Side Effects Following Administration of the First Dose of Oxford-AstraZeneca’s Covishield Vaccine in Bangladesh: A Cross-Sectional Study
Abstract
:1. Introduction
2. Methods
3. Result
3.1. Socio-Demographic Characteristics
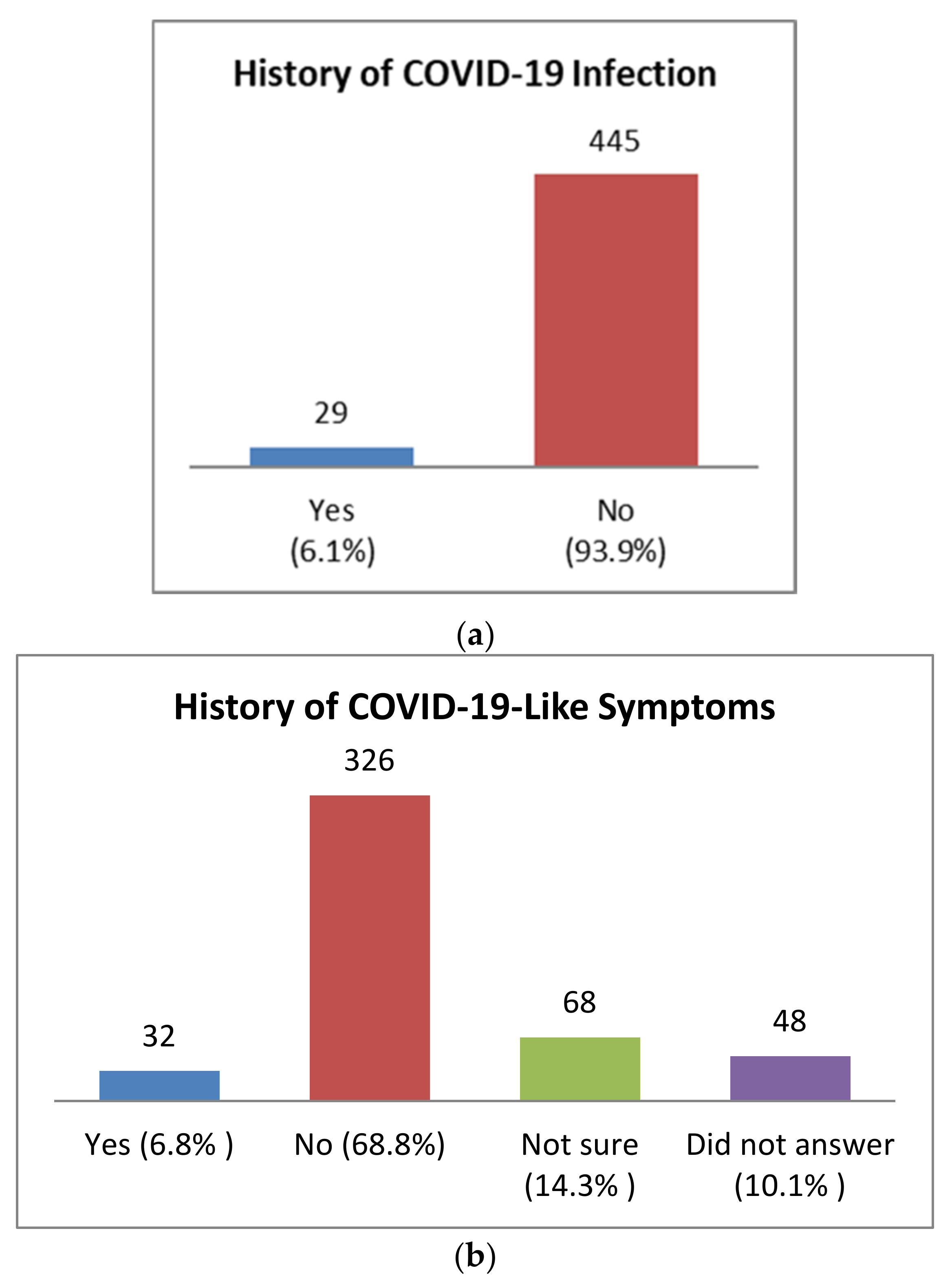
3.2. Past Medical History of Health
3.3. Perception and Awareness of Participants Regarding AstraZeneca Covishield Vaccine
3.4. Self-Reported Solicited Side Effects after Covishield Vaccination
3.5. Association between the Number of Reported COVID-19 Vaccine Side Effects and Participants’ Demographic Characteristics
3.6. Association of Individual Post-Vaccination Side Effects with Gender and Age
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sohrabi, C.; Alsafi, Z.; O’Neill, N.; Khan, M.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, R. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). Int. J. Surg. 2020, 76, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Coronavirus Disease (COVID-19) Update. Available online: https://www.who.int/bangladesh/emergencies/coronavirus-disease-(covid-19)-update (accessed on 28 March 2021).
- Islam, M.T.; Talukder, A.K.; Siddiqui, M.N.; Islam, T. Tackling the COVID-19 pandemic: The Bangladesh perspective. J. Public Health Res. 2020, 9, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Nasrullah, M.; Hosen, M.J. COVID-19 and Bangladesh: Challenges and How to Address Them. Front. Public Health 2020, 8, 154. [Google Scholar] [CrossRef] [PubMed]
- Siddique, R.; Bai, Q.; Shereen, M.A.; Nabi, G.; Han, G.; Rashid, F.; Ahmed, S.; Benzhanova, A.; Xue, M.; Khan, S. Evidence and speculations: Vaccines and therapeutic options for COVID-19 pandemic. Hum. Vaccines Immunother. 2021, 17, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Accelerating a Safe and Effective COVID-19 Vaccine. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/accelerating-a-safe-and-effective-covid-19-vaccine (accessed on 24 September 2021).
- “The Wait Is Over”: Bangladesh Begins COVID-19 Vaccinations|Reuters. Available online: https://www.reuters.com/article/us-health-coronavirus-bangladesh-vaccine-idUSKBN2A70I0 (accessed on 28 March 2021).
- Oxford-AstraZeneca COVID Vaccine Induces Cell Spikes Similar to SARS-CoV-2’s. Available online: https://www.news-medical.net/news/20210409/Oxford-AstraZeneca-COVID-vaccine-induces-cell-spikes-similar-to-SARS-CoV-2s.aspx (accessed on 25 September 2021).
- Covid-19 Vaccination: How’s Bangladesh Doing Compared to the Rest of the World? Available online: https://www.thedailystar.net/coronavirus-deadly-new-threat/news/covid-19-vaccination-hows-bangladesh-doing-compared-the-rest-the-world-2049289 (accessed on 5 July 2021).
- Jahan, N.; Archie, S.R.; Al Shoyaib, A.; Kabir, N.; Cheung, K. Recent approaches for solid dose vaccine delivery. Sci. Pharm. 2019, 87, 27. [Google Scholar] [CrossRef] [Green Version]
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet 2020, 396, 1979–1993. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- What We Know about COVID-19 Vaccine Development. Available online: https://www.who.int/publications/m/item/what-we-know-aboutcovid-19-vaccine-development (accessed on 28 March 2021).
- Abedin, M.; Islam, M.A.; Rahman, F.N.; Reza, H.M.; Hossain, M.Z.; Hossain, M.A.; Arefin, A.; Hossain, A. Willingness to vaccinate against COVID-19 among Bangladeshi adults: Understanding the strategies to optimize vaccination coverage. PLoS ONE 2021, 16, e0250495. [Google Scholar] [CrossRef] [PubMed]
- Chen, W. Promise and challenges in the development of COVID-19 vaccines. Hum. Vaccines Immunother. 2020, 16, 2604–2608. [Google Scholar] [CrossRef] [PubMed]
- Dhanda, S.; Osborne, V.; Lynn, E.; Shakir, S. Postmarketing studies: Can they provide a safety net for COVID-19 vaccines in the UK? BMJ Evid. Based Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Machingaidze, S.; Wiysonge, C.S. Understanding COVID-19 vaccine hesitancy. Nat. Med. 2021, 27, 1338–1339. [Google Scholar] [CrossRef] [PubMed]
- Side Effects of the Coronavirus Vaccines|The Coronavirus (COVID-19) Vaccine. Available online: https://www.nhsinform.scot/covid-19-vaccine/the-vaccines/side-effects-of-the-coronavirus-vaccines (accessed on 14 June 2021).
- Choe, Y.J.; Blatt, D.B.; Lee, H.J.; Choi, E.H. Associations between geographic region and immune response variations to pneumococcal conjugate vaccines in clinical trials: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020, 92, 261–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Shitany, N.A.; Harakeh, S.; Badr-Eldin, S.M.; Bagher, A.M.; Eid, B.; Almukadi, H.; Alghamdi, B.S.; Alahmadi, A.A.; Hassan, N.A.; Sindi, N.; et al. Minor to moderate side effects of pfizer-biontech COVID-19 vaccine among saudi residents: A retrospective cross-sectional study. Int. J. Gen. Med. 2021, 14, 1389–1401. [Google Scholar] [CrossRef] [PubMed]
- Su, J.R.; Moro, P.L.; Ng, C.S.; Lewis, P.W.; Said, M.A.; Cano, M.V. Anaphylaxis after vaccination reported to the Vaccine Adverse Event Reporting System, 1990–2016. J. Allergy Clin. Immunol. 2019, 143, 1465–1473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halsey, N.A.; Griffioen, M.; Dreskin, S.C.; Dekker, C.L.; Wood, R.; Sharma, D.; Jones, J.F.; LaRussa, P.S.; Garner, J.; Berger, M.; et al. Immediate hypersensitivity reactions following monovalent 2009 pandemic influenza A (H1N1) vaccines: Reports to VAERS. Vaccine 2013, 31, 6107–6112. [Google Scholar] [CrossRef] [PubMed]
- Potluri, T.; Fink, A.L.; Sylvia, K.E.; Dhakal, S.; Vermillion, M.S.; vom Steeg, L.; Deshpande, S.; Narasimhan, H.; Klein, S.L. Age-associated changes in the impact of sex steroids on influenza vaccine responses in males and females. NPJ Vaccines 2019, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Di Resta, C.; Ferrari, D.; Viganò, M.; Moro, M.; Sabetta, E.; Minerva, M.; Ambrosio, A.; Locatelli, M.; Tomaiuolo, R. The Gender Impact Assessment among Healthcare Workers in the SARS-CoV-2 Vaccination-An Analysis of Serological Response and Side Effects. Vaccines 2021, 9, 522. [Google Scholar] [CrossRef] [PubMed]
- United Nations Population Fund. Available online: https://www.unfpa.org/data/world-population/BD (accessed on 27 September 2021).
- Mass Vaccination: Age Bar Brought Down to 40 Now|The Daily Star. Available online: https://www.thedailystar.net/frontpage/news/mass-vaccination-age-bar-brought-down-40-now-2041445 (accessed on 3 July 2021).
- Adhikari, P.; Adhikari, K.; Gauli, B.; Sitaula, D. Acceptance of COVID-19 vaccine and pattern of side effects in Nepalese context: A post-vaccine cross-sectional study among health care workers in a tertiary care hospital. J. Chitwan Med. Coll. 2021, 11, 34–38. [Google Scholar]
- Riad, A.; Pokorná, A.; Attia, S.; Klugarová, J.; Koščík, M.; Klugar, M. Prevalence of COVID-19 Vaccine Side Effects among Healthcare Workers in the Czech Republic. J. Clin. Med. 2021, 10, 1428. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.M.A.; Rahman, M.L.; Hossian, M.; Matin, K.F.; Nabi, M.H.; Saha, S.; Hasan, M.; Manna, R.M.; Barsha, S.Y.; Hasan, S.M.R.; et al. Acceptance of COVID-19 vaccine and its determinants: Evidence from a large sample study in Bangladesh. Heliyon 2021, 7, e07376. [Google Scholar] [CrossRef] [PubMed]
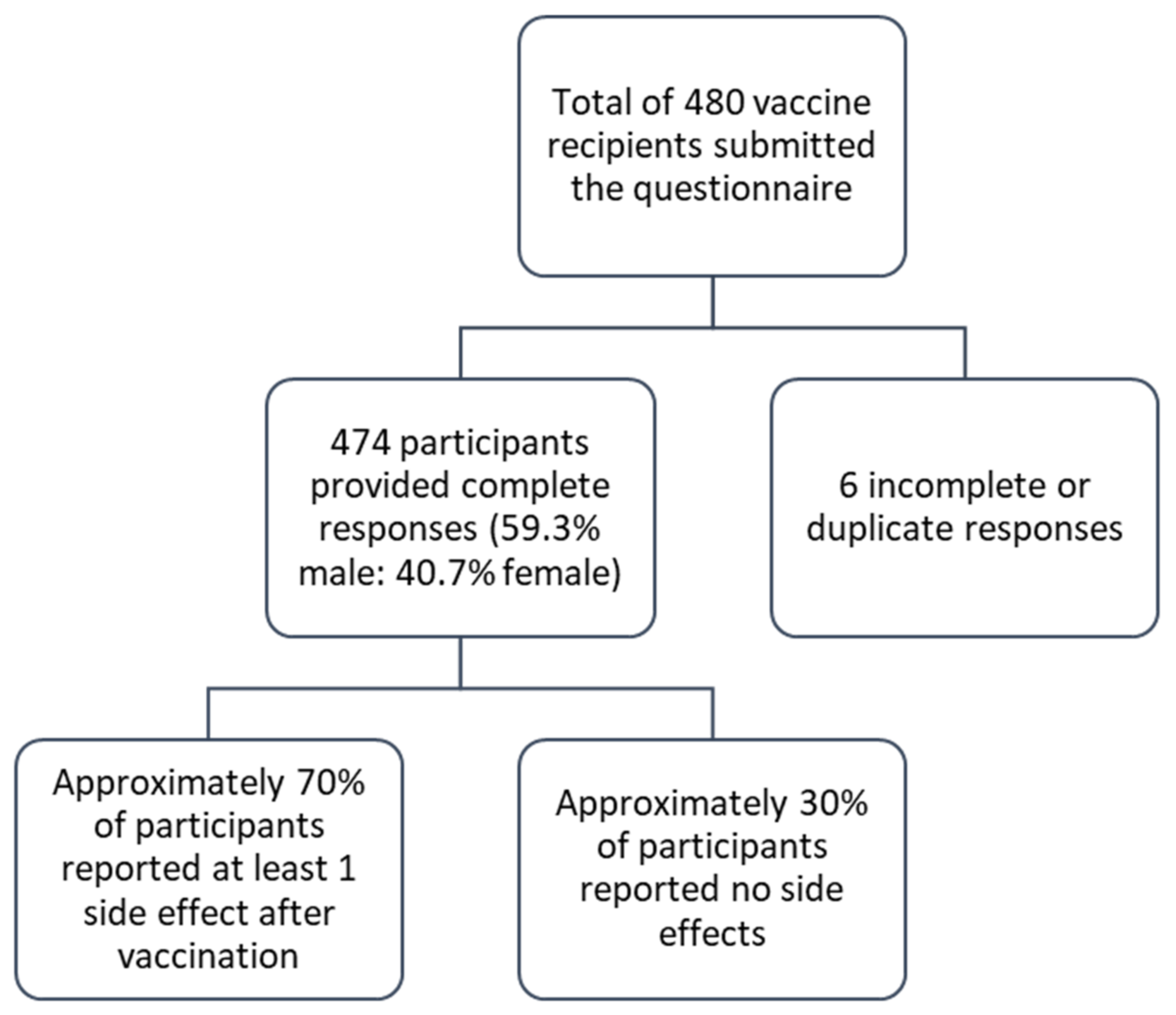



| Variable | Outcome | Frequency (n and %) |
|---|---|---|
| Gender | Male | 281 (59.3) |
| Female | 193 (40.7) | |
| Age | 18–30 years | 59 (12.4) |
| 31–40 years | 66 (13.9) | |
| 41–50 years | 158 (33.3) | |
| 51–60 years | 123 (25.9) | |
| 61–70 years | 51 (10.8) | |
| More than 70 years | 17 (3.6) | |
| Educational Qualification | Illiterate | 5 (1.1) |
| Primary | 20 (4.2) | |
| Secondary | 43 (9.1) | |
| Higher secondary | 68 (14.3) | |
| Undergraduate or higher | 338 (71.3) | |
| Occupation | Day labor | 4 (0.8) |
| Service holder (govt./private) | 98 (20.7) | |
| Frontline workers | 4 (0.8) | |
| Healthcare workers | 24 (5.1) | |
| Teachers/students | 163 (34.4) | |
| Business holder | 59 (12.4) | |
| Freelancer | 3 (0.6) | |
| Unemployed | 25 (5.3) | |
| Housewife | 93 (19.6) | |
| Other | 1 (0.2) | |
| Area of Residence | Rural | 189 (39.9) |
| Urban | 285 (60.1) | |
| Location of Vaccination Centre | Dhaka | 240 (50.6) |
| Chittagong | 24 (5.1) | |
| Rajshahi | 27 (5.7) | |
| Khulna | 111 (23.4) | |
| Barisal | 6 (1.3) | |
| Sylhet | 3 (0.6) | |
| Mymensingh | 8 (1.7) | |
| Rangpur | 51 (10.8) | |
| Body Mass Index (BMI) | <18.5 kg/m2 (underweight) | 7 (1.5) |
| 18.5–24.9 kg/m2 (normal weight) | 220 (46.4) | |
| 25–29.9 kg/m2 (overweight) | 177 (37.3) | |
| >30 kg/m2 (obese) | 40 (8.4) | |
| Not known | 30 (6.3) | |
| Comorbid Conditions * | None | 222 (46.8) |
| Hypertension | 158 (33.3) | |
| Diabetes | 116 (24.5) | |
| Heart disease | 40 (8.4) | |
| Lung disease | 38 (8.0) | |
| Kidney disease | 14 (2.2) | |
| Liver disease | 2 (0.4) | |
| Cancer | 1 (0.2) | |
| Stroke | 20 (4.2) | |
| Other | 21 (4.4) |
| Variables | No Side Effects | 1–3 Side Effects | 4–6 Side Effects | >6 Side Effects | χ2 (df) | OR | 95% CI | p-Value |
|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | p-Value | ||||
| Gender | ||||||||
| Male | 93 (33.1) | 146 (52.0) | 36 (12.8) | 6 (2.1) | 10.334 (3) | Ref. | Ref. | Ref. |
| Female | 50 (25.9) | 95 (49.2) | 35 (18.1) | 13 (6.7) | 0.016 | 1.506 | 0.97–2.34 | 0.067 |
| Age | ||||||||
| 18–30 years | 13 (22.0) | 24 (40.7) | 17 (28.8) | 5 (8.5) | 28.252 (15) 0.02 | 8.557 | 2.49–29.43 | 0.001 |
| 31–40 years | 16 (24.2) | 36 (54.5) | 11 (16.7) | 3 (4.5) | 5.052 | 1.57–16.24 | 0.007 | |
| 41–50 years | 46 (29.1) | 84 (53.2) | 24 (15.2) | 4 (2.5) | 4.078 | 1.39–11.98 | 0.011 | |
| 51–60 years | 43 (35.0) | 60 (48.8) | 15 (12.2) | 5 (4.1) | 3.768 | 1.26–11.29 | 0.018 | |
| 61–70 years | 15 (29.4) | 31 (60.8) | 4 (7.8) | 1 (2.0) | 3.687 | 1.13–12.09 | 0.031 | |
| >70 years | 10 (58.8) | 6 (35.3) | 0 (0.0) | 1 (5.9) | Ref. | Ref. | Ref. |
| Side Effect | Total n (%) | Male n (%) | Female n (%) | χ2 (df) | 18–30 Years n (%) | 31–40 Years n (%) | 41–50 Years n (%) | 51–60 Years n (%) | 61–70 Years n (%) | >70 Years n (%) | χ2 (df) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| p-Value | p-Value | ||||||||||
| Pain at site of injection | 232 (48.9) | 136 (48.4) | 96 (49.7) | 0.083 (1) 0.774 | 35 (59.3) | 38 (57.6) | 70 (44.3) | 56 (45.5) | 27 (52.9) | 6 (35.3) | 8.04 (5) 0.154 |
| Feverish | 134 (28.3) | 79 (28.1) | 55 (28.5) | 0.008 (1) 0.927 | 21 (35.6) | 16 (24.2) | 43 (27.2) | 33 (26.8) | 18 (35.3) | 3 (17.6) | 4.488 (5) 0.482 |
| Fever | 115 (24.3) | 65 (23.1) | 50 (25.9) | 0.48 (1) 0.489 | 16 (27.1) | 19 (28.8) | 50 (31.6) | 23 (18.7) | 4 (7.8) | 3 (17.6) | 15.644 (5) 0.008 |
| Myalgia | 110 (23.2) | 59 (21.0) | 51 (26.4) | 1.892 (1) 0.169 | 18 (30.5) | 18 (27.3) | 36 (22.8) | 31 (25.2) | 4 (7.8) | 3 (17.6) | 9.718 (5) 0.084 |
| Fatigue | 83 (17.5) | 41 (14.6) | 42 (21.8) | 4.073 (1) 0.044 | 16 (27.1) | 15 (22.7) | 22 (13.9) | 21 (17.1) | 8 (15.7) | 1 (5.9) | 8.146 (5) 0.148 |
| Headache | 65 (13.7) | 28 (10.0) | 37 (19.2) | 8.196 (1) 0.004 | 16 (27.1) | 11 (16.7) | 21 (13.3) | 14 (11.4) | 2 (3.9) | 1 (5.9) | 15.049 (5) 0.01 |
| Drowsiness | 44 (9.3) | 20 (7.1) | 24 (12.4) | 3.842 (1) 0.05 | 15 (25.4) | 4 (6.1) | 13 (8.2) | 9 (7.3) | 3 (5.9) | 0 (0.0) | 22.28 (5) 0.000 |
| Dizziness | 38 (8.0) | 17 (6.0) | 21 (10.9) | 3.621 (1) 0.05 | 9 (15.3) | 7 (10.6) | 10 (6.3) | 9 (7.3) | 2 (3.9) | 1 (5.9) | 6.748 (5) 0.24 |
| Joint pain | 29 (6.1) | 14 (5.0) | 15 (7.8) | 1.55 (1) 0.213 | 4 (6.8) | 2 (3.0) | 11 (7.0) | 9 (7.3) | 2 (3.9) | 1 (5.9) | 2.074 (5) 0.839 |
| Nausea or vomiting | 23 (4.9) | 5 (1.8) | 18 (9.3) | 14.115 (1) 0.000 | 5 (8.5) | 3 (4.5) | 4 (2.5) | 7 (5.7) | 3 (5.9) | 1 (5.9) | 3.877 (5) 0.567 |
| Swelling | 21 (4.4) | 6 (2.1) | 15 (7.8) | 8.586 (1) 0.003 | 5 (8.5) | 5 (7.6) | 5 (3.2) | 3 (2.4) | 2 (3.9) | 1 (5.9) | 5.687 (5) 0.338 |
| Itching | 20 (4.2) | 9 (3.2) | 11 (5.7) | 1.765 (1) 0.184 | 1 (1.7) | 3 (4.5) | 7 (4.4) | 5 (4.1) | 3 (5.9) | 1 (5.9) | 1.438 (5) 0.92 |
| Decreased appetite | 15 (3.2) | 9 (3.2) | 6 (3.1) | 0.003 (1) 0.954 | 3 (5.1) | 2 (3.0) | 5 (3.2) | 3 (2.4) | 1 (2.0) | 1 (5.9) | 1.576 (5) 0.904 |
| Irritation at injection site | 9 (1.9) | 6 (2.1) | 3 (1.6) | 0.207 (1) 0.649 | 1 (1.7) | 1 (1.5) | 3 (1.9) | 1 (0.8) | 2 (3.9) | 1 (5.9) | 3.412 (5) 0.637 |
| Diarrhea | 5 (1.1) | 3 (1.1) | 2 (1.0) | 0.001 (1) 0.974 | 1 (1.7) | 2 (3.0) | 1 (0.6) | 0 (0.0) | 1 (2.0) | 0 (0.0) | 4.862 (5) 0.433 |
| Difficulties in breathing | 4 (0.8) | 3 (1.1) | 1 (0.5) | 0.413 (1) 0.521 | 0 (0.0) | 1 (1.5) | 1 (0.6) | 0 (0.0) | 1 (2.0) | 1 (5.9) | 7.906 (5) 0.161 |
| Abdominal cramps | 4 (0.8) | 3 (75.0) | 1 (25.0) | 0.413 (1) 0.521 | 0 (0.0) | 0 (0.0) | 1 (0.6) | 1 (0.8) | 1 (2.0) | 1 (5.9) | 7.067 (5) 0.216 |
| Seizure | 3 (0.6) | 2 (0.7) | 1 (0.5) | 0.068 (1) 0.794 | 0 (0.0) | 0 (0.0) | 2 (1.3) | 0 (0.0) | 0 (0.0) | 1 (5.9) | 10.36 (5) 0.066 |
| Total | 331 (69.8) | 188 (66.9) | 143 (74.1) | 2.807 (1) 0.094 | 46 (78.0) | 50 (75.8) | 112 (70.9) | 80 (65.0) | 36 (70.6) | 7 (41.2) | 11.017 (5) 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jahan, N.; Rahman, F.I.; Saha, P.; Ether, S.A.; Roknuzzaman, A.; Sarker, R.; Kalam, K.T.; Haq, K.; Nyeen, J.; Himi, H.Z.; et al. Side Effects Following Administration of the First Dose of Oxford-AstraZeneca’s Covishield Vaccine in Bangladesh: A Cross-Sectional Study. Infect. Dis. Rep. 2021, 13, 888-901. https://doi.org/10.3390/idr13040080
Jahan N, Rahman FI, Saha P, Ether SA, Roknuzzaman A, Sarker R, Kalam KT, Haq K, Nyeen J, Himi HZ, et al. Side Effects Following Administration of the First Dose of Oxford-AstraZeneca’s Covishield Vaccine in Bangladesh: A Cross-Sectional Study. Infectious Disease Reports. 2021; 13(4):888-901. https://doi.org/10.3390/idr13040080
Chicago/Turabian StyleJahan, Nishat, Fahad Imtiaz Rahman, Poushali Saha, Sadia Afruz Ether, ASM Roknuzzaman, Rapty Sarker, Khondoker Tashya Kalam, Kashfa Haq, Julkar Nyeen, Humayra Zaman Himi, and et al. 2021. "Side Effects Following Administration of the First Dose of Oxford-AstraZeneca’s Covishield Vaccine in Bangladesh: A Cross-Sectional Study" Infectious Disease Reports 13, no. 4: 888-901. https://doi.org/10.3390/idr13040080
APA StyleJahan, N., Rahman, F. I., Saha, P., Ether, S. A., Roknuzzaman, A., Sarker, R., Kalam, K. T., Haq, K., Nyeen, J., Himi, H. Z., Hossain, M. N., Chowdhury, M. H., Uddin, M. M., & Alam, N. H. (2021). Side Effects Following Administration of the First Dose of Oxford-AstraZeneca’s Covishield Vaccine in Bangladesh: A Cross-Sectional Study. Infectious Disease Reports, 13(4), 888-901. https://doi.org/10.3390/idr13040080






