Effectiveness of Medilac-S as an Adjuvant to Conventional Irritable Bowel Syndrome Treatments: A Systematic Review with Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
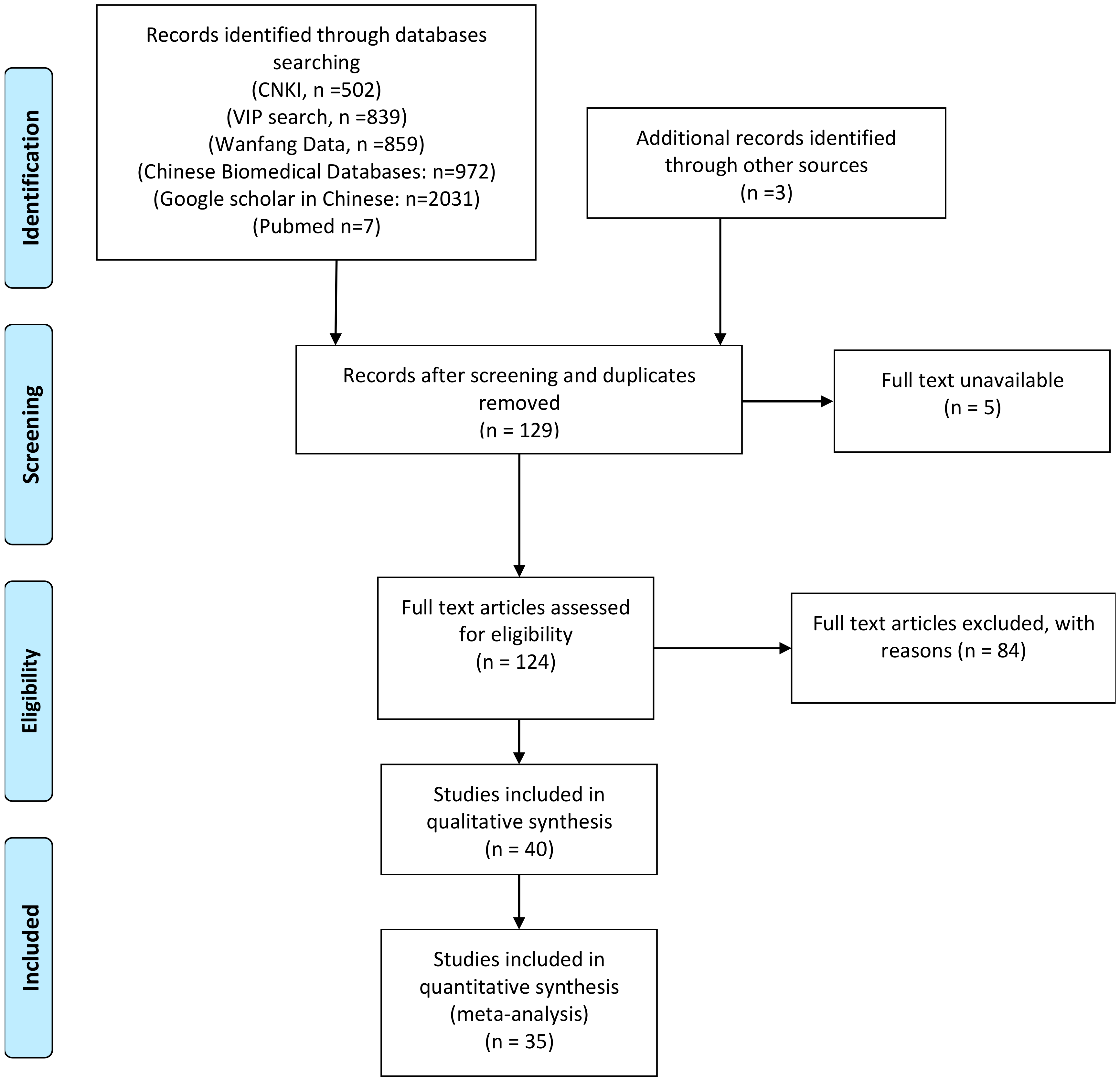
2.1. Systematic Search and Screening of Studies
2.2. Data Synthesis and Statistical Analyses
2.3. Quality Assessment of Studies
3. Results
3.1. Study Design and Population Characteristics
3.2. Probiotic Regimen and Standard IBS Medications
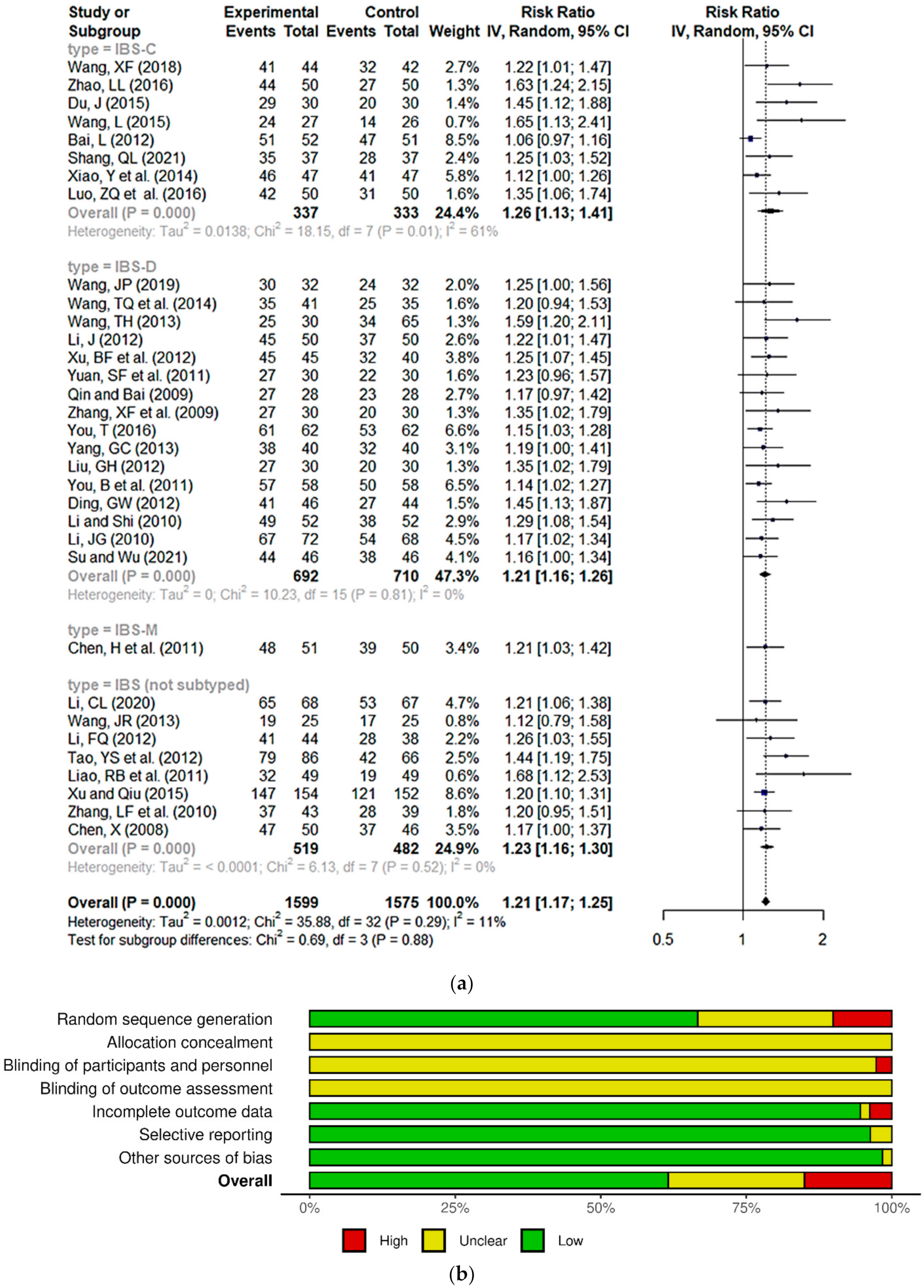
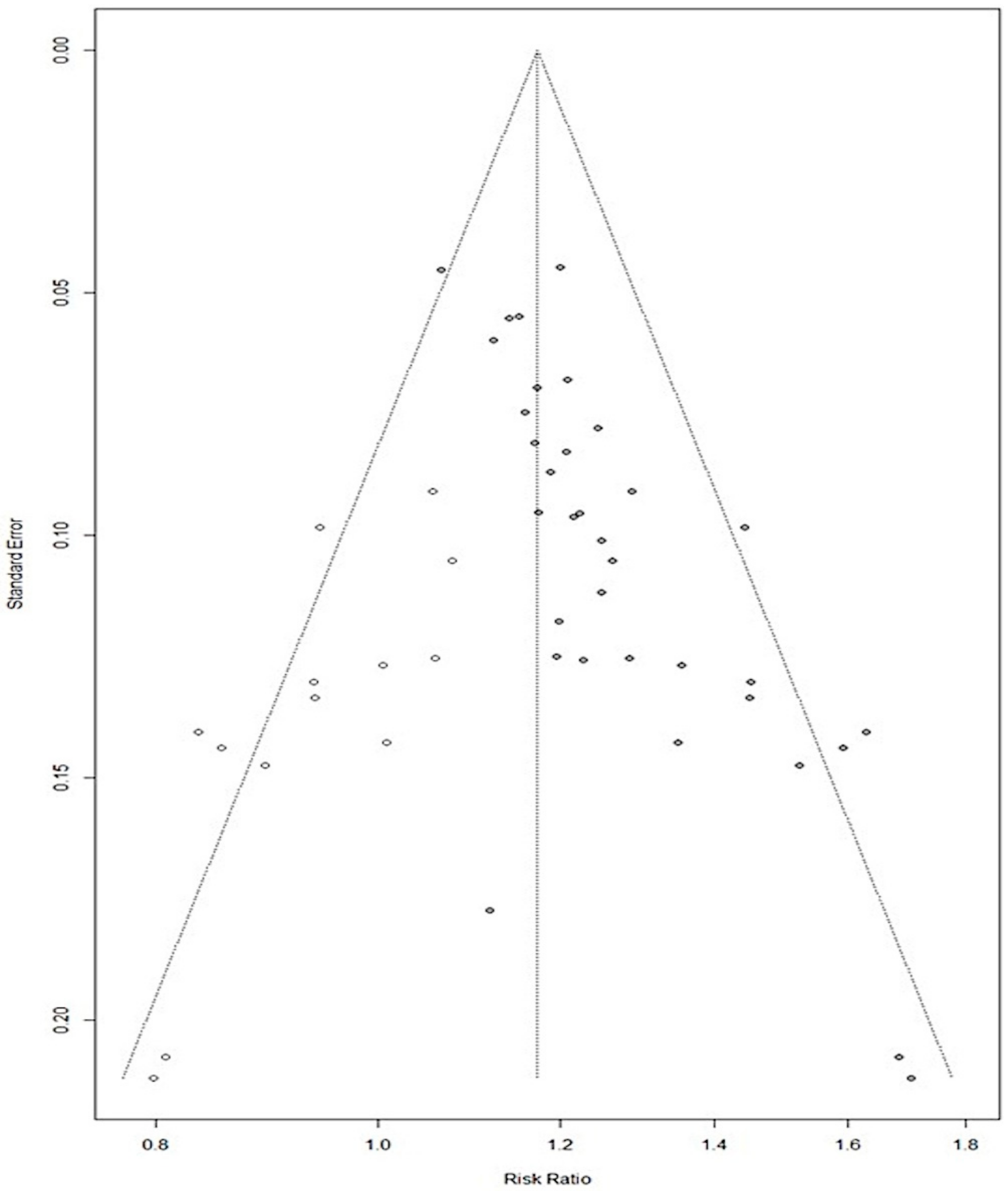
3.3. Meta-Analysis and Risk of Bias Analysis
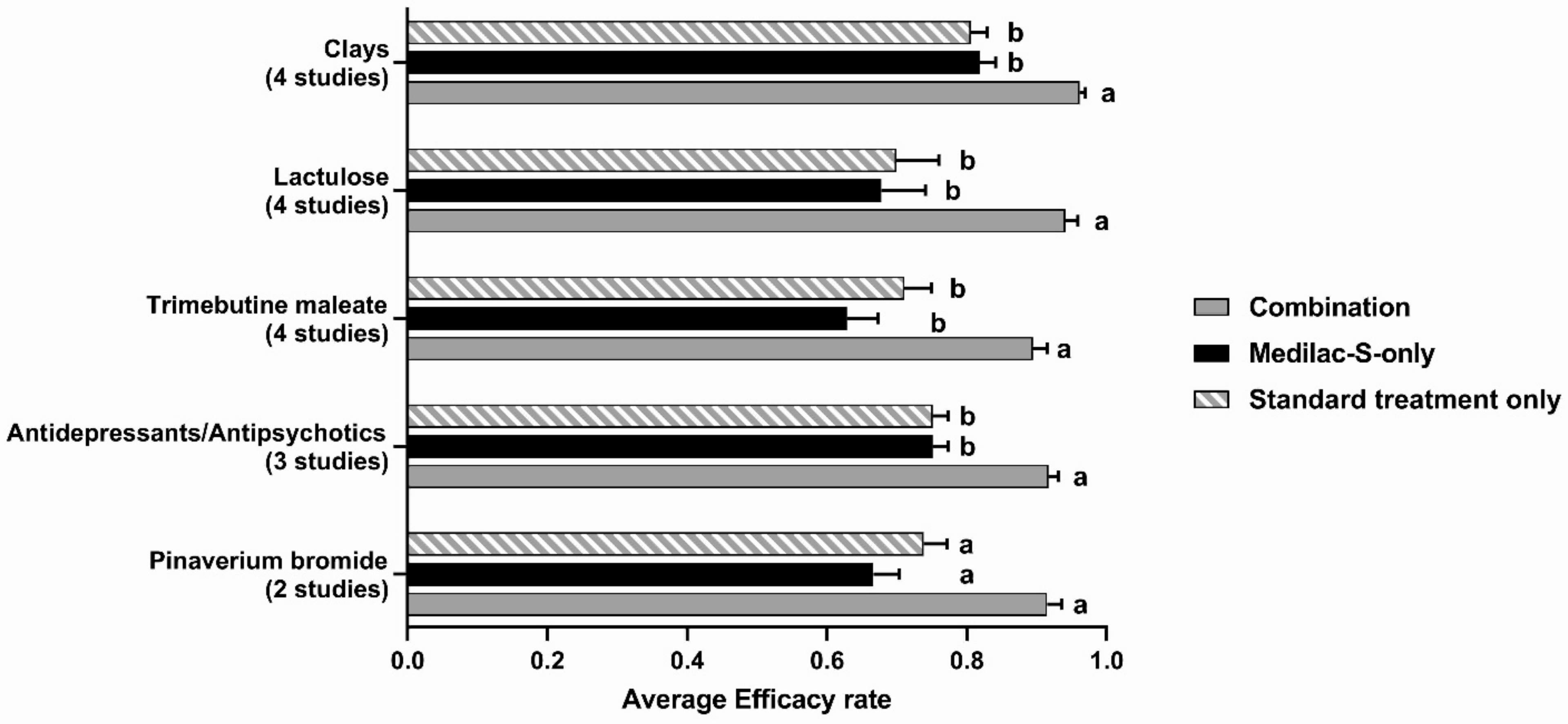
3.4. Other Outcome Measures of Efficacy
3.5. Subgroup Analysis of Studies with a Medilac-S-Only Arm
3.6. Safety and Adverse Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmulson, M.J.; Drossman, D.A. What Is New in Rome IV. J. Neurogastroenterol. Motil. 2017, 23, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M. Diagnosis and Treatment of Irritable Bowel Syndrome: A Review. JAMA 2021, 325, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Drossman, D.A. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features, and Rome IV. Gastroenterology 2016, 150, 1262–1279.e1262. [Google Scholar] [CrossRef] [PubMed]
- Sperber, A.D.; Bangdiwala, S.I.; Drossman, D.A.; Ghoshal, U.C.; Simren, M.; Tack, J.; Whitehead, W.E.; Dumitrascu, D.L.; Fang, X.; Fukudo, S.; et al. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology 2021, 160, 99–114.e113. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Liu, J.-S. Irritable bowel syndrome in China: A review on the epidemiology, diagnosis, and management. Chin. Med. J. 2021, 134, 1396–1401. [Google Scholar] [CrossRef] [PubMed]
- Goodoory, V.C.; Houghton, L.A.; Yiannakou, Y.; Black, C.J.; Ford, A.C. Natural History and Disease Impact of Rome IV vs. Rome III Irritable Bowel Syndrome: A Longitudinal Follow-Up Study. Clin. Gastroenterol. Hepatol. 2021, 20, 569–577.e3. [Google Scholar] [CrossRef] [PubMed]
- Vork, L.; Weerts, Z.; Mujagic, Z.; Kruimel, J.W.; Hesselink, M.A.M.; Muris, J.W.M.; Keszthelyi, D.; Jonkers, D.; Masclee, A.A.M. Rome III vs. Rome IV criteria for irritable bowel syndrome: A comparison of clinical characteristics in a large cohort study. Neurogastroenterol. Motil. 2018, 30, e13189. [Google Scholar] [CrossRef] [PubMed]
- Barberio, B.; Houghton, L.A.; Yiannakou, Y.; Savarino, E.V.; Black, C.J.; Ford, A.C. Symptom Stability in Rome IV vs. Rome III Irritable Bowel Syndrome. Off. J. Am. Coll. Gastroenterol. 2021, 116, 362–371. [Google Scholar] [CrossRef]
- Palsson, O.S.; Whitehead, W.; Törnblom, H.; Sperber, A.D.; Simren, M. Prevalence of Rome IV Functional Bowel Disorders Among Adults in the United States, Canada, and the United Kingdom. Gastroenterology 2020, 158, 1262–1273.e1263. [Google Scholar] [CrossRef]
- Ghoshal, U.C. Pros and Cons While Looking Through an Asian Window on the Rome IV Criteria for Irritable Bowel Syndrome: Pros. J. Neurogastroenterol. Motil. 2017, 23, 334–340. [Google Scholar] [CrossRef]
- Jung, K.W.; Myung, S.-J. An Asian perspective on irritable bowel syndrome. Intest. Res. 2022, 21, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Loosen, S.H.; Kostev, K.; Jördens, M.S.; Luedde, T.; Roderburg, C. Overlap between irritable bowel syndrome and common gastrointestinal diagnoses: A retrospective cohort study of 29,553 outpatients in Germany. BMC Gastroenterol. 2022, 22, 48. [Google Scholar] [CrossRef] [PubMed]
- Black, C.J.; Ford, A.C. Best management of irritable bowel syndrome. Frontline Gastroenterol. 2021, 12, 303–315. [Google Scholar] [CrossRef]
- Cangemi, D.J.; Lacy, B.E. Management of irritable bowel syndrome with diarrhea: A review of nonpharmacological and pharmacological interventions. Ther. Adv. Gastroenterol. 2019, 12, 1756284819878950. [Google Scholar] [CrossRef] [PubMed]
- Iacovou, M.; Tan, V.; Muir, J.G.; Gibson, P.R. The Low FODMAP Diet and Its Application in East and Southeast Asia. J. Neurogastroenterol. Motil. 2015, 21, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Ireton-Jones, C. The low FODMAP diet: Fundamental therapy in the management of irritable bowel syndrome. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 414–419. [Google Scholar] [CrossRef]
- Li, C.Y.; Li, S.C. Treatment of irritable bowel syndrome in China: A review. World J. Gastroenterol. 2015, 21, 2315–2322. [Google Scholar] [CrossRef] [PubMed]
- Gwee, K.A.; Gonlachanvit, S.; Ghoshal, U.C.; Chua, A.S.B.; Miwa, H.; Wu, J.; Bak, Y.-T.; Lee, O.Y.; Lu, C.-L.; Park, H.; et al. Second Asian Consensus on Irritable Bowel Syndrome. J. Neurogastroenterol. Motil. 2019, 25, 343–362. [Google Scholar] [CrossRef]
- Marlicz, W.; Skonieczna-Żydecka, K.; Krynicka, P.; Łoniewski, I.; Rydzewska, G. Probiotics in irritable bowel syndrome-is the quest for the right strain over? Rapid review of existing guidelines and recommendations. Prz. Gastroenterol. 2021, 16, 369–382. [Google Scholar] [CrossRef]
- Vasant, D.H.; Paine, P.A.; Black, C.J.; Houghton, L.A.; Everitt, H.A.; Corsetti, M.; Agrawal, A.; Aziz, I.; Farmer, A.D.; Eugenicos, M.P.; et al. British Society of Gastroenterology guidelines on the management of irritable bowel syndrome. Gut 2021, 70, 1214–1240. [Google Scholar] [CrossRef]
- Chen, L.-J.; Liu, L.-L.; Hu, Z.-B.; Lu, H.-B. Meta Analysis of the therapeutic effects of Medilac-S on IBS. China Pharm. 2012, 23, 2857–2860. [Google Scholar]
- Sohail, G.; Xu, X.; Christman, M.C.; Tompkins, T.A. Probiotic Medilac-S(®) for the induction of clinical remission in a Chinese population with ulcerative colitis: A systematic review and meta-analysis. World J. Clin. Cases 2018, 6, 961–984. [Google Scholar] [CrossRef] [PubMed]
- Balduzzi, S.; Rucker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, G. Meta: An R Package for Meta-Analysis. R. News 2007, 7, 40–45. Available online: http://cran.r-project.org/doc/Rnews/Rnews_2007-3.pdf (accessed on 20 October 2023).
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Zhu, Y.; Meng, X.; Tan, C.; Yin, H. Effect of probiotics on constipation-induced irritable bowel syndrome and its effect on plasma gastrointestinal hormone levels. Mod. Dig. Interv. 2016, 21. Available online: https://m.fx361.com/news/2016/0909/17565121.html (accessed on 20 October 2023).
- Wang, X. Clinical observation of lactulose combined with probiotics on constipation-type irritable bowel syndrome. China Naturop. 2018, 26. [Google Scholar] [CrossRef]
- Zhao, L. Analysis on the effectiveness and safety of combined use of Mechangan and lactulose in the treatment of constipated irritable bowel syndrome. Contemp. Med. Symp. 2016, 14, 11–12. [Google Scholar]
- Du, J. Clinical observation of Medilac-S combined with lactulose in the treatment of constipation-type irritable bowel syndrome. Chin. J. Integr. Trad. West. Med. Dig. 2015, 23. [Google Scholar] [CrossRef]
- Wang, L.; Gao, H.; Yao, P. Mechangan combined with lactulose treatment Observation of Therapeutic Effect of Elderly Irritable Bowel Syndrome with Constipation. Chin. J. Microecol. 2015, 27. Available online: http://www.cqvip.com/qk/96631x/2015008/665887940.html (accessed on 20 October 2023).
- Bai, L. Clinical Observation on Medilac-S Enteric-coated Capsule Combined with Lactulose in the Treatment of Constipation Type Irritable Bowel Syndrome. China Pharm. 2012, 23. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, Y.; Han, X.; Zhu, Z. Mechangan combined with mosapride in the treatment of irritable bowel syndrome with constipation in the elderly. Contemp. Med. Forum 2014, 12. Available online: http://www.cqvip.com/QK/60533A/201417/81898765504849524955484852.html (accessed on 20 October 2023).
- Shang, Q. Bacillus subtilis double live bacteria enteric-coated capsules combined with mosapride and lactulose oral solution The effect of treating constipation-type irritable bowel syndrome. Henan Med. Res. 2021, 30. [Google Scholar] [CrossRef]
- Yang, D.; Li, F.; Kang, Y.; Yu, L.; Zhao, J.; Zhang, H.; Ma, X.; Wei, L.S.; Liu, X.; Ren, Z.; et al. Effect of lactulose combined with microecological preparation on constipation-type irritable bowel syndrome Observation of curative effect. Chin. J. Clin. Gastroenterol. 2016, 28, 32. [Google Scholar]
- Sun, M.; Chen, Z.; Zheng, Y. Observation of the Effects of Lactulose Combined with Medilac-S in the Treatment of Irritable Bowel Syndrome with Constipation. 2013. Available online: http://www.cqvip.com/QK/88776X/201312/46095928.html (accessed on 20 October 2023).
- Xu, J.; Cheng, J.; Du, Y.; Hu, S. Lactulose Combined with Bacillus subtilis Double Live Bacteria Therapy Clinical Evaluation of Constipation-Type Irritable Bowel Syndrome. 2014. Available online: http://www.cqvip.com/qk/88515X/201422/661904551.html (accessed on 20 October 2023).
- Wang, J. Treatment of Bacillus subtilis double live bacteria enteric-coated capsules combined with trimebutine maleate Observation of Therapeutic Effect of Diarrheal Irritable Bowel Syndrome. Chin. J. Clin. Ration. Drug Use 2019, 12. [Google Scholar] [CrossRef]
- Wang, T.; Yin, S.; Zhang, R. Clinical analysis of Medilac-S combined with trimebutin in the treatment of diarrhea-predominant irritable bowel syndrome. In Proceedings of the 6th Chinese Medical Doctor Association Infectious Diseases Physician Conference and Infectious Disease Diagnosis and Treatment Summit Forum, the 14th Chinese Preventive Medicine Association Microecology Academic Conference, Dalian, China, 9 October 2014. [Google Scholar]
- Wang, T.-H. A Clinical Observation of Diarrhea-predominant Irritable Bowel Syndrome Treated by Medilac-S Capsules Combined with Trimebutine Maleate. Chin. J. Med. 2013, 11, 474–479. Available online: http://www.cqvip.com/QK/86373X/201302/44729714.html (accessed on 20 October 2023).
- Li, J. Clinical observation of combined application of Deshute and Medilac-S in the treatment of diarrhea-type IBS. J. China Tradit. Chin. Med. Inf. 2012, 4. Available online: http://industry.wanfangdata.com.cn/dl/Detail/Periodical?id=Periodical_zgzyyzx201201183 (accessed on 20 October 2023).
- Xu, B.; Tang, X.; Zhong, G.; Fan, H. Clinical efficacy of pivavironium combined with Medilac-S in the treatment of diarrhea-type IBS and its effects on IL-1B and IL-10. Jiangxi Med. J. 2012, 47. Available online: http://www.cqvip.com/QK/90121X/201205/42693737.html (accessed on 20 October 2023).
- Yuan, S.-F.; Zhang, J.-B.; Chen, F. Treatment of irritable bowel syndrome with trimebute maleate combined with Medilac-S. Chin. J. New Drugs 2011, 20. Available online: http://www.cqvip.com/QK/97417A/201107/37366274.html (accessed on 20 October 2023).
- Qin, J.-J.; Bai, P.-P. The Effect of Combination of Medilac-S with Trimebutine Maleate in Patients with Diarrhea-predominant Irritable Bowel Syndrome. Guide China Med. 2009, 7. Available online: http://www.cqvip.com/QK/86373X/200904/29604601.html (accessed on 20 October 2023).
- Zhang, X.-F.; Jiang, Y.-A.; Fang, C.-H. Efficacy of Medilac-S on Diarrhea-Dominant Irritable Bowel Syndrome. J. Clin. Dig. Dis. 2009, 2. Available online: https://d.wanfangdata.com.cn/periodical/lcxhbzz200901008 (accessed on 20 October 2023).
- You, T. Observation on the therapeutic effect of 186 cases of diarrhea-type irritable bowel syndrome with Smecta and Medilac-S. Psychol. Clin. Res. 2016, 22. Available online: https://www.qikan.wang/thesis/detail/4003212 (accessed on 20 October 2023).
- Yang, G. Medilac-S Capsules combined with Dictahedral Smectite Powder in Treating Diarrhea Type Irritable Bowel Syndrome Clinical Curative Effect Observation. Asia-Pac. Tradit. Med. 2013, 9. Available online: http://wx.gdinfo.net/articles/article_detail.aspx?id=44512629 (accessed on 20 October 2023).
- Liu, G.H. Therapeutic effect of Medilac-S combined with Smecta on diarrhea-predominant irritable bowel syndrome. China Health Nutr. Middle 2012. Available online: http://industry.wanfangdata.com.cn/dl/Detail/Periodical?id=Periodical_zgbjyy-z201205174 (accessed on 20 October 2023).
- You, B.; Zou, L.; Liu, Z.; Zhang, F. Clinical observation of Medilac-S combined with montmorillonite powder in treating diarrhea-predominant irritable bowel syndrome. Clin. Med. China 2011, 27. [Google Scholar] [CrossRef]
- Ding, G. Mei Changan combined with montmorillonite powder in the treatment of diarrhea-type irritable bowel syndrome: Clinical observation. Chin. J. Mod. Drug Appl. 2012, 6. [Google Scholar] [CrossRef]
- Li, C.; Shi, L. Effect of Doxepin Combined with Mei Changan on Diarrhea Irritable Bowel Syndrome. Natl. Med. Front. China 2010, 5. [Google Scholar] [CrossRef]
- Li, J.G. Therapeutic effect of Medilac-S combined with loperamide on diarrhea-predominant irritable bowel syndrome. Med. Inf. Drug Res. 2010. [Google Scholar] [CrossRef]
- Su, Y.; Wu, X. Bacillus subtilis double live bacteria in the diarrhea type with abdominal distension Application effect in irritable bowel syndrome. Inn. Mong. Med. J. 2021, 53. [Google Scholar] [CrossRef]
- Lu, D.; Dong, Y. Efficacy of combination of Smectite with Medilac-S for irritable bowel syndrome. Chin. J. New Drugs 2007, 16, 319. [Google Scholar] [CrossRef]
- Chen, H.; Zeng, Q.; Huang, H. Clinical study of trimebutine maleate combined with Mechangan capsules in the treatment of irritable bowel syndrome. China Med. Front. 2011, 6. [Google Scholar] [CrossRef]
- Li, C. Bacillus subtilis double live bacteria enteric-coated capsules combined with trimebutine maleate tablets Clinical effect of treatment of irritable bowel syndrome. Chronic Pathematology 2020, 21. [Google Scholar] [CrossRef]
- Wang, J. Bacillus subtilis double live bacteria enteric-coated capsules, trimebutine maleate combined with fluoxetine hydrochloride Observation of Therapeutic Effect on Irritable Bowel Syndrome. Jilin Med. 2013, 33. Available online: http://industry.wanfangdata.com.cn/dl/Detail/Periodical?id=Periodical_jilinyx201334009 (accessed on 20 October 2023).
- Li, F. Efficacy evaluation of Deshute combined with Mechangan in the treatment of irritable bowel syndrome. World Health Dig. 2012, 9, 154–155. [Google Scholar]
- Tao, Y.; Liu, J.; Yang, J. Curative Effect Observation of Trimebutin Combined with Bacillus subtilis Double Huo Yin Enteric-coated Capsules in Treating Irritable Bowel Syndrome. J. Clin. Mil. Med. 2012, 40. [Google Scholar] [CrossRef]
- Liao, R.B.; Su, Y.; Tang, J.; Liu, X. Mechangan Therapy Evaluation of clinical effect of irritable bowel syndrome. Acta Med. Sin. 2011, 24. [Google Scholar] [CrossRef]
- Xu, L.; Qiu, Y. Dicetel combined with Deanxit and Medilac-S on treatment of irritable bowel syndrome. North. Pharm. 2015, 12. Available online: http://industry.wanfangdata.com.cn/dl/Detail/Periodical?id=Periodical_bfyx201506097 (accessed on 20 October 2023).
- Zhang, L.; Chang, T.; Li, J. Bacillus subtilis double live bacteria enteric-coated capsules combined with low-dose amitriptyline Observation of Therapeutic Effect on Irritable Bowel Syndrome. Med. Innov. China 2010, 7. [Google Scholar] [CrossRef]
- Chen, X. Observation on the efficacy of Deanxit combined with Mechangan in the treatment of irritable bowel syndrome. Chin. Foreign Med. Treat. 2008. [Google Scholar] [CrossRef]
- Agachan, F.; Chen, T.; Pfeifer, J.; Reissman, P.; Wexner, S.D. A constipation scoring system to simplify evaluation and management of constipated patients. Dis. Colon. Rectum 1996, 39, 681–685. [Google Scholar] [CrossRef]
- Tompkins, T.A.; Hagen, K.E.; Wallace, T.D.; Fillion-Forté, V. Safety evaluation of two bacterial strains used in Asian probiotic products. Can. J. Microbiol. 2008, 54, 391–400. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2020, 12, 55–61. [Google Scholar] [CrossRef]
- Tremblay, A.; Xu, X.; Colee, J.; Tompkins, T.A. Efficacy of a Multi-Strain Probiotic Formulation in Pediatric Populations: A Comprehensive Review of Clinical Studies. Nutrients 2021, 13, 1908. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, A.; Skrzydło-Radomańska, B.; Mulak, A.; Lipiński, M.; Małecka-Panas, E.; Reguła, J.; Rydzewska, G. Guidelines on the management of irritable bowel syndrome: In memory of Professor Witold Bartnik. Prz. Gastroenterol. 2018, 13, 259–288. [Google Scholar] [CrossRef] [PubMed]
- Moayyedi, P.; Andrews, C.N.; MacQueen, G.; Korownyk, C.; Marsiglio, M.; Graff, L.; Kvern, B.; Lazarescu, A.; Liu, L.; Paterson, W.G.; et al. Canadian Association of Gastroenterology Clinical Practice Guideline for the Management of Irritable Bowel Syndrome (IBS). J. Can. Assoc. Gastroenterol. 2019, 2, 6–29. [Google Scholar] [CrossRef]
- Fukudo, S.; Okumura, T.; Inamori, M.; Okuyama, Y.; Kanazawa, M.; Kamiya, T.; Sato, K.; Shiotani, A.; Naito, Y.; Fujikawa, Y.; et al. Evidence-based clinical practice guidelines for irritable bowel syndrome 2020. J. Gastroenterol. 2021, 56, 193–217. [Google Scholar] [CrossRef]
- Patel, S.; Doerfler, B.; Boutros, K.; Ng, S.; Manuel, M.; DeSimone, E. Review of Treatment Options for Irritable Bowel Syndrome with Constipation and Chronic Idiopathic Constipation. Int. J. Gen. Med. 2021, 14, 1457–1468. [Google Scholar] [CrossRef]
- Lacy, B.E.; Pimentel, M.; Brenner, D.M.; Chey, W.D.; Keefer, L.A.; Long, M.D.; Moshiree, B. ACG Clinical Guideline: Management of Irritable Bowel Syndrome. Off. J. Am. Coll. Gastroenterol. 2021, 116, 17–44. [Google Scholar] [CrossRef]
- FDA. FDA Warns Consumers Not to Use “Best Bentonite Clay”. 2016. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-consumers-not-use-best-bentonite-clay (accessed on 25 October 2023).
- Health Sciences Authority (HSA), Government of Singapore. Smecta®: Restriction of Treatment of Acute Diarrhoea to Children > 2 Years of Age and Adults, and Recommendation against Use in Pregnant and Breastfeeding Women. 2020. Available online: https://www.hsa.gov.sg/announcements/dear-healthcare-professional-letter/smecta-restriction-of-treatment-of-acute-diarrhoea-to-children-2-years-of-age-and-adults-and-recommendation-against-use-in-pregnant-and-breastfeeding-women (accessed on 25 October 2023).
- Alonso-Cotoner, C.; Abril-Gil, M.; Albert-Bayo, M.; Mall, J.-P.G.; Expósito, E.; González-Castro, A.M.; Lobo, B.; Santos, J. The Role of Purported Mucoprotectants in Dealing with Irritable Bowel Syndrome, Functional Diarrhea, and Other Chronic Diarrheal Disorders in Adults. Adv. Ther. 2021, 38, 2054–2076. [Google Scholar] [CrossRef] [PubMed]
- Legan, T.B. Tryptophan-Synthesizing Bacteria Enhance Intestinal Serotonin Signaling and Motility. Master’s Thesis, Department of Neuroscience, The University of Vermont, Burlington, VT, USA. Available online: https://scholarworks.uvm.edu/graddis/1615 (accessed on 25 October 2023).
- Huang, J.; Huang, J.; Yin, T.; Lv, H.; Zhang, P.; Li, H. Enterococcus faecium R0026 Combined with Bacillus subtilis R0179 Prevent Obesity-Associated Hyperlipidemia and Modulate Gut Microbiota in C57BL/6 Mice. J. Microbiol. Biotechnol. 2021, 31, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Xiong, J.; Ni, J.; Chen, C.; Wang, K. Live Combined B. subtilis and E. faecium Alleviate Liver Inflammation, Improve Intestinal Barrier Function, and Modulate Gut Microbiota in Mice with Non-Alcoholic Fatty Liver Disease. Med. Sci. Monit. 2021, 27, e931143. [Google Scholar] [CrossRef] [PubMed]
- Pi, X.; Teng, W.; Fei, D.; Zhao, G.; Liu, W. Effects of Live Combined Bacillus subtilis and Enterococcus faecium on Gut Microbiota Composition in C57BL/6 Mice and in Humans. Front. Cell Infect. Microbiol. 2022, 12, 821662. [Google Scholar] [CrossRef]
| Reference | Study Dates | Population, Age Diagnostic Criteria | Arms, n | Probiotic Regimen | Outcome Measures | Adverse Events |
|---|---|---|---|---|---|---|
| IBS-C | ||||||
| Luo, ZQ et al. (2016) [26] | February 2014–February 2015 | 100 participants, 20–77 years old (average ~40 y) Rome III | Trimebutine maleate, n = 50 Trimebutine maleate + Medilac-S, n = 50 | 2 caps. TID (3 × 109 cfu/d) For 4 weeks | Clinical efficacy rate (categorical scale; see Figure 2). Symptoms severity scores (abdominal pain, bloating, constipation, BM number) were significantly lower compared to the control group (p < 0.05). ‡ GI hormones levels significantly improved over the control group (all, p < 0.05). Motilin ↑, vasoactive intestinal peptide ↓, Somatostatin ↓. | Mild, self-resolving diarrhea in 3 participants of the combination group. |
| Wang, XF (2018) [27] | March 2015–February 2016 | 86 participants, 21–57 years old (average ~37 y) Diagnostic guideline used (no source) | Lactulose, n = 42 Lactulose + Medilac-S, n = 44 | 1 cap. TID (1.5 × 109 cfu/d) For 4 weeks | Clinical efficacy rate (categorical scale). BSS and number of BM improved significantly and similarly in both groups. | None observed. |
| Zhao, LL (2016) [28] | February 2014–February 2016 | 150 participants, 27–63 years old (average ~39 y) Diagnostic guideline used (no source) | Lactulose, n = 50 Medilac-S, n = 50 Lactulose + Medilac-S, n = 50 | 2 caps. TID (3.0 × 109 cfu/d) For 4 weeks | Clinical efficacy rate (categorical scale). The improvement in individual symptom scores (abdominal pain, decreased appetite) was significantly superior in the combination group (all, p < 0.05). ‡ | Similar AE incidence in all groups (mild, self-resolving diarrhea: 1 case in lactulose and combination groups; abdominal distension: 1 case in the lactulose group; no adverse event in the Medilac-S group). |
| Du, J (2015) [29] | 2010–2012 | 90 participants, 26–65 years old Rome III | Lactulose, n = 30 Medilac-S, n = 30 Lactulose + Medilac-S, n = 30 | 2 caps. TID (3.0 × 109 cfu/d) For 5 weeks | Clinical efficacy rate (categorical scale). | Similar AE incidence in all groups (mild, self-resolving diarrhea: 2 cases in lactulose and combination group; abdominal distension: 4 cases in the lactulose group; no adverse event in the Medilac-S group). |
| Wang, L (2015) [30] | September 2012–September 2014 | 79 participants, 61–87 years old (average ~71 y) Rome III | Lactulose, n = 26 Medilac-S, n = 26 Lactulose + Medilac-S, n = 27 | 2 caps. TID (3.0 × 109 cfu/d) For 4 weeks | Clinical efficacy rate (categorical scale). Faster treatment onset in the combination group (p < 0.05). Superior relief of abdominal pain and distension in the combination group (p < 0.05). ‡ | Similar AE incidence in all groups. All cases resolved without medication. (Combination group: 3 cases of diarrhea (11.1%), lactulose group: 3 cases of bloating/abdominal distension (11.5%), Medilac-S group: 2 cases of bloating (7.6%)). |
| Bai, L (2012) [31] | March 2010–April 2012 | 204 participants, 22–85 years old (average ~39 y) Rome III | Lactulose, n = 51 Medilac-S, n = 51 Lactulose + Medilac-S, n = 52 (Phenolphthalein, n = 50) | 2 caps. TID (3.0 × 109 cfu/d) For 4 weeks | Clinical efficacy rate (categorical scale). Except Phenolphthalein, post-treatment symptom scores improved significantly in all groups (p < 0.05). The improvement was significantly better in the combination group vs. the three other groups (p < 0.05). ‡ | None observed. |
| Xiao, Y et al. (2014) [32] | April 2013–February 2014 | 94 participants, 52–84 years old (average ~69 y) Diagnostic criteria used (no source) | Mosapride, n = 47 Mosapride + Medilac-S, n = 47 | 4 caps. TID (6.0 × 109 cfu/d) For 4 weeks | Clinical efficacy rate (categorical scale). The abdominal pain (stomachache) and discomfort score was significantly lower in the combination group after treatment (p < 0.05). ‡ | None observed. |
| Shang, QL (2021) [33] | March 2018–October 2019 | 74 participants, 24–73 years old (average ~44 y) No guideline specified for the diagnosis | Mosapride + Lactulose, n = 37 Mosapride + Lactulose + Medilac-S, n = 37 | 2 caps. TID (3.0 × 109 cfu/d) For 4 weeks | Clinical efficacy rate (categorical scale). Individual symptom scores (bloating, abdominal pain, constipation) significantly improved in both groups (p < 0.05).‡ GI hormones (vasoactive intestinal peptide (VIP) and somatostatin (SS)) decreased after treatment and were lower in the combination group. | n.r. |
| Yang et al., 2016 [34] | October 2008–December 2011 | 231 participants, 16–63 years old (median age ~37 y) Rome III | Lactulose, n = 84 Lactulose + Medilac-S, n = 71 (Mosapride Citrate, n = 76) | 2 caps. TID (3.0 × 109 cfu/d) 8 weeks | Superior improvement in constipation score§ in the combination group (p < 0.05). ‡ BSS and number of BM improved significantly in all groups. | Similar AE incidence in all groups, all self-resolving. (Mild diarrhea: 14 in lactulose group, 10 cases in combination group; mild abdominal distension: 20 cases in the lactulose group, 23 cases in the combination group). |
| Sun et al., 2013 [35] | February 2010–August 2011 | 120 participants (average ~52 y *) Rome II | Lactulose, n = 40 Lactulose + Medilac-S, n = 40 (Mosapride, n = 40) | 2 caps. TID (3.0 × 109 cfu/d) 2 weeks | Bristol Stool Scale score: significant improvement (before–after) in all groups (p < 0.05); no significant difference in the number of participants with stool scores IV-VI in Lactulose + Medilac-S vs. Lactulose at the end of intake. | Similar AE incidence in both groups; all symptoms resolved after reducing lactulose intake. (Mild diarrhea: 3 in lactulose group, 1 case in combination group; bloating: 4 cases in the lactulose group, 1 case in the combination group). No abnormalities on the routine blood, urine, and hepatic and kidney function parameters. |
| Xu et al., 2014 [36] | January 2011–September 2013 | 98 participants, 23–60 y old Rome III | Mosapride + Lactulose, n = 33 Mosapride + Medilac-S, n = 30 Mosapride + Lactulose + Medilac-S, n = 35 | 2 caps. TID (3 × 109 cfu/d) 2 weeks | Except for the Bristol Stool Scale score and bloating that improved similarly in all groups, the improvement in abdominal pain ‡ and quality of life (Chinese SF-36) were significantly superior in the combination group (p < 0.05). | n.r. |
| IBS-D | ||||||
| Wang, JP (2019) [37] | September 2016–September 2017 | 64 participants, 20–68 years old (average ~41 y) Diagnostic guideline used (no source) | Trimebutine maleate, n = 32 Trimebutine maleate + Medilac-S, n = 32 | 2 caps. TID (3 × 109 cfu/d) For 12 weeks | Clinical efficacy (categorical scale). Significantly superior reduction in abdominal pain ‡ and diarrhea symptoms in the combination group (p < 0.05). Significant improvement in Bristol score (↑ types III and IV, ↓ types V-VII) in the combination group (p < 0.05). | AEs were similar in both groups: 2 cases in the control group (loss of appetite, nausea) and 3 cases in the combination group (loss of appetite, nausea, constipation). |
| Wang, TQ et al. (2014) [38] | n.r. | 76 participants, average 37 years old * Rome III | Trimebutine maleate, n = 35 Trimebutine maleate + Medilac-S, n = 41 | 2 caps. TID (3 × 109 cfu/d) For 2–4 weeks | Clinical efficacy (categorical scale). | There were 4 cases and 3 cases of dizziness, abdominal pain, and other adverse reactions in combination and control groups, respectively. Only 1 case in the combination group discontinued treatment. |
| Wang, TH (2013) [39] | September 2008–February 2012 | 117 participants, 18–65 years old (average 36.5 y) Rome III | Trimebutine maleate, n = 65 Medilac-S, n = 22 Trimebutine maleate + Medilac-S, n = 30 | 2 caps. TID (3 × 109 cfu/d) For 4 weeks | Clinical efficacy (categorical scale). | n.r. |
| Li, J (2012) [40] | September 2010–April 2011 | 150 participants, 23–59 years old, (average ~38 y) Rome II | Pinaverium bromide, n = 50 Medilac-S, n = 50 Pinaverium bromide + Medilac-S, n = 50 | 2 caps. TID (3 × 109 cfu/d) For 4 weeks | Clinical efficacy (categorical scale) | No abnormality in blood, urine, stool routine, liver and kidney function tests, and electrocardiogram. One case of mild, self-resolving constipation in the combination group. |
| Xu, BF et al. (2012) [41] | 2010–2011 | 85 participants, 19–76 years old (average 45.2 y) Rome III | Pinaverium bromide, n = 40 Pinaverium bromide + Medilac-S, n = 45 | 1 cap TID (1.5 × 109 cfu/d) For 4 weeks | Clinical efficacy (categorical scale). IL-1B decreased and IL-10 increased in the combination group (p < 0.05). No change in the control group. | Symptoms of drowsiness and dizziness in 9 patients in the combination group. All liver and kidney function parameters in normal range. |
| Yuan, SF et al. (2011) [42] | 2005–2010 | 60 participants, average 43 years old * Chinese guideline (2003) | Trimebutine maleate, n = 30 Trimebutine maleate + Medilac-S, n = 30 | 2 caps. TID (3 × 109 cfu/d) For 4 weeks | Clinical efficacy (categorical scale). | None observed. |
| Qin and Bai (2009) [43] | August 2007–February 2008 | 86 participants, 19–66 years old (average 41 y) Rome III | Trimebutine maleate, n = 28 Medilac-S, n = 30 Trimebutine maleate + Medilac-S, n = 28 | 2 caps. TID (3 × 109 cfu/d) For 2 weeks + 4-week follow-up | Clinical efficacy (categorical scale). Recurrence rate after 4-week follow-up, lower in combination (4/28; 14.3%) and Medilac-S (5/30; 16.7%) groups compared to trimebutine maleate (9/28; 32.1%) (p < 0.01). | There were no obvious adverse drug reactions deemed associated with Medilac-S. No abnormal laboratory test results in the three groups. In the trimebutine maleate and combination groups: 3 and 5 cases, respectively, displayed symptoms such as numbness in the limbs, dizziness, and thirst, which all disappeared after reducing the dose of trimebutine maleate. |
| Zhang, XF et al. (2009) [44] | September 2007–April 2008 | 60 participants, 20–66 years old (average 35.5 y) Rome III | Pinaverium bromide, n = 30 Pinaverium bromide + Medilac-S, n = 30 | 2 caps. TID (3 × 109 cfu/d) For 4 weeks | Clinical efficacy (categorical scale). Superior Bristol score improvement in the combination group (p = 0.016). Superior reduction in abdominal symptom scores in the combination group (p = 0.004). ‡ | A few participants reported common side effects of pinaverium bromide (mild gastrointestinal discomfort, and rash-like allergic reactions). No abnormal results in routine blood tests and biochemical tests. |
| You, T (2016) [45] | March 2015–March 2016 | 186 participants, 21–65 years old (average ~31 y) No guideline specified for the diagnosis | Smecta, n = 62 Medilac-S, n = 62 Smecta + Medilac-S, n = 62 | 2 caps. TID (3 × 109 cfu/d) For 2 weeks + 2 weeks follow-up | Clinical efficacy (categorical scale). Superior improvements in abdominal pain and diarrhea frequency, ‡ and shorter hospital stay in the combination group (p < 0.05). Note: absence of recurrence during follow-up included in the markedly effective definition. | n.r. |
| Yang, GC (2013) [46] | April 2011–April 2012 | 120 participants, 19–75 years old (average 43.5 y) Rome III | Montmorillonite, n = 40 Medilac-S, n = 40 Montmorillonite + Medilac-S, n = 40 | 2 caps. TID (3 × 109 cfu/d) For 2 weeks | Clinical efficacy (categorical scale). Superior improvements in individual symptoms (abdominal pain or discomfort, bloating, defecation pattern, defecation process ‡; all, p < 0.05) in the combination group. | All AEs reported were mild. In the Medilac-S group: 2 cases of nausea and 1 case of dizziness; in the combination group: 1 case of nausea. |
| Liu, GH (2012) [47] | 2010–2011 | 90 participants, 19–65 years old (average 38 y) Rome III | Smecta, n = 30 Medilac-S, n = 30 Smecta + Medilac-S, n = 30 | 2 caps. TID (3 × 109 cfu/d) For 4 weeks | Clinical efficacy (categorical scale). | None observed. |
| You, B et al. (2011) [48] | January 2008–April 2010 | 176 participants, 18–63 years old (average 41 y) Rome III | Montmorillonite, n = 58 Medilac-S, n = 60 Montmorillonite + Medilac-S, n = 58 | 2 caps. TID (3 × 109 cfu/d) For 2 weeks + 4-week follow-up | Clinical efficacy (categorical scale). More participants in the combination group reported improvements in abdominal pain and diarrhea scores at 3 days and 14 days of treatment (p < 0.05). ‡ Recurrence rate 4 weeks after treatment was significantly lower in the combination (10.3%) and Medilac-S (11.6%) groups compared to standard treatment (32.7%); p < 0.01. | AEs were similar between groups (drowsiness, dizziness, and sleepiness) in 5 (combination), 4 (Medilac-S), and 3 (Montmorillonite) participants. All values for blood, urine, stool panel, and liver and kidney functions were within normal range. |
| Ding, GW (2012) [49] | January 2009–August 2011 | 134 participants, 18–60 years old (average 39.5 y) Rome III | Flupentixol + Melitracen + Montmorillonite, n = 44 Flupentixol + Melitracen + Medilac-S, n = 44 Flupentixol + Melitracen + Montmorillonite + Medilac-S, n = 46 | 2 caps. TID (3 × 109 cfu/d) For 4 weeks | Clinical efficacy (categorical scale). | n.r. |
| Li and Shi (2010) [50] | April 2009–April 2010 | 156 participants, 18–67 y old (average 35 y) Rome III | Doxepin, n = 52 Medilac-S, n = 52 Doxepin + Medilac, n = 52 | 2 caps. TID (3 × 109 cfu/d) For 4 weeks + 12 weeks follow-up | Clinical efficacy (categorical scale). Note: absence of recurrence included in the markedly effective definition. | All AEs were mild and disappeared with a reduction in dosage. Three cases in the doxepin group (dry mouth, dizziness, lethargy), one case of headache in the Medilac-S group. |
| Li, JG (2010) [51] | 140 participants, 16–60 y oldRome III | Loperamide, n = 68 Loperamide + Medilac-S, n = 72 | 2 caps. TID (3 × 109 cfu/d) For 4 weeks | Clinical efficacy (categorical scale). | None observed. | |
| Su and Wu (2021) [52] | February 2019–February 2020 | 92 participants, 23–69 y old (average ~41.5 y) (Note: IBS-D with abdominal distension) Rome III-like diagnostic criteria described (no source) | Itopride, n = 46 Itopride + Medilac-S, n = 46 | 1 cap. TID (1.5 × 109 cfu/d) For 4 weeks | Clinical efficacy (categorical scale). Significantly superior improvement in IBS-SSS score and bloating score in the combination group (p < 0.001). ‡ Significantly higher Lactobacilli and Enterococci and lower yeast-like fungi counts in the combination group. | AEs were similar between groups: 3 cases (loss of appetite, nausea/vomiting, constipation) in the combination group; 5 cases in the Itopride group (2 loss of appetite, 3 nausea/vomiting). |
| Lu and Dong, 2007 [53] | January 2005–October 2006 | 60 participants, 18–63 years (average 27 y) Chinese guideline (2003) | Smecta, n = 18 Medilac-S, n = 21 Smecta + Medilac-S, n = 21 | 2 caps. TID (3 × 109 cfu/d) 1 week | Faster resolution (starting on day 3) and significantly lower scores after 7 days in the combination group (abdominal symptom scores, ‡ daily average bowel movements, and Bristol stool traits; all p < 0.05). | None observed. |
| IBS-M | ||||||
| Chen, H et al. (2011) [54] | April 2006–August 2008 | 151 participants, 19–65 years old (average ~42 y) Rome III | Trimebutine maleate, n = 50 Medilac-S, n = 50 Trimebutine maleate + Medilac-S, n = 51 | 2 caps. TID (3 × 109 cfu/d) For 8 weeks | Clinical efficacy (categorical scale). | AEs were mild and self-resolving. Two cases (dizziness, headache) in the combination group; two cases in the trimebutine maleate group (dizziness, headache); and one case of nausea in the Medilac-S group. |
| IBS (not subtyped) | ||||||
| Li, CL (2020) [55] | April 2016–April 2018 | 135 participants, 18–54 years old (average 36.5 y) Diagnostic guideline used (no source) | Trimebutine maleate, n = 67 Trimebutine maleate + Medilac-S, n = 68 | 1 cap. TID (1.5 × 109 cfu/d) For 12 weeks | Clinical efficacy (categorical scale). After 12 weeks, significant ↑ in Bristol stool types Ⅲ-IV and ↓ types V-VII in the combination group vs. trimebutine maleate (p < 0.05). | AE incidence rate was 4.41% (3/68; headache, nausea, thirst) in the combination group, compared with 2.99% (2/67; thirst, dizziness) in the control group (n.s.). |
| Wang, JR (2013) [56] | January 2009–December 2011 | 102 participants, 21–71 years old (average 42.5 y) Rome III | Trimebutine maleate, n = 25 Medilac-S, n = 26 Trimebutine maleate + Medilac-S, n = 25 (Trimebutine maleate + fluoxetine hydrochloride + Medilac-S, n= 26) | 2 caps. TID (3 × 109 cfu/d) For 4 weeks | Clinical efficacy (categorical scale). | None observed. No abnormalities in liver and kidney function tests. |
| Li, FQ (2012) [57] | March 2010–March 2012 | 113 participants, average 40 years old * Rome III | Pinaverium bromide, n = 38 Medilac-S, n = 31 Pinaverium bromide + Medilac-S, n = 44 | 2 caps. TID (3 × 109 cfu/d) For 2 weeks | Clinical efficacy (categorical scale). | All AEs were mild and self-resolving. Pinaverium bromide group (2 cases; nausea and loss of appetite), Medilac-S (1 case; nausea), combination group (1 case; dizziness). |
| Tao, YS et al. (2012) [58] | August 2008–August 2011 | 152 participants, 18–62 years old (average 40 y) Rome III | Trimebutine maleate, n = 66 Trimebutine maleate + Medilac-S, n = 86 | 2 caps TID (3 × 109 cfu/d) For 4 weeks | Clinical efficacy (categorical scale). | n.r. |
| Liao, RB et al. (2011) [59] | August 2009–November 2010 | 98 participants, average 37.5 years old Rome III | Pinaverium bromide, n = 49 Pinaverium bromide + Medilac-S, n = 49 | 2 caps. TID (3 × 109 cfu/d) For 4 weeks | Clinical efficacy (categorical scale). | n.r. |
| Xu and Qiu (2015) [60] | January 2012–December 2014 | 457 participants (average 61 years old *) Rome III | Flupentixol + Melitracen + Pinaverium bromide, n = 152 Flupentixol + Melitracen + Pinaverium bromide + Medilac-S, n = 154 (Pinaverium Bromide + Medilac-S, n = 151) | 2 caps. TID (3 × 109 cfu/d) For 2 weeks | Clinical efficacy (categorical scale). | Three subjects reported nausea and loss of appetite in the combination group, and three subjects from control group reported (nausea and dizziness). |
| Zhang, LF et al. (2010) [61] | June 2008–June 2009 | 123 participants, adults * Rome III | Amitriptyline, n = 39 Medilac-S, n = 41 Amitriptyline + Medilac-S, n = 43 | 2 caps. TID (3 × 109 cfu/d) For 4 weeks | Clinical efficacy (categorical scale). | n.r. |
| Chen, X (2008) [62] | January 2006–January 2008 | 144 participants, 22–73 years old (average 41.3 y) Rome II | Flupentixol + Melitracen, n = 46 Medilac-S, n = 48 Flupentixol + Melitracen + Medilac-S, n = 50 | 2 caps. TID (3 × 109 cfu/d) For 4 weeks | Clinical efficacy (categorical scale). | None observed. No abnormalities on kidney and liver function. |
| Concomitant Medication (Class; Main Mode of Action) | IBS-D | IBS-C | IBS-M | IBS (Not SubTyped) | Total |
|---|---|---|---|---|---|
| Smectite and Montmorillonite (Intestinal adsorbent; clays) | 5 | 5 | |||
| Trimebutine Maleate (Antispasmodic; antimuscarinic and weak µ-opioid receptor agonist) | 5 | 1 | 1 | 3 | 10 |
| Pinaverium Bromide (Antispasmodic; Ca2+ channel blocker) | 3 | 2 | 5 | ||
| Mosapride or Itopride (Gastroprokinetics; serotonin type-4 receptor agonist or antidopaminergic and anti-acetylcholinesterasic actions) | 1 * | 1 | 2 | ||
| Lactulose (Laxative; osmotic) | 7 | 7 | |||
| Loperamide (Antidiarrheal; µ-opioid receptor agonist) | 1 | 1 | |||
| Doxepin or Amitriptyline (Tricyclic antidepressants (TCA)) | 1 | 1 | 2 | ||
| Flupentixol + Melitracen (Antipsychotic + TCA) | 1 | 1 | |||
| Other combination of medications | 1 a | 2 b | 1 c | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tremblay, A.; Xu, X.; Colee, J.; Tompkins, T.A.; Binda, S. Effectiveness of Medilac-S as an Adjuvant to Conventional Irritable Bowel Syndrome Treatments: A Systematic Review with Meta-Analysis. Gastroenterol. Insights 2023, 14, 491-514. https://doi.org/10.3390/gastroent14040036
Tremblay A, Xu X, Colee J, Tompkins TA, Binda S. Effectiveness of Medilac-S as an Adjuvant to Conventional Irritable Bowel Syndrome Treatments: A Systematic Review with Meta-Analysis. Gastroenterology Insights. 2023; 14(4):491-514. https://doi.org/10.3390/gastroent14040036
Chicago/Turabian StyleTremblay, Annie, Xiaoyu Xu, James Colee, Thomas A. Tompkins, and Sylvie Binda. 2023. "Effectiveness of Medilac-S as an Adjuvant to Conventional Irritable Bowel Syndrome Treatments: A Systematic Review with Meta-Analysis" Gastroenterology Insights 14, no. 4: 491-514. https://doi.org/10.3390/gastroent14040036
APA StyleTremblay, A., Xu, X., Colee, J., Tompkins, T. A., & Binda, S. (2023). Effectiveness of Medilac-S as an Adjuvant to Conventional Irritable Bowel Syndrome Treatments: A Systematic Review with Meta-Analysis. Gastroenterology Insights, 14(4), 491-514. https://doi.org/10.3390/gastroent14040036







