Variants in Neurotransmitter-Related Genes Are Associated with Alzheimer’s Disease Risk and Cognitive Functioning but Not Short-Term Treatment Response
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. DNA Extraction and Genotyping
2.3. Drug Response Assessment
2.4. Statistical Analysis
3. Results
3.1. Clinical and Demographic Data in Responder and Non-Responder Groups
3.2. Study of Genetic Variants in Patients with Alzheimer’s Disease and Cognitive Performance
3.3. Study of Pharmacogenetic Variants with the Short-Term Response to Donepezil, Galantamine, Rivastigmine, and Memantine
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| ChEI | Cholinesterase inhibitor |
| DPZ | Donepezil |
| GAL | Galantamine |
| RIV | Rivastigmine |
| NMDA | N-methyl-D-aspartate antagonist |
| CYP2D6 | Cytochrome P450 family 2 subfamily D member 6 |
| CYP3A4 | Cytochrome P450 family 3 subfamily A member 4 |
| CYP3A5 | Cytochrome P450 family 3 subfamily A member 5 |
| NR1I2 | Nuclear receptor subfamily 1 Group I member 2 |
| ABCB1 | ATP binding cassette subfamily B member 1 |
| APOE | Apolipoprotein E |
| ACHE | Acetylcholinesterase |
| BCHE | Butyrylcholinesterase |
| CHAT | Choline O-acetyltransferase |
| MEM | Memantine |
| MM | Mexican Mestizo |
| INNN | Instituto Nacional de Neurología y Neurocirugía |
| HRALM | Hospital Regional Adolfo López Mateos |
| CHRNA7 | Cholinergic receptor nicotinic alpha 7 subunit |
| POR | Cytochrome P450 oxidoreductase |
| MMSE | Mini-Mental State Examination |
| GDS | Global Deterioration Scale |
| PVF | Phonological Verbal Fluency test |
| SVF | Semantic Verbal Fluency test |
| ADRs | Adverse drug reactions |
| ADL | Activities of daily living |
| SAH | Systemic arterial hypertension |
| T2DM | Type 2 diabetes mellitus |
| MAF | Minor allele frequency |
| CI | Confidence interval |
| OR | Odds ratio |
References
- Wortmann, M. Dementia: A global health priority—Highlights from an ADI and World Health Organization report. Alzheimers Res. Ther. 2012, 4, 40. Available online: http://www.ncbi.nlm.nih.gov/pubmed/22995353 (accessed on 24 October 2018). [CrossRef] [PubMed]
- Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimer’s Dement 2018, 14, 367–429. [Google Scholar] [CrossRef]
- Gauthier, S.; Rosa-Neto, P.; Morais, J.A. WC World Alzheimer Report 2021: Journey Through the Diagnosis of Dementia. 2021. Available online: https://www.alzint.org/u/World-Alzheimer-Report-2021.pdf (accessed on 14 July 2022).
- Crews, L.; Masliah, E. Molecular mechanisms of neurodegeneration in Alzheimer’s disease. Hum. Mol. Genet. 2010, 19, R12–R20. Available online: http://www.ncbi.nlm.nih.gov/pubmed/20413653 (accessed on 25 October 2018). [CrossRef]
- Becker, J.T.; Boller, F.; Lopez, O.L.; Saxton, J.; McGonigle, K.L. The natural history of Alzheimer’s disease. Description of study cohort and accuracy of diagnosis. Arch. Neurol. 1994, 51, 585–594. Available online: http://www.ncbi.nlm.nih.gov/pubmed/8198470 (accessed on 25 October 2018). [CrossRef]
- López, O.L.; DeKosky, S.T. Clinical symptoms in Alzheimer’s disease. Handb. Clin. Neurol. 2008, 89, 207–216. Available online: https://www.sciencedirect.com/science/article/pii/S0072975207012195 (accessed on 25 October 2018).
- Cammisuli, D.M.; Danti, S.; Bosinelli, F.; Cipriani, G. Non-pharmacological interventions for people with Alzheimer’s Disease: A critical review of the scientific literature from the last ten years. Eur. Geriatr. Med. 2016, 7, 57–64. Available online: https://www.sciencedirect.com/science/article/pii/S1878764916000048 (accessed on 25 October 2018). [CrossRef]
- Zúniga, T.; Trujillo, Z.; Cortés, G.; Acosta, I.; Sosa, A.L. Impacto de los Programas de Estimulación en Adultos Mayores con Demencia que Asisten a un Centro de Día. Arch. Neurocien. 2014, 19, 192–198. Available online: http://www.scirp.org/(S(i43dyn45teexjx455qlt3d2q))/reference/ReferencesPapers.aspx?ReferenceID=1608001 (accessed on 23 May 2017). [CrossRef]
- Alzheimer’s Disease International. World Alzheimer Report 2018—The State of the Art of Dementia Research: New Frontiers. Available online: https://www.alz.co.uk/research/WorldAlzheimerReport2018.pdf?2 (accessed on 25 October 2018).
- Campos, C.; Rocha, N.B.; Vieira, R.T.; Rocha, S.A.; Telles-Correia, D.; Paes, F.; Yuan, T.; Nardi, A.E.; Arias-Carrión, O.; Machado, S.; et al. Treatment of Cognitive Deficits in Alzheimer’s disease: A psychopharmacological review. Psychiatr. Danub. 2016, 28, 2–12. Available online: http://www.ncbi.nlm.nih.gov/pubmed/26938815 (accessed on 15 August 2016).
- Miranda, L.F.J.R.; Gomes, K.B.; Silveira, J.N.; Pianetti, G.A.; Byrro, R.M.; Peles, P.R.; Pereira, F.H.; Santos, T.R.; Assini, A.G.; Ribeiro, V.V.; et al. Predictive factors of clinical response to cholinesterase inhibitors in mild and moderate Alzheimer’s disease and mixed dementia: A one-year naturalistic study. J. Alzheimer’s Dis. 2015, 45, 609–620. Available online: http://www.ncbi.nlm.nih.gov/pubmed/25589728 (accessed on 20 April 2017). [CrossRef]
- US Department of Health and Human Services Food and Drug Administration. E15 Definitions for Genomic Biomarkers, Pharmacogenomics, Pharmacogenetics, Genomic Data and Sample Coding Categories. 2008. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/e15-pharmacogenomics-definitions-and-sample-coding (accessed on 6 December 2018).
- Zúñiga Santamaría, T.; Yescas Gómez, P.; Fricke Galindo, I.; González, M.G.; Vázquez, A.O.; López, M.L. Pharmacogenetic studies in Alzheimer disease. Neurol. (Engl. Ed.) 2022, 37, 287–303. [Google Scholar] [CrossRef]
- Wattmo, C.; Wallin, Å.K.; Londos, E.; Minthon, L. Long-term Outcome and Prediction Models of Activities of Daily Living in Alzheimer Disease With Cholinesterase Inhibitor Treatment. Alzheimer Dis. Assoc. Disord. 2011, 25, 63–72. Available online: http://journals.lww.com/00002093-201101000-00010 (accessed on 20 August 2020). [CrossRef] [PubMed]
- Wattmo, C.; Wallin, Å.K.; Londos, E.; Minthon, L. Predictors of long-term cognitive outcome in Alzheimer’s disease. Alzheimer’s Res. Ther. 2011, 3, 23. Available online: https://alzres.biomedcentral.com/articles/10.1186/alzrt85 (accessed on 20 August 2020). [CrossRef] [PubMed]
- Lopez, O.L.; Becker, J.T.; Wisniewski, S.; Saxton, J.; Kaufer, D.I.; DeKosky, S.T. Cholinesterase inhibitor treatment alters the natural history of Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2002, 72, 310–314. Available online: www.jnnp.com (accessed on 20 August 2020). [CrossRef] [PubMed]
- Birks, J.S. Cholinesterase inhibitors for Alzheimer’s disease. In Cochrane Database of Systematic Reviews; Birks, J.S., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2006; p. CD005593. Available online: http://www.ncbi.nlm.nih.gov/pubmed/16437532 (accessed on 14 December 2018).
- Blanco-Silvente, L.; Capellà, D.; Garre-Olmo, J.; Vilalta-Franch, J.; Castells, X. Predictors of discontinuation, efficacy, and safety of memantine treatment for Alzheimer’s disease: Meta-analysis and meta-regression of 18 randomized clinical trials involving 5004 patients. BMC Geriatr. 2018, 18, 168. Available online: http://www.ncbi.nlm.nih.gov/pubmed/30041625 (accessed on 14 December 2018). [CrossRef]
- McKhann, G.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E.M. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984, 34, 939–944. Available online: http://www.ncbi.nlm.nih.gov/pubmed/6610841 (accessed on 9 March 2018). [CrossRef]
- Dubois, B.; Feldman, H.H.; Jacova, C.; Dekosky, S.T.; Barberger-Gateau, P.; Cummings, J.; Delacourte, A.; Galasko, D.; Gauthier, S.; Jicha, G.; et al. Research criteria for the diagnosis of Alzheimer’s disease: Revising the NINCDS–ADRDA criteria. Lancet Neurol. 2007, 6, 734–746. Available online: http://www.ncbi.nlm.nih.gov/pubmed/17616482 (accessed on 9 March 2018). [CrossRef]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement 2011, 7, 263–269. Available online: http://www.ncbi.nlm.nih.gov/pubmed/21514250 (accessed on 9 March 2018). [CrossRef]
- Hixson, J.E.; Vernier, D.T. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J. Lipid Res. 1990, 31, 545–548. Available online: http://www.ncbi.nlm.nih.gov/pubmed/2341813 (accessed on 12 December 2018). [CrossRef]
- Varela, N.; Quiñones, L.A.; Stojanova, J.; Garay, J.; Cáceres, D.; Cespedes, S.; Sasso, J.; Miranda, C. Characterization of the CYP2D6 drug metabolizing phenotypes of the Chilean mestizo population through polymorphism analyses. Pharmacol. Res. 2015, 101, 124–129. [Google Scholar] [CrossRef]
- López-López, M.; Peñas-Lledó, E.; Dorado, P.; Ortega, A.; Corona, T.; Ochoa, A.; Yescas, P.; Alonso, E.; Llerena, A. CYP2D6 genetic polymorphisms in Southern Mexican Mayan Lacandones and Mestizos from Chiapas. Pharmacogenomics 2014, 15, 1859–1865. Available online: http://www.ncbi.nlm.nih.gov/pubmed/25495408 (accessed on 24 June 2015). [CrossRef]
- Dorado, P.; Cáceres, M.C.; Pozo-Guisado, E.; Wong, M.L.; Licinio, J.; Llerena, A. Development of a PCR-based strategy for CYP2D6 genotyping including gene multiplication of worldwide potential use. Biotechniques 2005, 39 (Suppl. S10), S571–S574. Available online: http://www.ncbi.nlm.nih.gov/pubmed/18957039 (accessed on 3 June 2016). [CrossRef]
- Gaedigk, A.; Simon, S.D.; Pearce, R.E.; Bradford, L.D.; Kennedy, M.J.; Leeder, J.S. The CYP2D6 activity score: Translating genotype information into a qualitative measure of phenotype. Clin. Pharmacol. Ther. 2008, 83, 234–242. Available online: http://www.ncbi.nlm.nih.gov/pubmed/17971818 (accessed on 17 June 2015). [CrossRef] [PubMed]
- Llerena, A.; Dorado, P.; Ramírez, R.; González, I.; Alvarez, M.; Peñas-Lledó, E.M.; Pérez, B.; Calzadilla, L.R. CYP2D6 genotype and debrisoquine hydroxylation phenotype in Cubans and Nicaraguans. Pharmacogenomics J. 2012, 12, 176–183. Available online: http://www.ncbi.nlm.nih.gov/pubmed/21135868 (accessed on 17 June 2015). [CrossRef]
- Acosta Quiroz, C.O.; García-Flores, R.; Echeverría-Castro, S.B. The Geriatric Depression Scale (GDS-15): Validation in Mexico and Disorder in the State of Knowledge. Int. J. Aging Hum. Dev. 2021, 93, 854–863. Available online: https://pubmed.ncbi.nlm.nih.gov/32960071/ (accessed on 14 July 2022). [CrossRef] [PubMed]
- Aguilar-Navarro, S.G.; Mimenza-Alvarado, A.J.; Samudio-Cruz, M.A.; Hernández-Contreras, F.J.; Gutiérrez-Gutiérrez, L.A.; Ramírez-González, F.; Avila-Funes, J.A. Validation of the Clock Drawing Test Scoring Method in older adults with neurocognitive disorder. Salud Ment. 2018, 41, 179–186. Available online: www.revistasaludmental.mx (accessed on 14 July 2022). [CrossRef]
- Franco-Marina, F.; García-González, J.J.; Wagner-Echeagaray, F.; Gallo, J.; Ugalde, O.; Sánchez-García, S.; Espinel-Bermúdez, C.; Juárez-Cedillo, T.; Rodríguez, M.A.; García-Peña, C. The Mini-mental State Examination revisited: Ceiling and floor effects after score adjustment for educational level in an aging Mexican population. Int. Psychogeriatr. 2010, 22, 72–81. Available online: https://pubmed.ncbi.nlm.nih.gov/19735592/ (accessed on 14 July 2022). [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.; Daly, M.J.; et al. PLINK: A toolset for whole-genome association and population-based linkage analysis. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R; Rstudio, PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 1 July 2023).
- Ortega-Vázquez, A.; Dorado, P.; Fricke-Galindo, I.; Jung-Cook, H.; Monroy-Jaramillo, N.; Martínez-Juárez, I.E.; Familiar-López, I.; Peñas-Lledó, E.; Llerena, A.; López-López, M. CYP2C9, CYP2C19, ABCB1 genetic polymorphisms and phenytoin plasma concentrations in Mexican-Mestizo patients with epilepsy. Pharmacogenomics J. 2015, 16, 286–292. Available online: http://www.ncbi.nlm.nih.gov/pubmed/26122019 (accessed on 9 July 2015). [CrossRef]
- Fricke-Galindo, I.; Ortega-Vázquez, A.; Monroy-Jaramillo, N.; Dorado, P.; Jung-Cook, H.; Peñas-Lledó, E.; Llerena, A.; López-López, M. Allele and genotype frequencies of genes relevant to anti-epileptic drug therapy in Mexican-Mestizo healthy volunteers. Pharmacogenomics 2016, 17, 1913–1930. [Google Scholar] [CrossRef]
- 1000 Genomes Project Consortium; Auton, A.; Brooks, L.D.; Durbin, R.M.; Garrison, E.P.; Kang, H.M.; Korbel, J.O.; Marchini, J.L.; McCarthy, S.; McVean, G.A.; et al. A global reference for human genetic variation. Nature 2015, 526, 68–74. Available online: https://www.nature.com/articles/nature15393 (accessed on 15 July 2022).
- Li, X.; Feng, X.; Sun, X.; Hou, N.; Han, F.; Liu, Y. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2019. Front Aging Neurosci. 2022, 14, 1120. [Google Scholar] [CrossRef]
- Rubin, L.; Ingram, L.A.; Resciniti, N.V.; Ashford-Carroll, B.; Leith, K.H.; Rose, A.; Ureña, S.; McCollum, Q.; Friedman, D.B. Genetic Risk Factors for Alzheimer’s Disease in Racial/Ethnic Minority Populations in the U.S.: A Scoping Review. Front. Public. Health 2021, 9, 2078. [Google Scholar] [CrossRef] [PubMed]
- Bellenguez, C.; Küçükali, F.; Jansen, I.E.; Kleineidam, L.; Moreno-Grau, S.; Amin, N.; Naj, A.C.; Campos-Martin, R.; Grenier-Boley, B.; Andrade, V.; et al. New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat. Genet. 2022, 54, 412–436. Available online: https://www.nature.com/articles/s41588-022-01024-z (accessed on 6 March 2023). [CrossRef] [PubMed]
- Elali, A.; Rivest, S. The role of ABCB1 and ABCA1 in beta-amyloid clearance at the neurovascular unit in Alzheimer’s disease. Front Physiol. 2013, 4, 45. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Liu, M.Y.; Sun, X.H.; Wei, M.J. Association between ABCB1 polymorphisms and haplotypes and Alzheimer’s disease: A meta-analysis. Sci. Rep. 2016, 6, 32708. Available online: https://www.nature.com/articles/srep32708 (accessed on 9 March 2023). [CrossRef]
- Ben Halla, S.; Tazzite, A.; Gazzaz, B.; Dehbi, H.; El Moutawakil, B. The impact of ABCB1 gene polymorphism (C3435T) and its expression on response to Donepezil in Moroccan patients with Alzheimer’s disease. Gene Rep. 2022, 26, 101443. [Google Scholar] [CrossRef]
- Yoon, H.; Myung, W.; Lim, S.W.W.; Kang, H.S.; Kim, S.; Won, H.H.; Carroll, B.J.; Kim, D.K. Association of the choline acetyltransferase gene with responsiveness to acetylcholinesterase inhibitors in alzheimer’s disease. Pharmacopsychiatry 2015, 48, 111–117. Available online: http://www.ncbi.nlm.nih.gov/pubmed/25730470 (accessed on 4 March 2016). [CrossRef]
- Sumirtanurdin, R.; Thalib, A.Y.; Cantona, K.; Abdulah, R. Effect of genetic polymorphisms on Alzheimer’s disease treatment outcomes: An update. Clin. Interv. Aging 2019, 14, 631. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC6445219/ (accessed on 10 March 2023). [CrossRef]
- Hálová, A.; Janoutová, J.; Ewerlingová, L.; Janout, V.; Bonczek, O.; Zeman, T.; Gerguri, T.; Balcar, V.J.; Šerý, O. CHAT gene polymorphism rs3810950 is associated with the risk of Alzheimer’s disease in the Czech population. J. Biomed. Sci. 2018, 25, 41. Available online: https://pubmed.ncbi.nlm.nih.gov/29759072/ (accessed on 10 March 2023). [CrossRef]
- Clarelli, F.; Mascia, E.; Santangelo, R.; Mazzeo, S.; Giacalone, G.; Galimberti, D.; Fusco, F.; Zuffi, M.; Fenoglio, C.; Franceschi, M.; et al. CHRNA7 Gene and Response to Cholinesterase Inhibitors in an Italian Cohort of Alzheimer’s Disease Patients. J. Alzheimer’s Dis. 2016, 52, 1203–1208. Available online: http://www.ncbi.nlm.nih.gov/pubmed/27104904 (accessed on 9 March 2018). [CrossRef]
- Russo, P.; Kisialiou, A.; Moroni, R.; Prinzi, G.; Fini, M. Effect of Genetic Polymorphisms (SNPs) in CHRNA7 Gene on Response to Acetylcholinesterase Inhibitors (AChEI) in Patients with Alzheimer’s Disease. Curr. Drug Targets 2017, 18, 1179–1190. Available online: https://pubmed.ncbi.nlm.nih.gov/26424395/ (accessed on 10 March 2023). [CrossRef] [PubMed]
- Ancín, I.; Barabash, A.; Vázquez-Álvarez, B.; Santos, J.L.; Sánchez-Morla, E.; Martínez, J.L.; Aparicio, A.; Peláez, J.C.; Díaz, J.A. Evidence for association of the non-duplicated region of CHRNA7 gene with bipolar disorder but not with Schizophrenia. Psychiatr. Genet. 2010, 20, 289–297. Available online: https://pubmed.ncbi.nlm.nih.gov/20463630/ (accessed on 10 March 2023). [CrossRef] [PubMed]
- Calabrò, M.; Mandelli, L.; Crisafulli, C.; Sidoti, A.; Jun, T.Y.; Lee, S.J.; Han, C.; Patkar, A.A.; Masand, P.S.; Pae, C.U.; et al. Genes Involved in Neurodevelopment, Neuroplasticity, and Bipolar Disorder: CACNA1C, CHRNA1, and MAPK1. Neuropsychobiology 2016, 74, 159–168. Available online: https://pubmed.ncbi.nlm.nih.gov/28494468/ (accessed on 10 March 2023). [CrossRef] [PubMed]
- Carson, R.; Craig, D.; Hart, D.; Todd, S.; McGuinness, B.; Johnston, J.A.; O’Neill, F.A.; Ritchie, C.W.; Passmore, A.P. Genetic variation in the alpha 7 nicotinic acetylcholine receptor is associated with delusional symptoms in Alzheimer’s disease. Neuromolecular Med. 2008, 10, 377–384. Available online: https://pubmed.ncbi.nlm.nih.gov/18696274/ (accessed on 10 March 2023). [CrossRef]
- Weng, P.H.; Chen, J.H.; Chen, T.F.; Sun, Y.; Wen, L.L.; Yip, P.K.; Chu, Y.M.; Chen, Y.C. CHRNA7 Polymorphisms and Dementia Risk: Interactions with Apolipoprotein ε4 and Cigarette Smoking. Sci. Rep. 2016, 6, 27231. Available online: https://www.nature.com/articles/srep27231 (accessed on 10 March 2023). [CrossRef]
- Woodland, C.; Huang, T.T.; Gryz, E.; Bendayan, R.; Fawcett, J.P. Expression, Activity and Regulation of CYP3A in Human and Rodent Brain. Drug Metab. Rev. 2008, 40, 149–168. [Google Scholar] [CrossRef]
- Rosales-Rimache, J.; Machado-Pereyra, P.; Bendezu-Quispe, G. Relationship between Butyrylcholinesterase Activity and Cognitive Ability in Workers Exposed to Chlorpyrifos. Safety 2023, 9, 12. Available online: https://www.mdpi.com/2313-576X/9/1/12/htm (accessed on 6 March 2023). [CrossRef]
- Ovejero-benito, M.C.; Ochoa, D.; Enrique-benedito, T.; Del Peso-Casado, M.; Zubiaur, P.; Navares, M.; Román, M.; Abad-Santos, F. Pharmacogenetics of Donepezil and Memantine in Healthy Subjects. J. Pers. Med. 2022, 12, 788. Available online: https://pubmed.ncbi.nlm.nih.gov/35629210/ (accessed on 23 March 2023). [CrossRef]
- Chamnanphon, M.; Wainipitapong, S.; Wiwattarangkul, T.; Chuchuen, P.; Nissaipan, K.; Phaisal, W.; Tangwongchai, S.; Sukasem, C.; Wittayalertpanya, S.; Gaedigk, A.; et al. CYP2D6 Predicts Plasma Donepezil Concentrations in a Cohort of Thai Patients with Mild to Moderate Dementia. Pharmgenomics Pers. Med. 2020, 13, 543–551. Available online: https://www.dovepress.com/cyp2d6-predicts-plasma-donepezil-concentrations-in-a-cohort-of-thai-pa-peer-reviewed-fulltext-article-PGPM (accessed on 23 March 2023). [CrossRef]

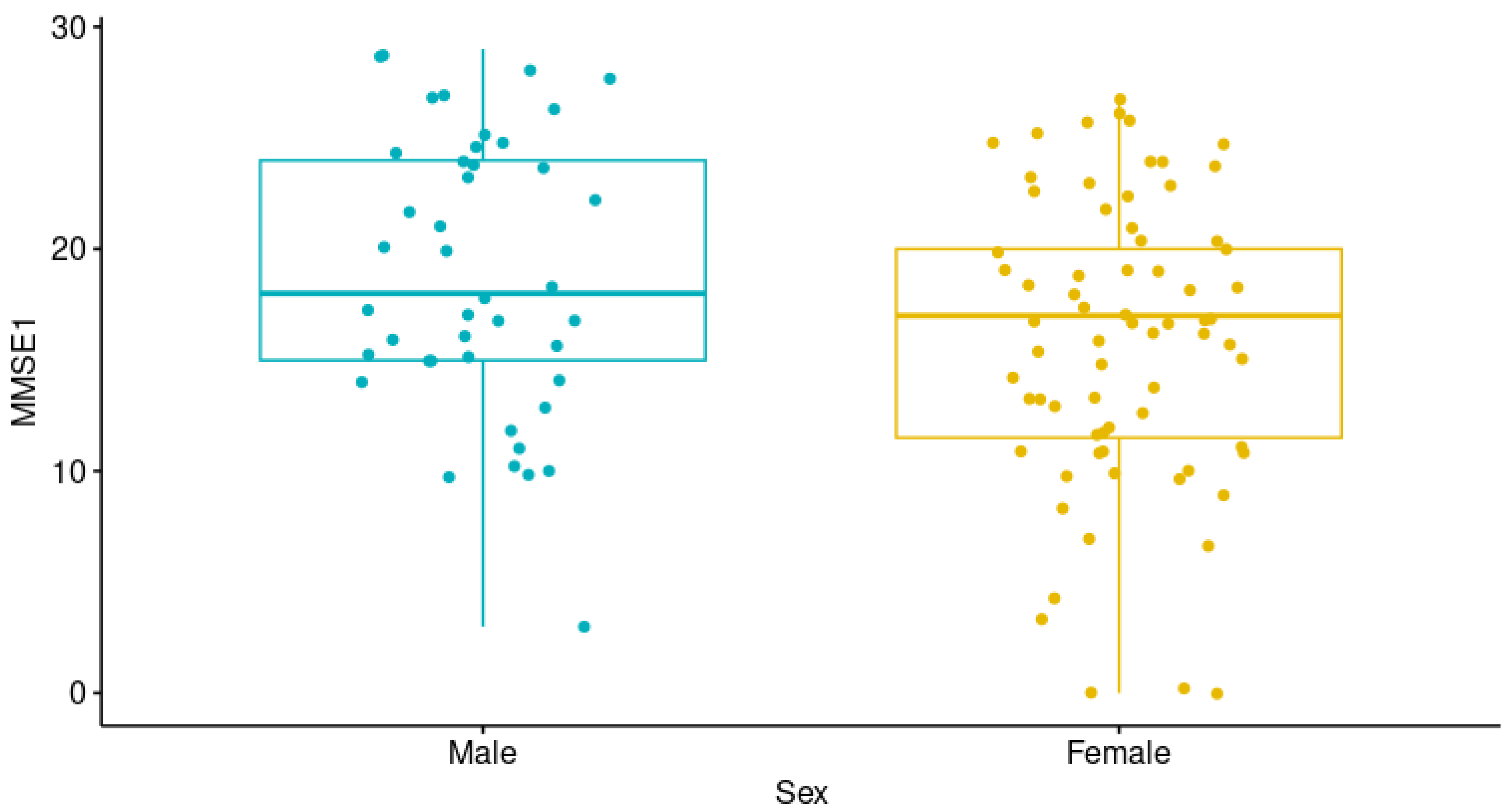
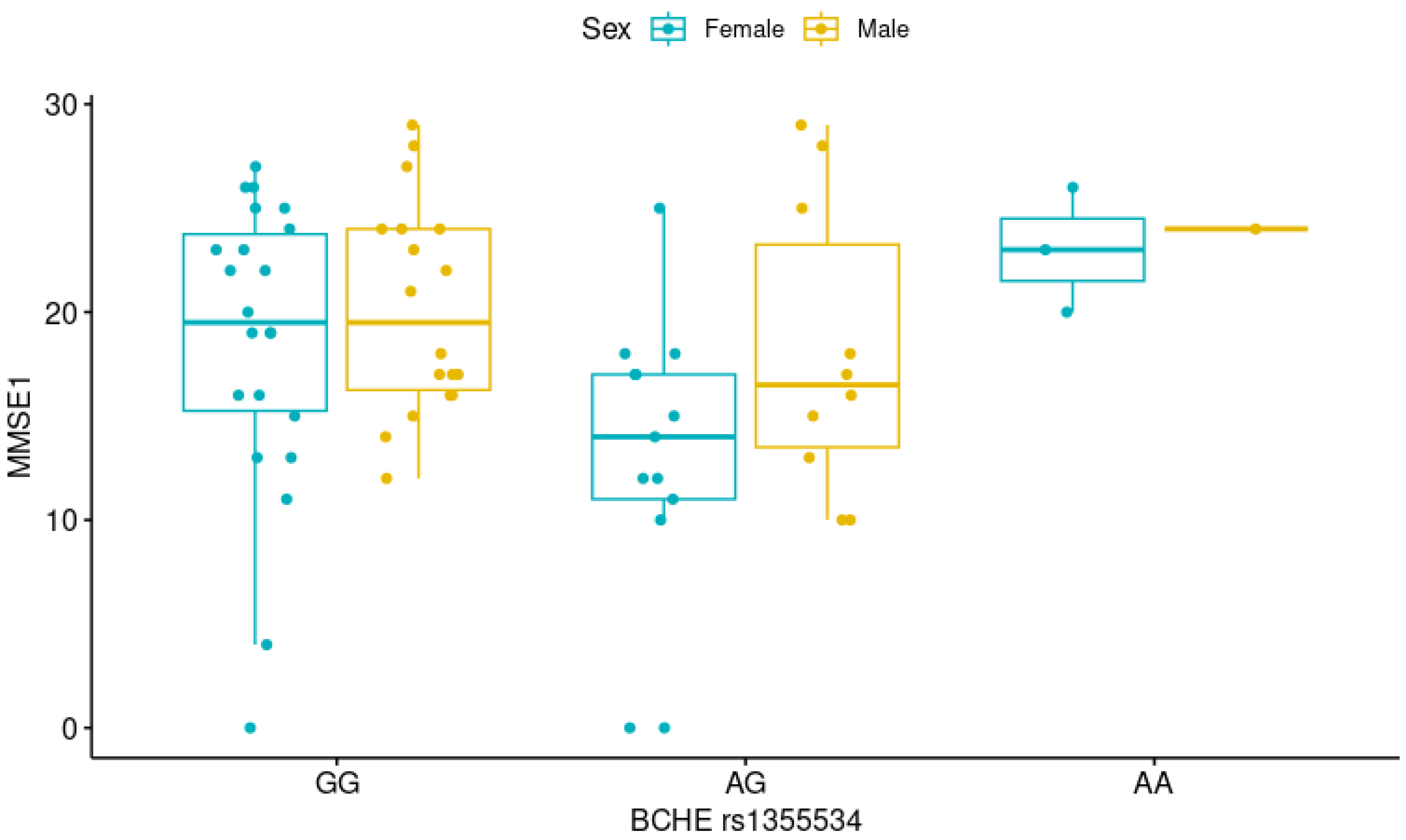
| Variable | Median (Interquartile Range (IQR)) |
|---|---|
| Age (years) | 64 (56–74) |
| Age at onset (years) | 59 (51–69) |
| Years of disease evolution | 5 (3–7) |
| Years of education | 9 (5–14) |
| Neuropsychological tests | |
| Time 1 a | |
| MMSE-1 | 17 (12–23) |
| SVF-1 | 7 (4–11) |
| PVF-1 | 3 (1–7) |
| CLOCK-1 | 0 (0–2) |
| GDS-1 | 4 (3–4) |
| Lawton Brody-1 | 4 (0–4) |
| Time 2 b | |
| MMSE-2 | 15 (10–20) |
| SVF-2 | 6 (3–10) |
| PVF-2 | 3 (0–6) |
| CLOCK-2 | 0 [0–1] |
| GDS-2 | 4 [3–5] |
| Lawton Brody-2 | 2 [0–4] |
| Gender male/female n (%) | 43 (36.8)/74 (63.2) |
| AD sporadic/familial | 49 (41.90)/68 (58.10) |
| AD early/late onset | 72 (61.50)/45 (38.50) |
| Co-morbidities | |
| Depression | 81 (69.20) |
| Hypertension | 39 (33.30) |
| Diabetes mellitus | 18 (15.40) |
| Treatment | |
| Monotherapy (DPZ, GAL, RIV, or MEM) | 80 (68.40) |
| DPZ + MEM | 20 (17.10) |
| GAL + MEM | 12 (10.30) |
| RIV + MEM | 5 (4.30) |
| Co-treatment | |
| Antidepressant | 81 (69.20) |
| Antipsychotic | 32 (27.40) |
| Responders/non-responders | 58 (49.60)/59 (50.40) |
| ADR/no ADR | 16 (13.70)/101 (86.30) |
| Gene | NCBI Reference | TaqMan Assay |
|---|---|---|
| ABCB1 | rs1128503 | C___7586662_10 |
| rs2032582 | C_11711720C_30 C_11711720D_40 | |
| rs1045642 | C__7586657_20 | |
| ACHE | rs1799806 | C_27168953_30 |
| rs17884589 | C_34446515_10 | |
| rs10953305 | C_2607820_20 | |
| BCHE | rs1803274 | C_27479669_20 |
| rs1355534 | C_8834703_20 | |
| CHAT | rs2177370 | C_224405_10 |
| rs3793790 | C_122323_20 | |
| CYP3A5 | rs776746 (CYP3A5*3) | C_26201809_30 |
| rs10264272 (CYP3A5*6) | C__30203950_10 | |
| NR1I2 | rs2461817 | C__15803606_20 |
| rs7643645 | C___1834250_10 | |
| rs3814055 | C__27504984_30 | |
| rs2276707 | C__15882324_10 | |
| rs3814058 | C__11231740_10 | |
| CHRNA7 | rs6494223 | C___1483016_10 |
| POR | rs1057868 | C___8890131_30 |
| Variable | Non-Responders n = 58 (%) | Responders n = 59 (%) | p-Value |
|---|---|---|---|
| Age, yrs | 61.5 ([55–71.5) | 67 (57–76) | 0.081 |
| Onset age, yrs | 56 (50.3–66.8) | 60 (53.5–69.5) | 0.148 |
| Scholarship, yrs | 9 (6–14) | 9 (4–15) | 0.363 |
| Sex | |||
| Male | 20 (34.5) | 23 (39.0) | 0.702 |
| Female | 38 (65.5) | 36 (61.0) | |
| AD type | |||
| Sporadic | 20 (34.5) | 29 (49.1) | 0.135 |
| Familial | 38 (65.5) | 30 (50.8) | |
| Onset AD | |||
| Early | 38 (65.5) | 34 (57.6) | 0.449 |
| Late | 20 (34.5) | 25 (42.4) | |
| Co-treatment | 0.333 | ||
| Monotherapy | 36 (62.1) | 43 (72.9) | 0.240 |
| DPZ + MEM | 13 (22.4) | 7 (11.9) | 0.148 |
| GAL + MEM | 5 (8.6) | 7 (11.9) | 0.762 |
| RIV + MEM | 4 (6.9) | 2 (3.4) | 0.439 |
| Comorbidities | |||
| Depression | 40 (69.0) | 42 (71.2) | 0.842 |
| SAH | 16 (27.6) | 23 (39.0) | 0.240 |
| T2DM | 7 (12.1) | 11 (18.6) | 0.443 |
| Antidepressant | |||
| Citalopram | 20 (34.5) | 24 (40.7) | 0.514 |
| Escitalopram | 5 (8.6) | 9 (1.5) | |
| None | 20 (34.5) | 16 (27.1) | |
| Other | 13 (22.4) | 10 (16.9) | |
| Antipsychotic | 19 (32.8) | 13 (22.0) | 0.218 |
| Neuropsychological tests | |||
| MMSE1 | 17 (12–22) | 17 (13–22) | 0.511 |
| SVF1 | 7 (3–10) | 7 (4–12) | 0.494 |
| PVF1 | 3 (1–6) | 4 (1–8.5) | 0.180 |
| CLOCK1 | 0 (0–1) | 0 (0–2) | 0.283 |
| GDS1 | 4 (3–5) | 3 (3–4) | 0.008 |
| Lawton–Brody 1 | 2 (0–4) | 4 (2–6) | 0.005 |
| KATZ1 | |||
| A-B | 27 (46.5) | 42 (71.2) | 0.022 |
| C-D | 27 (46.5) | 15 (25.4) | |
| E-F | 4 (7.0) | 2 (3.4) | |
| MMSE2 | 10 (7–16) | 20 (13–24) | <0.001 |
| SVF2 | 4 (0–7) | 7 (4–12) | <0.001 |
| PVF2 | 1 (0–3) | 4 (1–8) | 0.001 |
| CLOCK2 | 0 (0–0) | 0 (0–1) | 0.003 |
| GDS2 | 4 (4–5) | 3 (3–4) | <0.001 |
| Lawton–Brody 2 | 0 (0–2) | 3 (2–6) | <0.001 |
| KATZ2 | |||
| A-B | 20 (34.5) | 40 (67.8) | 0.001 |
| C-D | 27 (46.5) | 14 (23.7) | |
| E-F | 11 (19.0) | 5 (8.5) |
| Gene | Variant | MAF/AD | MAF/MM | Reference | p Value | OR (95% CI) |
|---|---|---|---|---|---|---|
| APOE | rs7412 (ε4) | 0.106 | 0.085 | [36] | 0.523 | - |
| ABCB1 | rs1128503 | 0.492 | 0.49 | [33] | 0.985 | - |
| rs1045642 | 0.613 | 0.49 | 0.014 | 1.34 (1.10–2.43) | ||
| rs2032582 (A/T) | 0.040/0.395 | 0.07/0.42 | 0.221 | - | ||
| ACHE | rs1799806 | 0.239 | 0.172 | Present work | 0.07 | - |
| rs17884589 | 0.261 | 0.147 | 0.001 | 2.08 (1.33–3.25) | ||
| rs10953305 | 0.194 | 0.243 | 0.223 | - | ||
| BCHE | rs1803274 | 0.142 | 0.11 | Present work | 0.298 | - |
| rs1355534 | 0.231 | 0.312 | 0.066 | - | ||
| CHAT | rs2177370 | 0.397 | 0.285 | Present work | 0.01 | 1.65 (1.12–2.43) |
| rs3793790 | 0.213 | 0.140 | 0.032 | 1.66 (1.04–2.66) | ||
| CHRNA7 | rs6494223 | 0.333 | 0.471 | Present work | <0.001 | 0.56 (0.41–0.77) |
| POR | rs1057868 | 0.275 | 0.259 | Present work | 0.704 | - |
| CYP3A5 | rs776746 | 0.135 | 0.263 | [34] | 0.003 | 0.44 (0.25–0.77) |
| rs10264272 | 0.025 | 0.005 | 0.094 | - | ||
| NR1I2 | rs2461817 | 0.391 | 0.348 | [34] | 0.288 | - |
| rs7643645 | 0.446 | 0.387 | 0.153 | - | ||
| rs2276707 | 0.141 | 0.175 | 0.284 | - | ||
| rs3814055 | 0.429 | 0.407 | 0.398 | - | ||
| rs3814058 | 0.141 | 0.175 | 0.284 | - |
| Genetic Variant (Allele/Genotype) | MAF Non-Responders | MAF Responders | p Value | p Value Adjusted for Sex |
|---|---|---|---|---|
| Donepezil | n = 22 | n = 22 | ||
| APOE rs7412 (ε4) | 0.068 | 0.105 | 0.839 | 0.768 |
| ABCB1 | ||||
| rs1128503 | 0.500 | 0.500 | 1.000 | 0.969 |
| rs1045642 | 0.476 | 0.316 | 0.144 | 0.185 |
| rs2032582 (A/T) | 0.500 | 0.395 | 0.345 | 0.420 |
| ACHE | ||||
| rs1799806 | 0.273 | 0.159 | 0.195 | 0.992 |
| rs17884589 | 0.182 | 0.250 | 0.437 | 0.769 |
| rs10953305 | 0.295 | 0.136 | 0.070 | 0.999 |
| BCHE | ||||
| rs1803274 | 0.114 | 0.250 | 0.097 | 0.680 |
| rs1355534 | 0.227 | 0.182 | 0.597 | 0.844 |
| CHAT | ||||
| rs2177370 | 0.341 | 0.409 | 0.509 | 0.391 |
| rs3793790 | 0.227 | 0.182 | 0.597 | 0.900 |
| CHRNA7 | ||||
| rs6494223 | 0.318 | 0.386 | 0.503 | 0.161 |
| POR | ||||
| rs1057868 | 0.318 | 0.159 | 0.080 | 0.513 |
| CYP3A5 | ||||
| rs776746 | 0.114 | 0.159 | 0.534 | 0.484 |
| rs10264272 | 0.023 | 0.045 | 0.557 | 0.541 |
| NR1I2 | ||||
| rs2461817 | 0.341 | 0.454 | 0.276 | 0.488 |
| rs7643645 | 0.432 | 0.545 | 0.286 | 0.529 |
| rs3814055 | 0.386 | 0.523 | 0.199 | 0.301 |
| rs2276707 | 0.091 | 0.114 | 0.725 | 0.767 |
| rs3814058 | 0.091 | 0.114 | 0.725 | 0.767 |
| Galantamine | n = 9 | n = 8 | ||
| APOE rs7412 (ε4) | 0.062 | 0.250 | 0.330 | 0.362 |
| ABCB1 | ||||
| rs1128503 | 0.500 | 0.500 | 1.000 | 0.566 |
| rs1045642 | 0.357 | 0.429 | 0.144 | 0.699 |
| rs2032582 (A/T) | 0.357 | 0.429 | 0.699 | 0.420 |
| ACHE | ||||
| rs1799806 | 0.143 | 0.437 | 0.079 | 0.286 |
| rs17884589 | 0.429 | 0.312 | 0.510 | 0.304 |
| rs10953305 | 0.000 | 0.125 | 0.171 | 0.999 |
| BCHE | ||||
| rs1803274 | 0.071 | 0.000 | 0.277 | NA |
| rs1355534 | 0.286 | 0.437 | 0.389 | 0.836 |
| CHAT | ||||
| rs2177370 | 0.437 | 0.437 | 1.000 | 1.000 |
| rs3793790 | 0.125 | 0.187 | 0.626 | 0.714 |
| CHRNA7 | ||||
| rs6494223 | 0.312 | 0.437 | 0.645 | 0.871 |
| POR | ||||
| rs1057868 | 0.312 | 0.437 | 0.465 | 0.871 |
| CYP3A5 | ||||
| rs776746 | 0.143 | 0.125 | 0.886 | 0.906 |
| rs10264272 | 0.000 | 0.000 | NA | NA |
| Rivastigmine | n = 5 | n = 3 | ||
| APOE rs7412 (ε4) | 0.200 | 0.000 | 0.696 | NA |
| ABCB1 | ||||
| rs1128503 | 0.500 | 0.333 | 0.492 | 1.000 |
| rs1045642 | 0.429 | 0.167 | 0.260 | 0.618 |
| rs2032582 (A/T) | 0.400 | 0.167 | 0.699 | 0.676 |
| ACHE | ||||
| rs1799806 | 0.214 | 0.167 | 0.807 | 0.718 |
| rs17884589 | 0.286 | 0.167 | 0.573 | 0.987 |
| rs10953305 | 0.286 | 0.167 | 0.573 | 1.000 |
| BCHE | ||||
| rs1803274 | 0.214 | 0.167 | 0.807 | 0.811 |
| rs1355534 | 0.071 | 0.167 | 0.515 | 0.547 |
| CHAT | ||||
| rs2177370 | 0.429 | 0.333 | 0.690 | 0.997 |
| rs3793790 | 0.357 | 0.167 | 0.394 | 0.934 |
| CHRNA7 | ||||
| rs6494223 | 0.312 | 0.437 | 0.645 | 0.871 |
| Memantine | n = 22 | n = 26 | ||
| CHRNA7 | ||||
| rs6494223 | 0.319 | 0.337 | 0.813 | 0.731 |
| NR1I2 | ||||
| rs2461817 | 0.419 | 0.370 | 0.517 | 0.731 |
| rs7643645 | 0.419 | 0.402 | 0.827 | 0.371 |
| rs3814055 | 0.351 | 0.413 | 0.417 | 0.480 |
| rs2276707 | 0.122 | 0.174 | 0.349 | 0.955 |
| rs3814058 | 0.122 | 0.174 | 0.349 | 0.955 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zúñiga-Santamaría, T.; Pérez-Aldana, B.E.; Fricke-Galindo, I.; González-González, M.; Trujillo-de los Santos, Z.G.; Boll-Woehrlen, M.C.; Rodríguez-García, R.; López-López, M.; Yescas-Gómez, P. Variants in Neurotransmitter-Related Genes Are Associated with Alzheimer’s Disease Risk and Cognitive Functioning but Not Short-Term Treatment Response. Neurol. Int. 2025, 17, 65. https://doi.org/10.3390/neurolint17050065
Zúñiga-Santamaría T, Pérez-Aldana BE, Fricke-Galindo I, González-González M, Trujillo-de los Santos ZG, Boll-Woehrlen MC, Rodríguez-García R, López-López M, Yescas-Gómez P. Variants in Neurotransmitter-Related Genes Are Associated with Alzheimer’s Disease Risk and Cognitive Functioning but Not Short-Term Treatment Response. Neurology International. 2025; 17(5):65. https://doi.org/10.3390/neurolint17050065
Chicago/Turabian StyleZúñiga-Santamaría, Tirso, Blanca Estela Pérez-Aldana, Ingrid Fricke-Galindo, Margarita González-González, Zoila Gloria Trujillo-de los Santos, Marie Catherine Boll-Woehrlen, Rosalía Rodríguez-García, Marisol López-López, and Petra Yescas-Gómez. 2025. "Variants in Neurotransmitter-Related Genes Are Associated with Alzheimer’s Disease Risk and Cognitive Functioning but Not Short-Term Treatment Response" Neurology International 17, no. 5: 65. https://doi.org/10.3390/neurolint17050065
APA StyleZúñiga-Santamaría, T., Pérez-Aldana, B. E., Fricke-Galindo, I., González-González, M., Trujillo-de los Santos, Z. G., Boll-Woehrlen, M. C., Rodríguez-García, R., López-López, M., & Yescas-Gómez, P. (2025). Variants in Neurotransmitter-Related Genes Are Associated with Alzheimer’s Disease Risk and Cognitive Functioning but Not Short-Term Treatment Response. Neurology International, 17(5), 65. https://doi.org/10.3390/neurolint17050065






