Pathophysiologic Mechanisms of Severe Spinal Cord Injury and Neuroplasticity Following Decompressive Laminectomy and Expansive Duraplasty: A Systematic Review
Abstract
1. Introduction
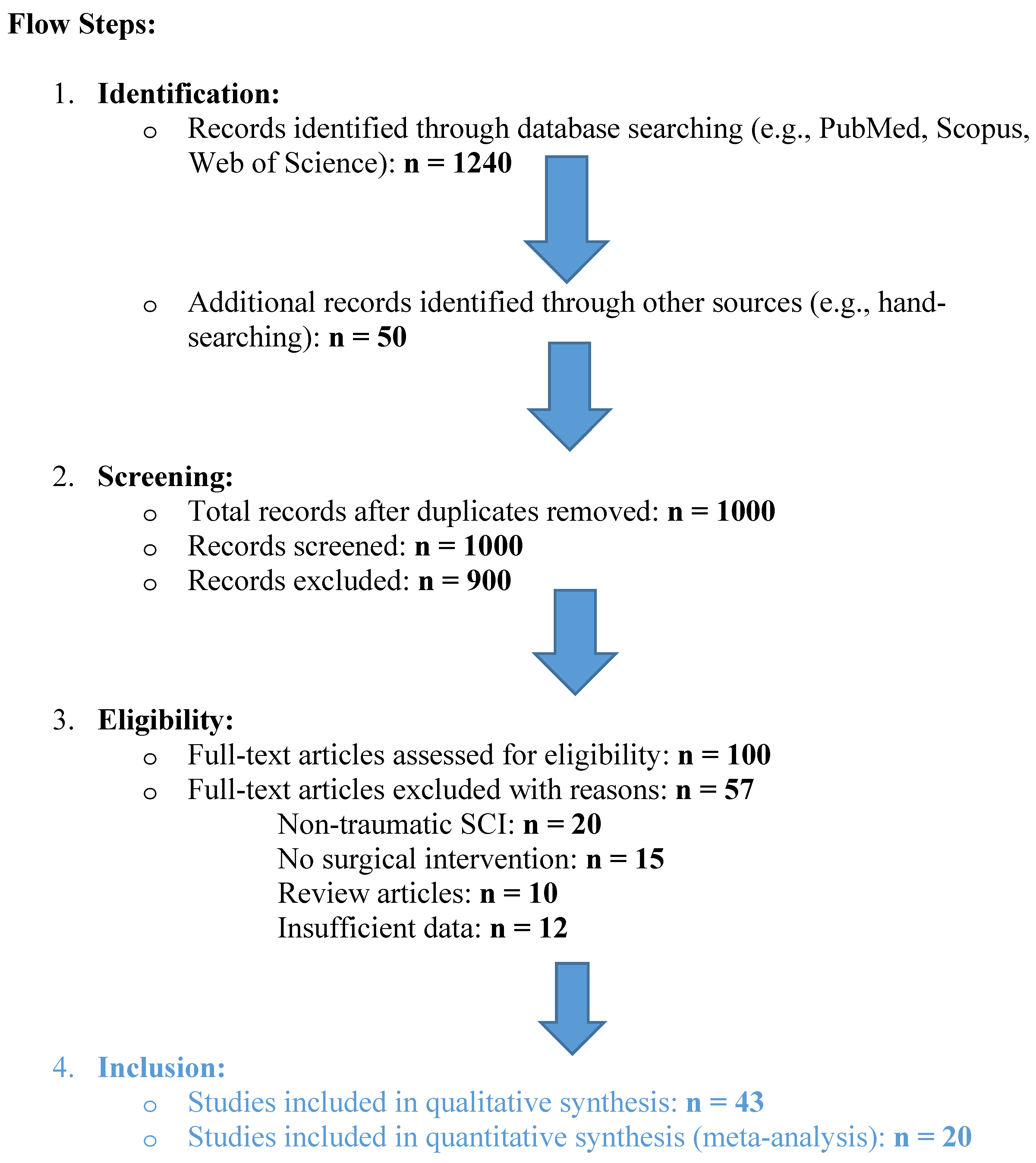
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
- -
- -
- -
- -
2.4. Quality Assessment
3. Results
3.1. Study Characteristics
3.2. Pathophysiology of SCI
| Timeline | SCI Mechanism | Neuroplasticity |
|---|---|---|
| Acute (<48 h) [1,2,3,5,10,15] | Primary Injury: Direct trauma leads to hemorrhage, axonal shearing, and cellular necrosis. Demyelination and Necrosis: Demyelination and neuronal cell death rapidly follow mechanical damage. Blood-Spinal Cord Barrier Disruption (BSCB): A breach in the BSCB leads to increased permeability, allowing immune cell infiltration, especially neutrophils, which release metalloproteinase-9 (MMP-9), worsening tissue breakdown. Inflammation: Early immune response with neutrophil and macrophage infiltration. Pro-inflammatory cytokines (TNF-α, IL-1β, IL-6) are upregulated, activating M1 microglia, releasing cytotoxic glutamate and nitric oxide, and increasing cell death. | Limited Neuroplasticity: Immediately following injury, neuroplasticity is significantly impaired due to the release of cytotoxic substances like glutamate. Synaptic circuits are abruptly disrupted, causing widespread loss of function. Glutamate Toxicity: Excessive glutamate release causes excitotoxic damage, inhibiting early neural regeneration. Neurotrophic Response: Limited neuroprotective responses, such as brain-derived neurotrophic factor (BDNF) upregulation, are present but insufficient to counteract acute damage. Axonal Injury: Axons near the injury site degenerate, reducing the potential for early plastic changes. |
| Subacute (2–14 days) [3,15,16,19,26,27,28] | Continued Inflammation: The immune response escalates, with macrophages, T cells, and lymphocytes infiltrating the injury site. The presence of pro-inflammatory cytokines continues, prolonging tissue damage and cell death. Astrocytic and Glial Activation: Astrocytes proliferate and become reactive, losing aquaporin-4 (AQP4) activity. This worsens BSCB permeability and disrupts glutamate reuptake, contributing to neurotoxicity. Formation of CSPGs: Reactive astrocytes secrete chondroitin sulfate proteoglycans (CSPGs), inhibiting axonal regrowth. Ependymal Cell Activation: Self-renewing ependymal cells migrate to the injury site, forming astrocytes and contributing to scar formation. Glial Scar Formation Begins: Scar tissue, formed by activated astrocytes and fibrotic tissue, acts as a physical and chemical barrier to axonal regeneration. | Early Plasticity: Some axonal sprouting occurs near the injury site, but neuroplasticity is primarily inhibited by CSPGs and the glial scar formation. Ependymal Cell Contribution: Ependymal cells activate and proliferate, but their differentiation is mostly glial-biased (towards astrocytes), which limits their ability to support neuronal regeneration. Axonal Sprouting and Circuit Reorganization: Axons near the lesion site begin sprouting, though inhibitory molecules like CSPGs largely block the growth. Maladaptive Changes: Initial signs of maladaptive neuroplasticity, such as aberrant sprouting or hyperexcitability, may appear, contributing to dysfunctional sensory and motor circuits. |
| Intermediate and Chronic Phase (>14 days/6 months) [3,10,15,16,20,29,30,31] | Consolidation of Glial Scar: The glial scar, consisting of reactive astrocytes, macrophages, and CSPGs, fully develops, surrounding the fibrotic core formed by type A pericytes. This scar severely limits any potential for axonal regrowth. Chronic Inflammation: Microglia and macrophages continue to release pro-inflammatory cytokines, perpetuating neuroinflammation and preventing tissue repair. Wallerian Degeneration: Axonal degeneration (Wallerian degeneration) occurs distal to the injury, contributing to the ongoing loss of neural tissue. Demyelination: Ongoing demyelination of surviving neurons results in further functional loss, and oligodendrocyte apoptosis impairs remyelination efforts. Neuroimmune Modulation: Some immune cells (e.g., CD4+ T lymphocytes) may help shift the immune environment towards a more neuroprotective state, promoting limited repair mechanisms. | Adaptive and Maladaptive Plasticity: Significant neuroplastic changes occur, with both beneficial (adaptive) and harmful (maladaptive) consequences. Adaptive Plasticity: Propriospinal neurons, which span different spinal cord segments, sprout and form new synaptic connections to bridge the injury site. These new circuits can support partial recovery of motor functions. Maladaptive Plasticity: Abnormal reorganization of spinal circuits may lead to spasticity, hyperreflexia, and sensory-evoked spasms, which worsen quality of life. Propriospinal Circuit Reorganization: Propriospinal neurons play a key role in forming compensatory circuits, enabling some recovery of locomotion, especially with rehabilitation interventions. Potential for Neurogenesis: Though limited, some endogenous neural stem/progenitor cells may contribute to neurogenesis, especially in the presence of factors like IL-4, which promote axonal growth and neurotrophic support. |
3.3. Surgical Technique of Decompressive Laminectomy and Expansive Duraplasty (Figure 2)
3.4. Effects of Decompressive Laminectomy and Expansive Duraplasty
3.5. Neuroplasticity Markers
3.6. Controversies and Future Directions
4. Discussion
Limitations
5. Conclusions
| Study | Study Design | Population | Intervention | Outcomes | |
|---|---|---|---|---|---|
| 1 | Garg et al., 2022 [25] | Clinical—Retrospective | 18 patients (SCI) | Decompressive laminectomy + duraplasty | Improved ITP, SCPP, and neuroplasticity markers. 1 AIS grade ↑ SCIM-III: +16.5 |
| 2 | Phang et al., 2015 [13] | Clinical—Observational | 25 patients (SCI) | Perfusion monitoring | Improved SCPP and pressure reactivity. WISCI II ≥ 5: 57% vs. 29% (control). No ASIA Score |
| 3 | Curt et al., 2008 [1] | Clinical—Review | Variable (SCI) | NA | Neuroplasticity mechanisms |
| 4 | Kornblith et al., 2013 [2] | Clinical—Multicenter | 150 patients (SCI) | Mechanical ventilation strategies | Improved extubation rates |
| 5 | Lenehan et al., 2012 [3] | Clinical—Epidemiological | Population-based | NA | Epidemiological insights |
| 6 | Thietje et al., 2011 [4] | Clinical—Retrospective | 62 patients (Deceased SCI) | Mortality analysis | Mortality and cause insights |
| 7 | Keefe et al., 2017 [5] | Preclinical—Animal | Rodent models | Neurotrophic factor modulation | Increased BDNF, NGF levels |
| 8 | Stoyanova et al., 2021 [6] | Preclinical—Animal | Rodent models | Ghrelin-mediated plasticity | Enhanced regeneration |
| 9 | Yue et al., 2020 [7] | Clinical—Prospective | 35 patients (SCI) | Perfusion protocols | Enhanced functional recovery |
| 10 | Saadoun et al., 2020 [8] | Clinical—Observational | 20 patients (SCI) | Targeted perfusion therapy | Reduced edema, improved outcomes |
| 11 | Leonard et al., 2015 [24] | Preclinical—Animal | Rodent models | Substance P modulation | Reduced inflammation and edema |
| 12 | Punjani et al., 2023 [15] | Preclinical—Review | Mixed human/animal data | Plasticity pathways | Highlighted neuroplasticity mechanisms |
| 13 | Zhu et al., 2019 [69] | Clinical—Retrospective | 30 patients (SCI) | Durotomy with duroplasty | Improved motor function and reduced intrathecal pressure. ASIA Motor Score Δ: +14.4. Bladder control: 73% |
| 14 | Ahuja et al., 2017 [8] | Clinical—Systematic Review | Variable population (SCI) | Repair and regeneration strategies | Insights on neuroplasticity and axonal repair |
| 15 | Leonard et al., 2013 [67] | Preclinical—Animal | Rodent models | Substance P modulation | Reduced inflammation and improved functional outcomes |
| 16 | Gotz et al., 2015 [19] | Preclinical—Animal | Rodent models | Astrocytic plasticity interventions | Enhanced synaptic remodeling and axonal regeneration |
| 17 | Lau et al., 2011 [26] | Preclinical—Animal | Lamprey brain models | Neurite sprouting post-SCI | Increased synapsin expression and sprouting |
| 18 | Anjum et al., 2020 [10] | Clinical—Observational | 50 patients (SCI) | Inflammation-targeted therapies | Reduced secondary damage and improved recovery |
| 19 | Dimou and Gallo, 2015 [64] | Preclinical—Review | Various animal models | NG2-glia functions | Insights into glial plasticity and neurogenesis |
| 20 | Guo et al., 2019 [70] | Preclinical—Animal | Mouse models | Gene expression modulation | Identification of genes promoting regeneration |
| 21 | Cafferty et al., 2010 [74] | Preclinical—Animal | Rodent models | Growth-associated genes | Enhanced axonal sprouting and plasticity |
| 22 | Cozzens et al., 2013 [27] | Clinical—Systematic Review | Variable population (SCI) | Cervical spine and spinal cord injury management | Guidelines for early intervention |
| 23 | Xing et al., 2022 [51] | Preclinical—Animal | Rat models | PI3K/AKT signaling pathways | Improved axonal growth and synaptogenesis |
| 24 | Bobinger et al., 2018 [75] | Preclinical—Review | Mixed models | Apoptotic pathways in neural injury | Insights on reducing cell death post-injury |
| 25 | Lee et al., 2010 [73] | Preclinical—Animal | Rodent models | Ghrelin for apoptosis inhibition | Improved functional recovery |
| 26 | Le Feber et al., 2016 [76] | Preclinical—In vitro | Neural cultures | Neuronal damage progression in ischemia | Modeling SCI-like ischemic conditions |
| 27 | Stoyanova et al., 2022 [77] | Preclinical—Animal | Rodent models | Hypoxia-induced Pax6 modulation | Enhanced neuronal survival and regeneration |
| 28 | Galtrey and Fawcett, 2007 [23] | Preclinical—Review | Mixed models | Role of CSPGs in regeneration | Reduction in inhibitory signaling |
| 29 | Saadoun et al., 2019 [19] | Clinical—Observational | 25 patients (SCI) | Perfusion-targeted therapies | Reduced edema and improved SCPP |
| 30 | Sun et al., 2018 [52] | Preclinical—Animal | Mouse models | Stem cells and exercise | Enhanced recovery via PI3K/AKT pathways |
| 31 | Grassner et al., 2018 [16] | Clinical—Review | Variable population | Spinal meninges in SCI | Neuroanatomical insights into recovery |
| 32 | Sharma et al., 2022 [37] | Clinical—Retrospective | 20 patients (SCI) | Magnetic resonance imaging in perfusion monitoring | Improved spinal cord perfusion visualization |
| 33 | Miao et al., 2023 [78] | Preclinical—Animal | Rodent models | Neuroplasticity via TrKA pathways | Enhanced neurite elongation and recovery |
| 34 | Werndle et al., 2014 [14] | Clinical—Observational | 30 patients (SCI) | Perfusion pressure monitoring | Reduced secondary injury through SCPP improvements |
| 35 | Kwon et al., 2009 [12] | Clinical—Randomized | 40 patients (SCI) | Intrathecal pressure monitoring | Improved outcomes via drainage protocols |
| 36 | Chen et al., 2012 [30] | Preclinical—Animal | Rat models | BDNF signaling in synaptogenesis | Enhanced recovery of motor function |
| 37 | Varsos et al., 2015 [11] | Clinical—Observational | 30 patients (SCI) | Spinal perfusion pressure dynamics | Reduced pressure-related damage |
| 38 | Leonard et al., 2015 [68] | Preclinical—Animal | Rodent models | Edema and hemorrhage contributions | Reduction in post-injury complications |
| 39 | Fehlings et al., 2006 [66] | Clinical—Systematic Review | Variable population (SCI) | Timing of intervention | Guidelines for early surgical decompression |
| 40 | Hu et al., 2023 [32] | Preclinical—Animal | Rodent models | Multi-molecular interactions post-SCI | Insights on recovery mechanisms |
| 41 | Hill et al., 2019 [79] | Preclinical—Animal | Rodent models | Reactive astrocyte modulation | Improved synaptic plasticity |
| 42 | Ahuja et al., 2017 [29] | Clinical—Retrospective | 50 patients (SCI) | Surgical repair strategies | Improved outcomes via axonal repair |
| 43 | Kheram et al., 2023 [80] | Clinical—Observational | 11 patients (SCI) | Perfusion-targeted interventions | Improved SCPP and reduced edema |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Curt, A.; Van Hedel, H.J.; Klaus, D.; Dietz, V. Recovery from a spinal cord injury: Significance of compensation, neural plasticity, and repair. J. Neurotrauma 2008, 25, 677–685. [Google Scholar] [CrossRef]
- Kornblith, L.Z.; Kutcher, M.E.; Callcut, R.A.; Redick, B.J.; Hu, C.K.; Cogbill, T.H.; Baker, C.C.; Shapiro, M.L.; Burlew, C.C.; Kaups, K.L.; et al. Mechanical ventilation weaning and extubation after spinal cord injury: A Western Trauma Association multicenter study. J. Trauma Acute Care Surg. 2013, 75, 1060–1069. [Google Scholar] [CrossRef] [PubMed]
- Lenehan, B.; Street, J.; Kwon, B.K.; Noonan, V.; Zhang, H.; Fisher, C.G.; Dvorak, M.F. The epidemiology of traumatic spinal cord injury in British Columbia, Canada. Spine 2012, 37, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Thietje, R.; Pouw, M.H.; Schulz, A.P.; Kienast, B.; Hirschfeld, S. Mortality in patients with traumatic spinal cord injury: Descriptive analysis of 62 deceased subjects. J. Spinal Cord Med. 2011, 34, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Keefe, K.M.; Sheikh, I.S.; Smith, G.M. Targeting Neurotrophins to Specific Populations of Neurons: NGF, BDNF, and NT-3 and Their Relevance for Treatment of Spinal Cord Injury. Int. J. Mol. Sci. 2017, 18, 548. [Google Scholar] [CrossRef]
- Stoyanova, I.; Lutz, D. Ghrelin-Mediated Regeneration and Plasticity After Nervous System Injury. Front. Cell Dev. Biol. 2021, 9, 595914. [Google Scholar] [CrossRef]
- Yue, J.K.; Hemmerle, D.D.; Winkler, E.A.; Thomas, L.H.; Fernandez, X.D.; Kyritsis, N.; Pan, J.Z.; Pascual, L.U.; Singh, V.; Weinstein, P.R.; et al. Clinical Implementation of Novel Spinal Cord Perfusion Pressure Protocol in Acute Traumatic Spinal Cord Injury at U.S. Level I Trauma Center: TRACK-SCI Study. World Neurosurg. 2020, 133, e391–e396. [Google Scholar]
- Saadoun, S.; Papadopoulos, M.C. Targeted Perfusion Therapy in Spinal Cord Trauma. Neurotherapeutics 2020, 17, 511–521. [Google Scholar] [CrossRef]
- Ahuja, C.S.; Schroeder, G.D.; Vaccaro, A.R.; Fehlings, M.G. Spinal Cord Injury—What Are the Controversies? J. Orthop. Trauma 2017, 31 (Suppl. 4), S7–S13. [Google Scholar] [CrossRef]
- Anjum, A.; Yazid, M.D. Spinal Cord Injury: Pathophysiology, Multimolecular Interactions, and Recovery Mechanisms. Mol. Neurobiol. 2020, 21, 7533. [Google Scholar] [CrossRef]
- Varsos, G.V.; Werndle, M.C.; Czosnyka, Z.H.; Smielewski, P.; Kolias, A.G.; Phang, I.; Saadoun, S.; Bell, B.A.; Zoumprouli, A.; Papadopoulos, M.C.; et al. Intraspinal pressure and spinal cord perfusion pressure after spinal cord injury: An observational study. J. Neurosurg. Spine 2015, 23, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Kwon, B.K.; Curt, A.; Belanger, L.M.; Bernardo, A.; Chan, D.; Markez, J.A.; Gorelik, S.; Slobogean, G.P.; Umedaly, H.; Giffin, M.; et al. Intrathecal pressure monitoring in acute spinal cord injury. J. Neurosurg. Spine 2009, 10, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Phang, I.; Werndle, M.C.; Saadoun, S.; Varsos, G.; Czosnyka, M.; Zoumprouli, A.; Papadopoulos, M.C. Expansion duroplasty improves intraspinal pressure, spinal cord perfusion pressure, and vascular pressure reactivity index in patients with traumatic spinal cord injury: Injured spinal cord pressure evaluation study. J. Neurotrauma 2015, 32, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Werndle, M.C.; Saadoun, S.; Phang, I.; Czosnyka, M.; Varsos, G.V.; Czosnyka, Z.H.; Smielewski, P.; Jamous, A.; Bell, B.A.; Zoumprouli, A.; et al. Monitoring of spinal cord perfusion pressure in acute spinal cord injury. Crit. Care Med. 2014, 42, 646–655. [Google Scholar] [CrossRef]
- Punjani, N.; Deska-Gauthier, D.; Hachem, L.D.; Abramian, M.; Fehlings, M.G. Neuroplasticity and regeneration after spinal cord injury. N. Am. Spine Soc. J. 2023, 15, 100235. [Google Scholar] [CrossRef]
- Grassner, L.; Grillhösl, A.; Griessenauer, C.J.; Thomé, C.; Bühren, V.; Strowitzki, M.; Winkler, P.A. Spinal Meninges and Their Role in Spinal Cord Injury: A Neuroanatomical Review. J. Neurotrauma 2018, 35, 403–410. [Google Scholar] [CrossRef]
- Zhu, F.; Yao, S.; Ren, Z. Spinal cord expansion duroplasty and outcomes. J. Neurotrauma 2020, 35, 235–240. [Google Scholar]
- Archavlis, E.; Palombi, D.; Konstantinidis, D.; Carvi y Nievas, M.; Trobisch, P.; Stoyanova, I.I. Pathophysiologic Mechanisms of Severe Spinal Cord Injury and Neuroplasticity Following Decompressive Laminectomy and Expansive Duraplasty: A Systematic Review Protocol. PROSPERO International Prospective Register of Systematic Reviews. CRD42024643470. 2024. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=42024643470 (accessed on 9 January 2025).
- Gotz, M.; Sirko, S.; Beckers, J.; Irmler, M. Reactive astrocytes as neural stem or progenitor cells: In vivo lineage, in vitro potential, and genome-wide expression analysis. Glia 2015, 63, 1452–1468. [Google Scholar] [CrossRef]
- Wei, Q.; Sun, X.; Huang, L.-Y.; Pan, H.-X.; Li, L.-J.; Wang, L.; Pei, G.-Q.; Wang, Y.; Zhang, Q.; Cheng, H.-X.; et al. Bone marrow mesenchymal stem cells and exercise restore motor function following spinal cord injury. Neural Regen. Res. 2023, 18, 1067–1075. [Google Scholar] [CrossRef]
- Saadoun, S.; Papadopoulos, M.C. Targeting spinal edema after injury. Neurotherapeutics 2019, 18, 122–130. [Google Scholar]
- Kirdajova, D.; Anderova, M. NG2 cells and their neurogenic potential. Curr. Opin. Pharmacol. 2020, 50, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Galtrey, C.M.; Fawcett, J.W. The role of chondroitin sulfate proteoglycans in regeneration and plasticity in the central nervous system. Brain Res. Rev. 2007, 54, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Leonard, A.V.; Thornton, E.; Vink, R. The relative contribution of edema and hemorrhage to raised intrathecal pressure after traumatic spinal cord injury. J. Neurotrauma 2015, 32, 397–402. [Google Scholar] [CrossRef]
- Garg, K.; Agrawal, D.; Hurlbert, R.J. Expansive Duraplasty—Simple Technique with Promising Results in Complete Cervical Spinal Cord Injury: A Preliminary Study. Neurol. India 2022, 70, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Lau, B.Y.; Foldes, A.E.; Alieva, N.O.; Oliphint, P.A.; Busch, D.J.; Morgan, J.R. Increased synapsin expression and neurite sprouting in lamprey brain after spinal cord injury. Exp. Neurol. 2011, 228, 283–293. [Google Scholar] [CrossRef]
- Cozzens, J.W.; Prall, J.A.; Holly, L. The 2012 Guidelines for the Management of Acute Cervical Spine and Spinal Cord Injury. Neurosurgery 2013, 72 (Suppl. 2), 2–3. [Google Scholar]
- Hu, J.; Jin, L.Q.; Selzer, M.E. Inhibition of central axon regeneration: Perspective from chondroitin sulfate proteoglycans. Neural Regen. Res. 2022, 17, 1955–1956. [Google Scholar]
- Ahuja, C.S.; Nori, S.; Tetreault, L.; Wilson, J.; Kwon, B.; Harrop, J.; Choi, D.; Fehlings, M.G. Traumatic Spinal Cord Injury—Repair and Regeneration. Neurosurgery 2017, 80, S9–S22. [Google Scholar] [CrossRef]
- Chen, T.; Wu, Y.; Wang, Y. Brain-Derived Neurotrophic Factor and Neural Stem Cells. Neurochem. Res. 2017, 42, 3073–3083. [Google Scholar] [CrossRef]
- Singh, P.L.; Agarwal, N.; Barrese, J.C.; Heary, R.F. Current therapeutic strategies for inflammation following traumatic spinal cord injury. Neural Regen. Res. 2012, 7, 1812–1821. [Google Scholar]
- Hu, X.; Xu, W.; Ren, Y.; Wang, Z.; He, X.; Huang, R.; Ma, B.; Zhao, J.; Zhu, R.; Cheng, L. Spinal cord injury: Molecular mechanisms and therapeutic inter-ventions. Signal Transduct. Target. Ther. 2023, 8, 245. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Guo, M.; Lv, X.; Wang, Z.; Yang, J.; Li, Y.; Yu, F.; Wen, X.; Feng, L.; Zhou, T. Role of Transient Receptor Potential Vanilloid 4 in Vascular Function. Front. Mol. Biosci. 2021, 8, 677661. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Wen, Z.; Cao, M.; Zhang, H.; Ling, S.K.; Fu, B.S.; Qin, L.; Xu, J.; Yung, P.S. The emerging role of Piezo1 in the musculoskeletal system and disease. Theranostics 2024, 14, 3963–3983. [Google Scholar] [CrossRef] [PubMed]
- Mirzaie, M.; Karimi, M.; Fallah, H.; Khaksari, M.; Nazari-Robati, M. Downregulation of Matrix Metalloproteinases 2 and 9 is Involved in the Protective Effect of Trehalose on Spinal Cord Injury. Int. J. Mol. Cell Med. 2018, 7, 8–16. [Google Scholar]
- Ishizawa, K.; Komori, T.; Shimada, T.; Arai, E.; Imanaka, K.; Kyo, S.; Hirose, T. Hemodynamic infarction of the spinal cord: Involvement of the gray matter plus the border-zone between the central and peripheral arteries. Spinal Cord. 2005, 43, 306–310. [Google Scholar] [CrossRef]
- Squair, J.W.; Gautier, M.; Mahe, L.; Soriano, J.E.; Rowald, A.; Bichat, A.; Cho, N.; Anderson, M.A.; James, N.D.; Gandar, J.; et al. Neuroprosthetic baroreflex controls haemodynamics after spinal cord injury. Nature 2021, 590, 308–314. [Google Scholar] [CrossRef]
- Sharma, S.; Neelavalli, J.; Shah, T.; Gupta, R.K. Susceptibility-weighted imaging: An emerging technique for evaluation of the spine and spinal cord. Br. J. Radiol. 2022, 95, 20211294. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Nakashima, H.; Nagoshi, N.; Chow, D.S.; Grossman, R.G.; Kopjar, B. Rationale, design and critical end points for the Riluzole in Acute Spinal Cord Injury Study (RISCIS): A randomized, double-blinded, placebo-controlled parallel multi-center trial. Spinal Cord 2016, 54, 8–15. [Google Scholar] [CrossRef]
- Montague-Cardoso, K.; Malcangio, M. Changes in blood-spinal cord barrier permeability and neuroimmune interactions in the underlying mechanisms of chronic pain. PAIN Rep. 2021, 6, e879. [Google Scholar] [CrossRef]
- Pang, Q.M.; Chen, S.Y.; Xu, Q.J.; Fu, S.P.; Yang, Y.C.; Zou, W.H.; Zhang, M.; Liu, J.; Wan, W.H.; Peng, J.C.; et al. Neuroinflammation and Scarring After Spinal Cord Injury: Therapeutic Roles of MSCs on Inflammation and Glial Scar. Front. Immunol. 2021, 12, 751021. [Google Scholar] [CrossRef]
- Luo, Y.; Yao, F.; Shi, Y.; Zhu, Z.; Xiao, Z.; You, X.; Liu, Y.; Yu, S.; Tian, D.; Cheng, L.; et al. Tocilizumab promotes repair of spinal cord injury by facilitating the restoration of tight junctions between vascular endothelial cells. Fluids Barriers CNS 2023, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Gao, C.; Gao, D.; Sun, R.; Li, W.; Wang, F.; Wang, Y.; Cao, H.; Zhou, G.; Zhang, J.; et al. Reduction in pericyte coverage leads to blood-brain barrier dysfunction via endothelial transcytosis following chronic cerebral hypoperfusion. Fluids Barriers CNS 2021, 18, 21. [Google Scholar] [CrossRef] [PubMed]
- Theodore, N.; Martirosyan, N.; Hersh, A.M.; Ehresman, J.; Ahmed, A.K.; Danielson, J.; Sullivan, C.; Shank, C.D.; Almefty, K.; Lemole, G.M., Jr.; et al. Cerebrospinal Fluid Drainage in Patients with Acute Spinal Cord Injury: A Multi-Center Randomized Controlled Trial. World Neurosurg. 2023, 177, e472–e479. [Google Scholar] [CrossRef] [PubMed]
- McCracken, E.; Valeriani, V.; Simpson, C.; Jover, T.; McCulloch, J.; Dewar, D. The lipid peroxidation by-product 4-hydroxynonenal is toxic to axons and oligodendrocytes. J. Cereb. Blood Flow Metab. 2000, 20, 1529–1536. [Google Scholar] [CrossRef]
- Bradbury, E.J.; Burnside, E.R. Moving beyond the glial scar for spinal cord repair. Nat. Commun. 2019, 10, 3879. [Google Scholar] [CrossRef]
- Li, X.; Jiao, K.; Liu, C.; Li, X.; Wang, S.; Tao, Y.; Cheng, Y.; Zhou, X.; Wei, X.; Li, M. Bibliometric analysis of the inflammation expression after spinal cord injury: Current research status and emerging frontiers. Spinal Cord 2024, 62, 609–618. [Google Scholar] [CrossRef]
- Kopper, T.J.; Gensel, J.C. Myelin as an inflammatory mediator: Myelin interactions with complement, macrophages, and microglia in spinal cord injury. J. Neurosci. Res. 2018, 96, 969–977. [Google Scholar] [CrossRef]
- Gál, L.; Bellák, T.; Marton, A.; Fekécs, Z.; Weissman, D.; Török, D.; Biju, R.; Vizler, C.; Kristóf, R.; Beattie, M.B.; et al. Restoration of Motor Function through Delayed Intraspinal Delivery of Human IL-10-Encoding Nucleoside-Modified mRNA after Spinal Cord Injury. Research 2023, 6, 0056. [Google Scholar] [CrossRef]
- Ritzel, R.M.; Li, Y.; Jiao, Y.; Lei, Z.; Doran, S.J.; He, J.; Shahror, R.A.; Henry, R.J.; Khan, R.; Tan, C.; et al. Brain injury accelerates the onset of a reversible age-related microglial phenotype associated with inflammatory neurodegeneration. Sci. Adv. 2023, 9, eadd1101. [Google Scholar] [CrossRef]
- Xing, C.; Jia, Z.; Qu, H.; Liu, S.; Jiang, W.; Zhong, H.; Zhou, M.; Zhu, S.; Ning, G.; Feng, S. Correlation Analysis Between Magnetic Resonance Imaging-Based Anatomical Assessment and Behavioral Outcome in a Rat Contusion Model of Chronic Thoracic Spinal Cord Injury. Front. Neurosci. 2022, 16, 838786. [Google Scholar] [CrossRef]
- Sun, G.; Yang, S.; Cao, G.; Wang, Q.; Hao, J.; Wen, Q.; Li, Z.; So, K.F.; Liu, Z.; Zhou, S.; et al. γδ T cells provide the early source of IFN-γ to aggravate lesions in spinal cord injury. J. Exp. Med. 2018, 215, 521–535. [Google Scholar] [CrossRef] [PubMed]
- Huie, J.R.; Stuck, E.D.; Lee, K.H.; Irvine, K.A.; Beattie, M.S.; Bresnahan, J.C.; Grau, J.W.; Ferguson, A.R. AMPA Receptor Phosphorylation and Synaptic Colocalization on Motor Neurons Drive Maladaptive Plasticity below Complete Spinal Cord Injury. eNeuro 2015, 2. [Google Scholar] [CrossRef] [PubMed]
- Hassannejad, Z.; Zadegan, S.A.; Vaccaro, A.R.; Rahimi-Movaghar, V.; Sabzevari, O. Biofunctionalized peptide-based hydrogel as an injectable scaffold for BDNF delivery can improve regeneration after spinal cord injury. Injury 2019, 50, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Kathe, C.; Skinnider, M.A.; Hutson, T.H.; Regazzi, N.; Gautier, M.; Demesmaeker, R.; Komi, S.; Ceto, S.; James, N.D.; Cho, N.; et al. The neurons that restore walking after paralysis. Nature 2022, 611, 540–547. [Google Scholar] [CrossRef]
- Eisdorfer, J.T.; Smit, R.D.; Keefe, K.M.; Lemay, M.A.; Smith, G.M.; Spence, A.J. Epidural Electrical Stimulation: A Review of Plasticity Mechanisms That Are Hypothesized to Underlie Enhanced Recovery From Spinal Cord Injury with Stimulation. Front. Mol. Neurosci. 2020, 13, 163. [Google Scholar] [CrossRef]
- Lee, S.E.; Jahng, T.A.; Chung, C.K.; Kim, H.J. Circumferential spinal cord decompression through a posterior midline approach with lateral auxiliary ports for lower thoracic compressive myelopathy. Neurosurgery 2012, 70 (Suppl. 2), 221–229. [Google Scholar] [CrossRef]
- Kahraman, M.A.; Senturk, S. The Necessity of Extensive Decompression for Spinal Epidural Hematoma: A Case Report and Literature Review. Cureus 2023, 15, e44192. [Google Scholar] [CrossRef]
- Vavrek, R.; Girgis, J.; Tetzlaff, W.; Hiebert, G.W.; Fouad, K. BDNF promotes connections of corticospinal neurons onto spared descending interneurons in spinal cord injured rats. Brain 2006, 129 Pt 6, 1534–1545. [Google Scholar] [CrossRef]
- Sparacia, G.; Parla, G.; Miraglia, R.; de Ville de Goyet, J. Brain Functional Connectivity Significantly Improves After Surgical Eradication of Porto-Systemic Shunting in Pediatric Patients. Life 2025, 15, 290. [Google Scholar] [CrossRef]
- Gee, C.M.; Kwon, B.K. Significance of spinal cord perfusion pressure following spinal cord injury: A systematic scoping review. J. Clin. Orthop. Trauma 2022, 34, 102024. [Google Scholar] [CrossRef]
- Ahadi, R.; Khodagholi, F.; Daneshi, A.; Vafaei, A.; Mafi, A.A.; Jorjani, M. Diagnostic Value of Serum Levels of GFAP, pNF-H, and NSE Compared With Clinical Findings in Severity Assessment of Human Traumatic Spinal Cord Injury. Spine 2015, 40, E823–E830. [Google Scholar] [CrossRef] [PubMed]
- Aarabi, B.; Chixiang, C.; Simard, J.M.; Chryssikos, T.; Stokum, J.A.; Sansur, C.A.; Crandall, K.M.; Olexa, J.; Oliver, J.; Meister, M.R.; et al. Proposal of a Management Algorithm to Predict the Need for Expansion Duraplasty in American Spinal Injury Association Impairment Scale Grades A-C Traumatic Cervical Spinal Cord Injury Patients. J. Neurotrauma 2022, 39, 1716–1726. [Google Scholar] [CrossRef] [PubMed]
- Dimou, L.; Gallo, V. NG2-glia and their functions in the central nervous system. Glia 2015, 63, 1429–1451. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Qiao, H.; Sun, Z.; Li, X. Effect of BDNF-plasma-collagen matrix controlled delivery system on the behavior of adult rats neural stem cells. J. Biomed. Mater. Res. A 2013, 101, 599–606. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Perrin, R.G. The timing of surgical intervention in the treatment of spinal cord injury: A systematic review of recent clinical evidence. Spine 2006, 31, S28–S35. [Google Scholar] [CrossRef]
- Leonard, A.V.; Thornton, E.; Vink, R. Substance P as a mediator of neurogenic inflammation after spinal cord injury. J. Neurotrauma 2013, 30, 1812–1823. [Google Scholar] [CrossRef]
- Leonard, A.V.; Vink, R. Reducing intrathecal pressure after traumatic spinal cord injury: A potential clinical target to promote tissue survival. Neural Regen. Res. 2015, 10, 380–382. [Google Scholar]
- Zhu, F.; Yao, S.; Ren, Z.; Telemacque, D.; Qu, Y.; Chen, K.; Yang, F.; Zeng, L.; Guo, X. Early durotomy with duroplasty for severe adult spinal cord injury without radiographic abnormality: A novel concept and method of surgical decompression. Spinal Cord 2019, 28, 2275–2282. [Google Scholar] [CrossRef]
- Guo, L.; Lv, J.; Huang, Y.F.; Hao, D.-J. Differentially expressed genes associated with spinal cord injury. Neural Regen. Res. 2019, 14, 1262–1270. [Google Scholar]
- Bhagwani, A.; Chopra, M.; Kumar, H. Spinal Cord Injury Provoked Neuropathic Pain and Spasticity, and Their GABAergic Connection. Neurospine 2022, 19, 646–668. [Google Scholar] [CrossRef]
- Yoo, J.S.; Ahn, J.; Buvanendran, A.; Singh, K. Multimodal analgesia in pain management after spine surgery. J. Spine Surg. 2019, 5 (Suppl. 2), S154–S159. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Chung, H.; Yoo, Y.S. Inhibition of apoptotic cell death by ghrelin improves recovery after spinal cord injury. Endocrinology 2010, 151, 3815–3826. [Google Scholar] [CrossRef] [PubMed]
- Cafferty, W.B.; Duffy, P.; Huebner, E.; Strittmatter, S.M. MAG and OMgp synergize with Nogo-A to restrict axonal growth and neurological recovery after spinal cord trauma. J. Neurosci. 2010, 30, 6825–6837. [Google Scholar] [CrossRef] [PubMed]
- Bobinger, T.; Burkardt, P.; BHuttner, H.; Manaenko, A. Programmed Cell Death after Intracerebral Hemorrhage. Curr Neuropharmacol. 2018, 16, 1267–1281. [Google Scholar] [CrossRef]
- Le Feber, J.; Tzafi Pavlidou, S.; Erkamp, N.; van Putten, M.J.; Hofmeijer, J. Progression of Neuronal Damage in an In Vitro Model of the Ischemic Penumbra. PLoS ONE 2016, 11, e0147231. [Google Scholar] [CrossRef]
- Stoyanova, I.I.; Klymenko, A.; Willms, J.; Doeppner, T.R.; Tonchev, A.B.; Lutz, D. Ghrelin Regulates Expression of Pax6 in Hypoxic Brain Progenitor Cells. Cells 2022, 11, 782. [Google Scholar] [CrossRef]
- Miao, L.; Qing, S.W.; Tao, L. Huntingtin-associated protein 1 ameliorates neurological function rehabilitation by facilitating neurite elongation through TrKA-MAPK pathway in mice spinal cord injury. Front Mol. Neurosci. 2023, 16, 1214150. [Google Scholar] [CrossRef]
- Hill, S.A.; Blaeser, A.S.; Coley, A.A.; Xie, Y.; Shepard, K.A.; Harwell, C.C.; Gao, W.J.; Garcia, A.D.R. Sonic hedgehog signaling in astrocytes mediates cell type-specific synaptic organization. Elife 2019, 8, e45545. [Google Scholar] [CrossRef]
- Kheram, N.; Boraschi, A.; Pfender, N.; Friedl, S.; Rasenack, M.; Fritz, B.; Kurtcuoglu, V.; Schubert, M.; Curt, A.; Zipser, C.M. Cerebrospinal Fluid Pressure Dynamics as a Bedside Test in Traumatic Spinal Cord Injury to Assess Surgical Spinal Cord Decompression: Safety, Feasibility, and Proof-of-Concept. Neurorehabilit. Neural Repair. 2023, 37, 171–182. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Archavlis, E.; Palombi, D.; Konstantinidis, D.; Carvi y Nievas, M.; Trobisch, P.; Stoyanova, I.I. Pathophysiologic Mechanisms of Severe Spinal Cord Injury and Neuroplasticity Following Decompressive Laminectomy and Expansive Duraplasty: A Systematic Review. Neurol. Int. 2025, 17, 57. https://doi.org/10.3390/neurolint17040057
Archavlis E, Palombi D, Konstantinidis D, Carvi y Nievas M, Trobisch P, Stoyanova II. Pathophysiologic Mechanisms of Severe Spinal Cord Injury and Neuroplasticity Following Decompressive Laminectomy and Expansive Duraplasty: A Systematic Review. Neurology International. 2025; 17(4):57. https://doi.org/10.3390/neurolint17040057
Chicago/Turabian StyleArchavlis, Eleftherios, Davide Palombi, Dimitrios Konstantinidis, Mario Carvi y Nievas, Per Trobisch, and Irina I. Stoyanova. 2025. "Pathophysiologic Mechanisms of Severe Spinal Cord Injury and Neuroplasticity Following Decompressive Laminectomy and Expansive Duraplasty: A Systematic Review" Neurology International 17, no. 4: 57. https://doi.org/10.3390/neurolint17040057
APA StyleArchavlis, E., Palombi, D., Konstantinidis, D., Carvi y Nievas, M., Trobisch, P., & Stoyanova, I. I. (2025). Pathophysiologic Mechanisms of Severe Spinal Cord Injury and Neuroplasticity Following Decompressive Laminectomy and Expansive Duraplasty: A Systematic Review. Neurology International, 17(4), 57. https://doi.org/10.3390/neurolint17040057








