google Scholar] [CrossRef]
- Desai, M.; Kalkach-Aparicio, M.; Sheikh, I.S.; Cormier, J.; Gallagher, K.; Hussein, O.M.; Cespedes, J.; Hirsch, L.J.; Westover, B.; Struck, A.F. Evaluating the Impact of Point-of-Care Electroencephalography on Length of Stay in the Intensive Care Unit: Subanalysis of the SAFER-EEG Trial. Neurocrit. Care 2024, 42, 108–117. [Google Scholar] [CrossRef]
- Gururangan, K.; Kozak, R.; Dorriz, P.J. Time Is Brain: Detection of Nonconvulsive Seizures and Status Epilepticus during Acute Stroke Evaluation Using Point-of-Care Electroencephalography. J. Stroke Cerebrovasc. Dis. 2025, 34, 108116. [Google Scholar] [CrossRef]
- Sheikh, Z.B.; Dhakar, M.B.; Fong, M.W.K.; Fang, W.; Ayub, N.; Molino, J.; Haider, H.A.; Foreman, B.; Gilmore, E.; Mizrahi, M.; et al. Accuracy of a Rapid-Response EEG’s Automated Seizure-Burden Estimator. Neurology 2025, 104, e210234. [Google Scholar] [CrossRef]
- Naunheim, R.S.; Treaster, M.; English, J.; Casner, T. Automated Electroencephalogram Identifies Abnormalities in the ED. Am. J. Emerg. Med. 2011, 29, 845–848. [Google Scholar] [CrossRef]
- Naunheim, R.S.; Treaster, M.; English, J.; Casner, T.; Chabot, R. Use of Brain Electrical Activity to Quantify Traumatic Brain Injury in the Emergency Department. Brain Inj. 2010, 24, 1324–1329. [Google Scholar] [CrossRef]
- McCrea, M.; Prichep, L.; Powell, M.R.; Chabot, R.; Barr, W.B. Acute Effects and Recovery After Sport-Related Concussion: A Neurocognitive and Quantitative Brain Electrical Activity Study. J. Head Trauma Rehabil. 2010, 25, 283–292. [Google Scholar]
- Barr, W.B.; Prichep, L.S.; Chabot, R.; Powell, M.R.; McCrea, M. Measuring Brain Electrical Activity to Track Recovery from Sport-Related Concussion. Brain Inj. 2012, 26, 58–66. [Google Scholar] [CrossRef]
- Prichep, L.S.; Ghosh-Dastidar, S.; Jacquin, A.; Koppes, W.; Miller, J.; Radman, T.; O’Neil, B.; Naunheim, R.; Huff, J.S. Classification Algorithms for the Identification of Structural Injury in TBI Using Brain Electrical Activity. Comput. Biol. Med. 2014, 53, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Prichep, L.S.; Naunheim, R.; Bazarian, J.; Mould, W.A.; Hanley, D. Identification of Hematomas in Mild Traumatic Brain Injury Using an Index of Quantitative Brain Electrical Activity. J. Neurotrauma 2015, 32, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, S.I.; Thomas, C.; Kulek, A.; Tolomello, R.; Mika, V.; Robinson, D.; Medado, P.; Pearson, C.; Prichep, L.S.; O’Neil, B.J. Comparison of Quantitative EEG to Current Clinical Decision Rules for Head CT Use in Acute Mild Traumatic Brain Injury in the ED. Am. J. Emerg. Med. 2015, 33, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Naunheim, R.; Covassin, T.; Jacquin, A.; Hanley, D.; Michelson, E. Using a Brain Electrical Activity Biomarker Could Aid in the Objective Identification of Mild Traumatic Brain Injury Patients. Am. J. Emerg. Med. 2018, 36, 142–143. [Google Scholar] [CrossRef]
- Vincent, A.S.; Bailey, C.M.; Cowan, C.; Cox-Fuenzalida, E.; Dyche, J.; Gorgens, K.A.; Krawczyk, D.C.; Young, L. Normative Data for Evaluating Mild Traumatic Brain Injury with a Handheld Neurocognitive Assessment Tool. Appl. Neuropsychol. Adult 2017, 24, 566–576. [Google Scholar] [CrossRef]
- Jacquin, A.; Kanakia, S.; Oberly, D.; Prichep, L.S. A Multimodal Biomarker for Concussion Identification, Prognosis and Management. Comput. Biol. Med. 2018, 102, 95–103. [Google Scholar] [CrossRef]
- Naunheim, R.; Konstantinovic Koscso, M.; Poirier, R. Reduction in Unnecessary CT Scans for Head-Injury in the Emergency Department Using an FDA Cleared Device. Am. J. Emerg. Med. 2019, 37, 1987–1988. [Google Scholar] [CrossRef]
- Michelson, E.; Huff, J.S.; Garrett, J.; Naunheim, R. Triage of Mild Head-Injured Intoxicated Patients Could Be Aided by Use of an Electroencephalogram-Based Biomarker. J. Neurosci. Nurs. 2019, 51, 62–66. [Google Scholar] [CrossRef]
- Covassin, T.; McGowan, A.L.; Bretzin, A.C.; Anderson, M.; Petit, K.M.; Savage, J.L.; Katie, S.L.; Elbin, R.J.; Pontifex, M.B. Preliminary Investigation of a Multimodal Enhanced Brain Function Index among High School and Collegiate Concussed Male and Female Athletes. Physician Sportsmed. 2020, 48, 442–449. [Google Scholar] [CrossRef]
- Bazarian, J.J.; Prichep, L.S. Validation of a Multimodal EEG-Based Index to Aid in Diagnosing and Tracking Concussion Among Athletes. Neurology 2022, 98, S20–S21. [Google Scholar] [CrossRef]
- Armañanzas, R.; Liang, B.; Kanakia, S.; Bazarian, J.J.; Prichep, L.S. Identification of Concussion Subtypes Based on Intrinsic Brain Activity. JAMA Netw. Open 2024, 7, e2355910. [Google Scholar] [CrossRef] [PubMed]
- Koreerat, N.R.; Giese, R. An Observation of Application of Structural Brain Injury Devices for Traumatic Brain Injury Evaluation in Austere Military Prehospital Settings. Mil. Med. 2021, 186, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Luster, J.D.; Hoffman, W.R.; Jordan, M.; Cacic, K.; Tchopev, Z.N.; Anderson, J.; Gissendanner, W.; Miranda, E.; Yuan, T.; Willis, A. Feasibility Assessment of Rapid Response EEG in the Identification of Nonconvulsive Seizures During Military Medical Air Transport. Mil. Med. 2024, 190, e479–e483. [Google Scholar] [CrossRef] [PubMed]
- Barton, D.J.; Coppler, P.J.; Talia, N.N.; Charalambides, A.; Stancil, B.; Puccio, A.M.; Okonkwo, D.O.; Callaway, C.W.; Guyette, F.X.; Elmer, J. Prehospital Electroencephalography to Detect Traumatic Brain Injury during Helicopter Transport: A Pilot Observational Cohort Study. Prehosp. Emerg. Care 2024, 28, 405–412. [Google Scholar] [CrossRef]
- Numan, T.; Van den Boogaard, M.; Kamper, A.M.; Rood, P.J.; Peelen, L.M.; Slooter, A.J.; Abawi, M.; van den Boogaard, M.; Claassen, J.A.; Coesmans, M.; et al. Delirium Detection Using Relative Delta Power Based on 1-Minute Single-Channel EEG: A Multicentre Study. Br. J. Anaesth. 2019, 122, 60–68. [Google Scholar] [CrossRef]
- Wijnen, V.J.M.; Oudewortel, L.; Van Luijtelaar, G.; Witlox, J.; Slooter, A.J.C.; Van Gool, W.A. Feasibility and Potential of a Bedside Mini-EEG for Diagnosing Delirium Superimposed on Dementia. Clin. Neurophysiol. 2022, 142, 181–189. [Google Scholar] [CrossRef]
- Mulkey, M.A.; Huang, H.; Albanese, T.; Kim, S.; Yang, B. Supervised Deep Learning with Vision Transformer Predicts Delirium Using Limited Lead EEG. Sci. Rep. 2023, 13, 7890. [Google Scholar] [CrossRef]
| Study Design | Feasibility | Diagnostic Performance | Clinical Implications and Cost-Effectiveness | |
|---|---|---|---|---|
| 1. | First author: Brenner [94], 2015, USA Sample size: 12 adult patients (median age: 51.5) Conditions: AMS with seizure history or witnessed seizure Setting: ED EEG System: Portable Brainmaster EEG device Comparison: conv-EEG (reference standard) EEG Interpretation: neurophysiologist (POC-EEG); neurologist (conv-EEG) | POC-EEG:
| Agreement between POC-EEG and conv-EEG:
| POC-EEG device cost: ~USD 2500 conv-EEG cost: ~USD 50,000 |
| 2. | First author: Muraja-Murro [95], 2015, Finland Sample size: 100 patients (18–90 aged) Conditions: unexplained AMS from various etiologies Setting: ED EEG System: Grass Technologies Comparison: fm-EEG (reference standard) EEG Interpretation: Three expert neurophysiologists, blinded to EEG type | POC-EEG:
| POC-EEG performance:
| |
| 3. | First author: Rittenberger [29], 2019, USA Sample size: 95 patients (mean age: 59) Condition: PCA Setting: Tertiary care cardiac arrest hospital EEG system: Cadwell 6-electrode POC-EEG EEG Interpretation: Epileptologist and neurointensivist Comparison: First 30 min of c-conv-EEG, performed after POC-EEG EEG interpretation: Epileptologist and neurointensivist | POC-EEG:
| Agreement between POC-EEG and c-conv-EEG:
| Survival to hospital discharge:
|
| 4. | First author: Egawa [96], 2020, Japan Sample size: 50 patients (median age: 72) Conditions: AMS from various etiologies (subarachnoid hemorrhage, cerebral hemorrhage, post-cardiac arrest (PCA) syndrome, SE, TBI) Setting: neuro-ICU EEG system: AE-120A EEG headset Comparison: fm-cEEG (immediately subsequent) EEG Interpretation: One neurointensivist and one board-certified neurophysiologist | POC-EEG:
| POC-EEG findings:
| |
| 5. | First author: Caricato [97], 2020, Italy Sample size: 40 patients
EEG system: CerebAir headset (study group) Comparison: 8-electrode rm-EEG (control group) (continuous) EEG Interpretation: Expert neurologist | EEG Application:
| POC-EEG findings: EEG abnormalities classified as
| EEG-related ASM initiation:
|
| 6. | First author: Meyer [98], 2021, Germany Patients: 52 patients (mean age: 63 years) Conditions: AMS due to SE, ischemic stroke, intracranial bleeding, meningitis, encephalitis, metabolic encephalopathies Setting: neuro-ICU EEG system: CerebAir monitoring Comparison: Routine conv-EEG (delayed) EEG Interpretation: Resident physician, supervised by a board-certified senior physician | POC-EEG:
| Diagnostic Performance:
| |
| 7. | First author: Welte [99], 2024, Germany Sample size: 100 patients Setting: Neurological ED Conditions: AMS or suspected seizures EEG system: CerebAir (minimum 10 min) EEG Interpretation: Neurology resident, supervised by an EEG expert Comparison: conv-rEEG (55 patients) performed immediately hours to days after POC-EEG EEG Interpretation: Specialized neurology residents, supervised by senior board-certified EEG experts | POC-EEG:
| Agreement between swEEG and first rEEG results (55 patients):
| Potential therapeutic intervention:
|
| 8. | First author: Hobbs [37], 2018, USA Sample size: 34 patients (mean age: 61) Setting: ICU Condition: AMS (GCS <12) due to mixed etiologies (metabolic encephalopathy, AIS, intracerebral hemorrhage, TBI, and autoimmune encephalitis) and requiring EEG monitoring EEG system: Ceribell rr-EEG system EEG Interpretation: Sonified EEG by neurointensivists without epilepsy training Comparison: conv-EEG performed after POC-EEG; reference standard EEG Interpretation: Two epileptologists reviewed the entire Ceribell EEG recording and correlated findings with conv-EEG reports | POC-EEG:
| Diagnostic Performance:
| Sonification tool impact:
|
| 9. | First author: Parvizi [31], 2018, USA Sample size: 84 EEG samples selected from patients Condition: AMS EEG system: Ceribell (visual + sonification) EEG Interpretation:
| EEG sonification:
| Diagnostic Performance:
| |
| 10. | First author: Yazbeck [32], 2019, USA Sample size: 10 patients (mean age: 59.7 years) Setting: neuro-ICU Condition: AMS at risk for NCSE EEG system: Ceribell rr-EEG system sound application; interpreted on-site by treating physicians using real-time sonification and visual review on the rr-EEG device Comparison: conv-EEG performed after POC-EEG in six patients | POC-EEG:
| Concordance with conv-EEG:
| POC-EEG Impact on treatment decision:
|
| 11. | First author: Kamousi [100], 2019, USA Sample size:
Conditions: AMS (ICU study); healthy subject component (controlled laboratory setting) EEG system: Ceribell rr-EEG system Study design:
| Laboratory Study (Healthy Subject):
| ||
| 12. | First author: Chen [101], 2020, USA Sample size: 5 patients Setting: ICU Conditions: AMS or suspected seizures or SE in critically ill adult patients with confirmed COVID-19 infection EEG System: Ceribell rr-EEG Comparison: conv-EEG performed in 2 patients for extended monitoring |
| ||
| 13. | First author: LaMonte [27], 2021, USA Sample size:
EEG system:
| POC-EEG:
| POC-EEG Diagnostic Performance:
| POC-EEG implication:
|
| 14. | First author: Vespa [9], 2020, USA Sample size: 181 patients (mean age: 58.6) Setting: ICUs from five academic hospitals Condition: AMS suspected of NCS EEG system: Ceribell rr-EEG system (30 s sonification per hemisphere + 60 s visual EEG review; real-time interpretation: treating physician; remote neurologist review) Comparison: conv-EEG performed immediately after POC-EEG; reference standard | POC-EEG:
| POC-EEG diagnostic performance (vs. initial clinical suspicion):
| POC-EEG clinical impact (after vs. before):
|
| 15. | First author: Wright [7], 2021, USA Sample size: 38 patients Setting: ED, two hospital sites (Community hospital, Academic hospital) Condition: suspected NCSE due to various etiologies were identified (e.g., SE, stroke, TBI, toxic-metabolic encephalopathies, and idiopathic AMS) EEG system: Ceribell rr-EEG + Brain Stethoscope EEG Interpretation:
| POC-EEG:
| POC-EEG Diagnostic Performance:
| Overall impact of POC-EEG across both sites:
|
| 16. | First author: Kamousi [33], 2021, USA Sample size: 353 rr-EEG recordings Condition: Adults with AMS requiring rr-EEG monitoring for suspected seizures Settings: ICUs and EDs across six academic and community hospitals EEG system: Ceribell monitoring; Clarity machine learning algorithm Reference standard: Ceribell review by two independent neurologists | POC-EEG:
| POC-EEG Diagnostic Performance:
| Potential POC-EEG application:
|
| 17. | First author: Kalkach-Aparicio [102], 2024, USA Sample size: 240 patients (median age: 64) Conditions: Persistent altered AMS, clinical concern for NCS, patients at risk for SE Setting: University hospital EEG system: Ceribell rr-EEG; interpretation by EEG expert Comparison: conv-EEG performed after POC-EEG; interpretation by EEG expert | POC-EEG:
| Seizure detection using 2HELPS2B score on rr-EEG vs. cEEG:
| Seizure risk prediction:
|
| 18. | First author: Madill [8], 2022, USA Sample size: 74 patients (mean age: 61.7 years) Conditions: Clinical events concerning seizures (49%), PCA (24%), and unexplained encephalopathy (27%), Settings: ICU and ED, community hospital affiliated with a university hospital EEG system: Ceribell rr-EEG; interpretation by on-site neurology and remote epileptologist via tele-EEG Comparison: Historical practice before rr-EEG implementation | POC-EEG:
| POC-EEG Diagnostic Performance:
| Inter-hospital Transfers:
|
| 19. | First author: Kurup [103], 2022, USA Sample size: 19 patients Setting: ICU Condition: Suspected NCS or NCSE EEG system: Ceribell rr-EEG Comparison: conv-EEG; interpreted by experienced epileptologists | POC-EEG:
| EEG Findings:
| |
| 20. | First author: Eberhard [5], 2023, USA Sample size: 164 EEGs (35 conv-EEGs pre-QI; 115 rr-EEGs post-QI) Condition: Suspected seizures Setting: Community hospital EEG system: Ceribell rr-EEG, real-time sonification and cloud-based EEG interpretation: Remote review by on-call neurologist Comparison (Reference Standard): Historical control group (pre-QI) | POC-EEG:
| Seizure Detection Rates (diagnostic yield):
| Patients discharged:
|
| 21. | First author: Ward [36], 2023, USA Sample size: 88 patients (mean age: 57) Conditions: Concern for NCSE (19% exhibited hyperkinetic movements PCA, 46% had a history of seizures and 35% were unresponsive) Setting: ICU and ED at a community hospital EEG system:
| POC-EEG:
| POC-EEG Findings:
| Hospital transfer for emergent EEG:
Financial impact:
|
| 22. | First author: Villamar [104], 2023, USA Sample size: 21 patients (median age: 64) Condition: Comatose PCA patients Setting: ICU EEG system: Ceribell rr-EEG monitoring as part of routine clinical care; Clarity algorithm (version 4.0) for automated seizure detection EEG Interpretation: Board-certified epileptologist retrospective review Comparison:
| Raw POC-EEG review findings:
| ||
| 23. | First author: Kozak [6], 2023, USA Sample size: 157 adult patients (mean age: 57.7 years) Conditions: Clinical suspicion of seizures, unexplained encephalopathy, or PCA Setting: ED from a community hospital EEG system: Ceribell rr-EEG, Clarity, reviewed by intensivists and neurologists Comparison: conv-EEG performed after POC-EEG in 51.6% of cases; interpretation: EEG-trained neurologist (reference standard) | POC-EEG:
| POC-EEG Findings:
| Treatment changes based on POC-EEG findings: 59.2% of cases POC-EEG findings associated with ASM management changes (p < 0.001):
|
| 24. | First author: Kamousi [35], 2024, USA Sample size: 665 POC-EEG recordings Setting: 11 hospitals EEG system: Ceribell Clarity analysis (two versions tested) Reference standard: EEG reviewed post hoc by at least two blinded epileptologists | POC-EEG:
| Clarity Diagnostic Performance:
| |
| 25. | First author: Dorriz [34], 2024, USA Sample size: 317 POC-EEG recordings Setting: U.S. community hospital EEG system: Ceribell Clarity outputs (for SE detection); monitoring Reference standard: EEG-trained neurologist’s interpretation of POC-EEG recordings | POC-EEG:
| Clarity concordance with neurologist:
| |
| 26. | First author: Desai [105], 2024, USA Sample size: 283 patients
EEG system: Ceribell rr-EEG Comparison: At least 4 h conv-EEG | POC-EEG:
| POC-EEG impact on ICU stay:
| |
| 27. | First author: Gururangan [106], 2025, USA Sample size: 70 patients (mean age: 75.0 years) Conditions: 38 stroke patients (54.3%: 73.7% ischemic, 15.8% hemorrhagic, 10.5% TIA); 32 stroke mimics (45.7%: 46.9% seizures, 28.1% toxic-metabolic encephalopathy, 12.5% hypertensive encephalopathy) Setting: Tertiary care community hospital EEG system: Ceribell rr-EEG used during stroke codes Reference standard: Final stroke vs. stroke mimic diagnosis based on
| POC-EEG:
| POC-EEG findings:
| POC-EEG Seizure Detection in Stroke Codes:
|
| 28. | First author: Sheikh [107], 2025, USA Sample size: 235 rr-EEG Setting: Three hospitals Condition: Neurologic conditions with a high risk of seizures Setting: ICU or ED EEG system: ClarityPro (v 6.0) Setting: Three hospitals Reference standard: Expert neurophysiologist consensus review of EEGs | POC-EEG:
| Performance of Clarity at different SzB thresholds:
| Clarity application:
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fratangelo, R.; Lolli, F.; Scarpino, M.; Grippo, A. Point-of-Care Electroencephalography in Acute Neurological Care: A Narrative Review. Neurol. Int. 2025, 17, 48. https://doi.org/10.3390/neurolint17040048
Fratangelo R, Lolli F, Scarpino M, Grippo A. Point-of-Care Electroencephalography in Acute Neurological Care: A Narrative Review. Neurology International. 2025; 17(4):48. https://doi.org/10.3390/neurolint17040048
Chicago/Turabian StyleFratangelo, Roberto, Francesco Lolli, Maenia Scarpino, and Antonello Grippo. 2025. "Point-of-Care Electroencephalography in Acute Neurological Care: A Narrative Review" Neurology International 17, no. 4: 48. https://doi.org/10.3390/neurolint17040048
APA StyleFratangelo, R., Lolli, F., Scarpino, M., & Grippo, A. (2025). Point-of-Care Electroencephalography in Acute Neurological Care: A Narrative Review. Neurology International, 17(4), 48. https://doi.org/10.3390/neurolint17040048






