Murine Typhus: A Review of a Reemerging Flea-Borne Rickettsiosis with Potential for Neurologic Manifestations and Sequalae
Abstract
1. Introduction
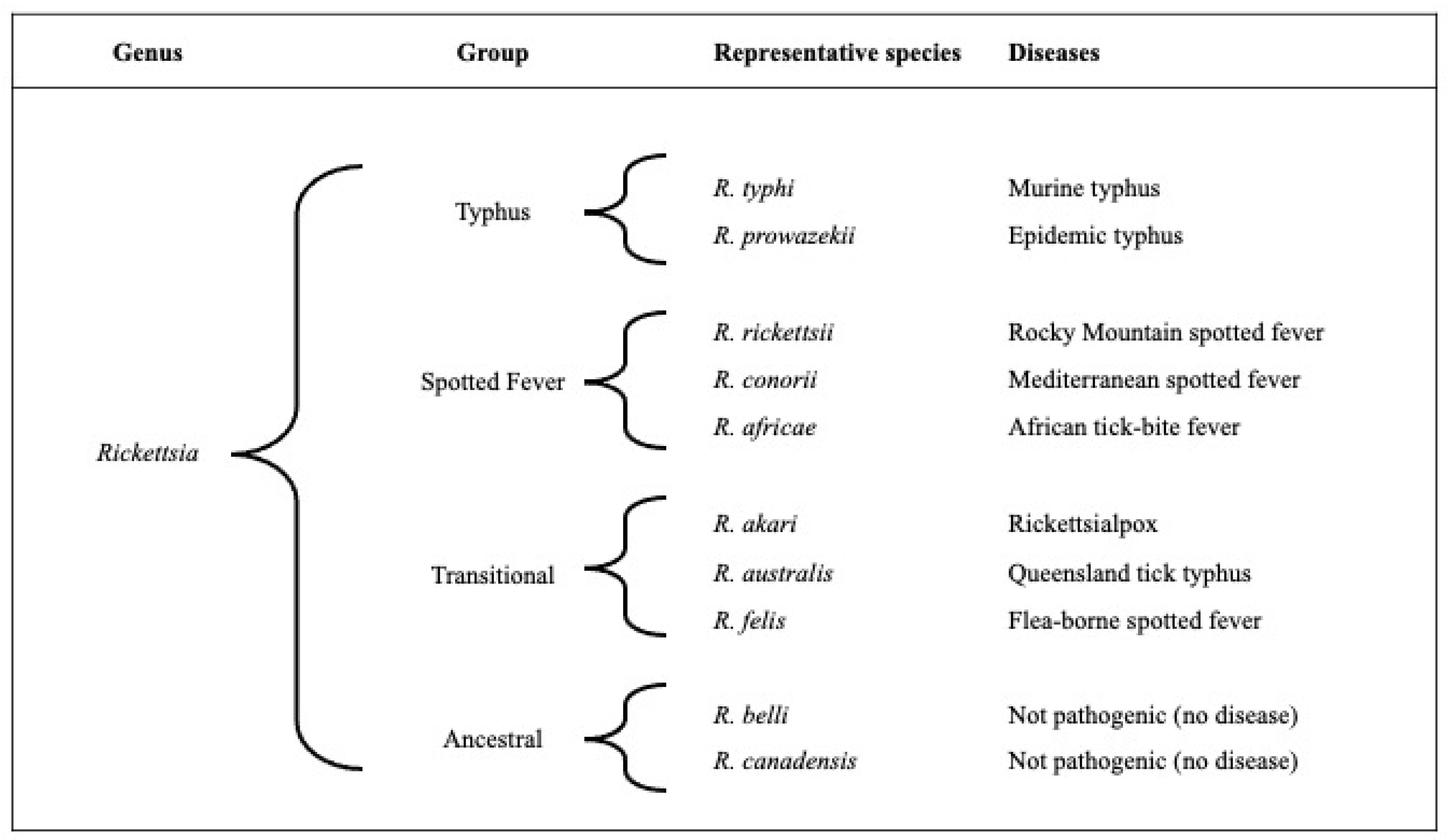
2. Microbiology
3. Pathology, Pathogenesis, and Immunity
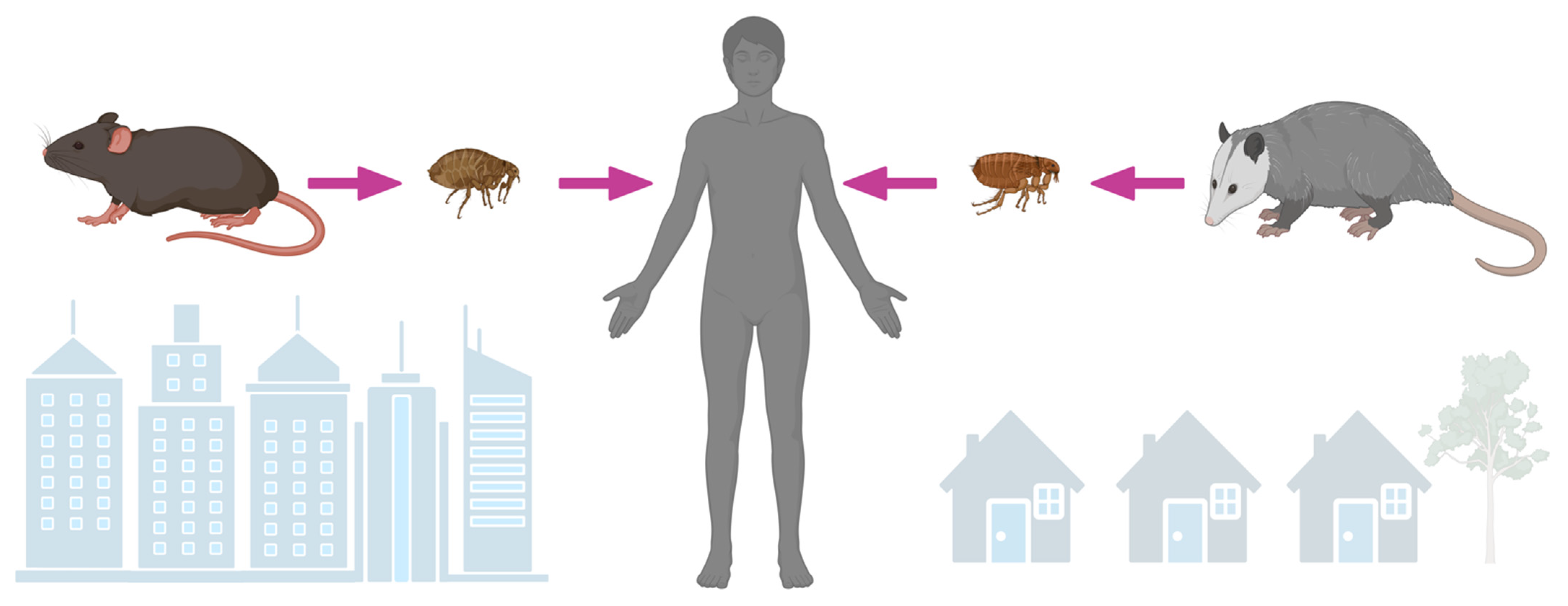
4. Ecology of an Emerging and Reemerging Infection
5. Epidemiology of an Underrecognized and Reemerging Cause of Infection
6. Clinical Manifestations of an Acute Undifferentiated Febrile Illness
7. Neurologic Manifestations and Sequelae
8. Diagnosis
9. Treatment and Prevention
10. Conclusions and Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blanton, L.S.; Dulmer, J.S.; Walker, D.H. Rickettsia typhi (Murine typhus). In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 9th ed.; Bennett, J.E., Dolin, R., Blaser, M.J., Eds.; Elsevier: Philadelphia, PA, USA, 2020; Volume 2, pp. 2372–2376. [Google Scholar]
- Yu, X.-J.; Walker, D.H.; Family, I. Rickettsiaceae. In Bergey’s Manual of Systematic Bacteriology, 2nd ed.; Brenner, D.J., Kreig, N.R., Stanley, J.T., Eds.; Springer: New York, NY, USA, 2005; Volume 2, pp. 96–116. [Google Scholar]
- Azad, A.F. Epidemiology of Murine Typhus. Annu. Rev. Entomol. 1990, 35, 553–569. [Google Scholar] [CrossRef] [PubMed]
- Civen, R.; Ngo, V. Murine typhus: An unrecognized suburban vectorborne disease. Clin. Infect. Dis. 2008, 46, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.F.; Radulovic, S.; Higgins, J.A.; Noden, B.H.; Troyer, J.M. Flea-borne rickettsioses: Ecologic considerations. Emerg. Infect. Dis. 1997, 3, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Dumler, J.S.; Taylor, J.P.; Walker, D.H. Clinical and laboratory features of Murine typhus in South Texas, 1980 through 1987. JAMA 1991, 266, 1365–1370. [Google Scholar] [CrossRef] [PubMed]
- Pieracci, E.G.; Evert, N.; Drexler, N.A.; Mayes, B.; Vilcins, I.; Huang, P.; Campbell, J.; Behravesh, C.B.; Paddock, C.D. Fatal flea-borne typhus in Texas: A retrospective case series, 1985–2015. Am. J. Trop. Med. Hyg. 2017, 96, 1088–1093. [Google Scholar] [CrossRef]
- Tsioutis, C.; Zafeiri, M.; Avramopoulos, A.; Prousali, E.; Miligkos, M.; Karageorgos, S.A. Clinical and laboratory characteristics, epidemiology, and outcomes of murine typhus: A systematic review. Acta Trop. 2017, 166, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Robaina-Bordon, J.M.; Carranza-Rodriguez, C.; Hernandez-Cabrera, M.; Bolanos-Rivero, M.; Pisos-Alamo, E.; Jaen-Sanchez, N.; Hernandez-Betancor, A.; Suarez-Hormiga, L.; Perez-Arellano, J.L. Murine typhus in Canary Islands, Spain, 1999–2015. Emerg. Infect. Dis. 2021, 27, 570–573. [Google Scholar] [CrossRef]
- Gillespie, J.J.; Beier, M.S.; Rahman, M.S.; Ammerman, N.C.; Shallom, J.M.; Purkayastha, A.; Sobral, B.S.; Azad, A.F. Plasmids and rickettsial evolution: Insight from Rickettsia felis. PLoS ONE 2007, 2, e266. [Google Scholar] [CrossRef]
- Gillespie, J.J.; Williams, K.; Shukla, M.; Snyder, E.E.; Nordberg, E.K.; Ceraul, S.M.; Dharmanolla, C.; Rainey, D.; Soneja, J.; Shallom, J.M.; et al. Rickettsia phylogenomics: Unwinding the intricacies of obligate intracellular life. PLoS ONE 2008, 3, e2018. [Google Scholar] [CrossRef]
- Parola, P. Rickettsia felis: From a rare disease in the USA to a common cause of fever in sub-Saharan Africa. Clin. Microbiol. Infect. 2011, 17, 996–1000. [Google Scholar] [CrossRef]
- Maina, A.N.; Luce-Fedrow, A.; Omulo, S.; Hang, J.; Chan, T.C.; Ade, F.; Jima, D.D.; Ogola, E.; Ge, H.; Breiman, R.F.; et al. Isolation and characterization of a novel Rickettsia species (Rickettsia asembonensis sp. nov.) obtained from cat fleas (Ctenocephalides felis). Int. J. Syst. Evol. Microbiol. 2016, 66, 4512–4517. [Google Scholar] [CrossRef] [PubMed]
- Mediannikov, O.; Aubadie-Ladrix, M.; Raoult, D. Candidatus ‘Rickettsia senegalensis’ in cat fleas in Senegal. New Microbes New Infect. 2015, 3, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, T.P.; Verhoeve, V.I.; Guillotte, M.L.; Lehman, S.S.; Rennoll, S.A.; Beier-Sexton, M.; Rahman, M.S.; Azad, A.F.; Gillespie, J.J. Wholly Rickettsia! Reconstructed metabolic profile of the quintessential bacterial parasite of eukaryotic cells. mBio 2017, 8, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- McLeod, M.P.; Qin, X.; Karpathy, S.E.; Gioia, J.; Highlander, S.K.; Fox, G.E.; McNeill, T.Z.; Jiang, H.; Muzny, D.; Jacob, L.S.; et al. Complete genome sequence of Rickettsia typhi and comparison with sequences of other rickettsiae. J. Bacteriol. 2004, 186, 5842–5855. [Google Scholar] [CrossRef] [PubMed]
- Kato, C.Y.; Chung, I.H.; Robinson, L.K.; Eremeeva, M.E.; Dasch, G.A. Genetic typing of isolates of Rickettsia typhi. PLoS Negl. Trop. Dis. 2022, 16, e0010354. [Google Scholar] [CrossRef] [PubMed]
- Sears, K.T.; Ceraul, S.M.; Gillespie, J.J.; Allen, E.D., Jr.; Popov, V.L.; Ammerman, N.C.; Rahman, M.S.; Azad, A.F. Surface proteome analysis and characterization of surface cell antigen (Sca) or autotransporter family of Rickettsia typhi. PLoS Pathog. 2012, 8, e1002856. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, T.; Kawano, H.; Kusuhara, Y. The major outer membrane protein rOmpB of spotted fever group rickettsiae functions in the rickettsial adherence to and invasion of Vero cells. Microbes Infect. 2006, 8, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.H.; Yu, X.J. Progress in rickettsial genome analysis from pioneering of Rickettsia prowazekii to the recent Rickettsia typhi. Ann. N.Y. Acad. Sci. 2005, 1063, 13–25. [Google Scholar] [CrossRef]
- Radulovic, S.; Troyer, J.M.; Beier, M.S.; Lau, A.O.; Azad, A.F. Identification and molecular analysis of the gene encoding Rickettsia typhi hemolysin. Infect. Immun. 1999, 67, 6104–6108. [Google Scholar] [CrossRef]
- Rennoll-Bankert, K.E.; Rahman, M.S.; Gillespie, J.J.; Guillotte, M.L.; Kaur, S.J.; Lehman, S.S.; Beier-Sexton, M.; Azad, A.F. Which way in? The RalF Arf-GEF orchestrates Rickettsia host cell invasion. PLoS Pathog. 2015, 11, e1005115. [Google Scholar] [CrossRef]
- Rahman, M.S.; Gillespie, J.J.; Kaur, S.J.; Sears, K.T.; Ceraul, S.M.; Beier-Sexton, M.; Azad, A.F. Rickettsia typhi possesses phospholipase A2 enzymes that are involved in infection of host cells. PLoS Pathog. 2013, 9, e1003399. [Google Scholar] [CrossRef] [PubMed]
- Voss, O.H.; Gillespie, J.J.; Lehman, S.S.; Rennoll, S.A.; Beier-Sexton, M.; Rahman, M.S.; Azad, A.F. Risk1, a phosphatidylinositol 3-kinase effector, promotes Rickettsia typhi intracellular survival. mBio 2020, 11, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Simser, J.A.; Macaluso, K.R.; Azad, A.F. Functional analysis of secA homologues from rickettsiae. Microbiology 2005, 151 Pt 2, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.J.; Rahman, M.S.; Ammerman, N.C.; Beier-Sexton, M.; Ceraul, S.M.; Gillespie, J.J.; Azad, A.F. TolC-dependent secretion of an ankyrin repeat-containing protein of Rickettsia typhi. J. Bacteriol. 2012, 194, 4920–4932. [Google Scholar] [CrossRef] [PubMed]
- Voss, O.H.; Cobb, J.; Gaytan, H.; Rivera Diaz, N.; Sanchez, R.; DeTolla, L.; Rahman, M.S.; Azad, A.F. Pathogenic, but not nonpathogenic, Rickettsia spp. evade inflammasome-dependent IL-1 responses to establish an intracytosolic replication niche. mBio 2022, 13, e0291821. [Google Scholar] [CrossRef] [PubMed]
- Mooser, H.; Castaneda, M.R. The multiplication of the virus of Mexican typhus fever in fleas. J. Exp. Med. 1932, 55, 307–323. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Walker, D.H.; Ismail, N. Emerging and re-emerging rickettsioses: Endothelial cell infection and early disease events. Nat. Rev. Microbiol. 2008, 6, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.H.; Parks, F.M.; Betz, T.G.; Taylor, J.P.; Muehlberger, J.W. Histopathology and immunohistologic demonstration of the distribution of Rickettsia typhi in fatal murine typhus. Am. J. Clin. Pathol. 1989, 91, 720–724. [Google Scholar] [CrossRef]
- Stephens, B.E.; Thi, M.; Alkhateb, R.; Agarwal, A.; Sharkey, F.E.; Dayton, C.; Anstead, G.M. Case report: Fulminant murine typhus presenting with status epilepticus and multi-organ failure: An autopsy case and a review of the neurologic presentations of murine typhus. Am. J. Trop. Med. Hyg. 2018, 99, 306–309. [Google Scholar] [CrossRef]
- Binford, C.H.; Ecker, H.D. Endemic (murine) typhus; report of autopsy findings in three cases. Am. J. Clin. Pathol. 1947, 17, 797–806. [Google Scholar] [CrossRef]
- Alarcon, J.; Sanosyan, A.; Contreras, Z.A.; Ngo, V.P.; Carpenter, A.; Hacker, J.K.; Probert, W.S.; Terashita, D.; Balter, S.; Halai, U.A. Fleaborne typhus-associated deaths—Los Angeles County, California, 2022. MMWR Morb. Mortal Wkly Rep. 2023, 72, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Rauch, J.; Muntau, B.; Eggert, P.; Tappe, D. Rickettsia typhi as cause of fatal encephalitic typhus in hospitalized patients, Hamburg, Germany, 1940–1944. Emerg. Infect. Dis. 2018, 24, 1982–1987. [Google Scholar] [CrossRef] [PubMed]
- Khairallah, M.; Ben Yahia, S.; Toumi, A.; Jelliti, B.; Loussaief, C.; Romdhane, F.B.; Messaoud, R.; Chakroun, M. Ocular manifestations associated with murine typhus. Br. J. Ophthalmol. 2009, 93, 938–942. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.H.; Popov, V.L.; Feng, H.M. Establishment of a novel endothelial target mouse model of a typhus group rickettsiosis: Evidence for critical roles for gamma interferon and CD8 T lymphocytes. Lab. Investig. 2000, 80, 1361–1372. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rollwagen, F.M.; Dasch, G.A.; Jerrells, T.R. Mechanisms of immunity to rickettsial infection: Characterization of a cytotoxic effector cell. J. Immunol. 1986, 136, 1418–1421. [Google Scholar] [CrossRef] [PubMed]
- Crist, A.E., Jr.; Wisseman, C.L., Jr.; Murphy, J.R. Characteristics of lymphoid cells that adoptively transfer immunity to Rickettsia mooseri infection in mice. Infect. Immun. 1984, 44, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.R.; Wisseman, C.L., Jr.; Fiset, P. Mechanisms of immunity in typhus infection: Analysis of immunity to Rickettsia mooseri infection of guinea pigs. Infect. Immun. 1980, 27, 730–738. [Google Scholar] [CrossRef]
- Brill, N. Pathological and experimental data derived from a further study of an acute infectious disease of unknown origin. Am. J. Med. Sci. 1911, 142, 196–218. [Google Scholar] [CrossRef]
- McNeil, H. Endemic typhus fever in South Texas. Tex. State J. Med. 1916, 12, 188–191. [Google Scholar]
- Paullin, J. Typhus fever with a report of cases. South. Med. J. 1913, 6, 36–43. [Google Scholar] [CrossRef][Green Version]
- Maxcy, K. An epidemiological study of endemic typhus (Brill’s disease) in the southeastern United States. Public Health Rep. 1926, 41, 2967–2995. [Google Scholar] [CrossRef]
- Dyer, R.E.; Rumreich, A.; Badger, L.F. Typhus fever: A virus of the typhus type derived from fleas collected from wild rats. Public Health Rep. 1931, 46, 334–338. [Google Scholar] [CrossRef]
- Dyer, R.; Workman, W.; Rumreich, A. Endemic typhus fever virus recovered from wild rat trapped at typhus focus in the United States. Public Health Reports 1932, 47, 2370–2372. [Google Scholar] [CrossRef]
- Mooser, H.; Ruiz Castaneda, M.; Zinsser, H. Rats as carriers of Mexican typhus fever. J. Am. Med. Assoc. 1931, 97, 231–232. [Google Scholar] [CrossRef]
- Traub, R.; Wisseman, C.L.; Farhang-Azad, A. The ecology of murine typhus-a critical review. Trop. Dis. Bull. 1978, 75, 237–317. [Google Scholar] [PubMed]
- Ito, S.; Vinson, J.W.; McGuire, T.J., Jr. Murine Typhus rickettsiae in the Oriental rat flea. Ann. N.Y. Acad. Sci. 1975, 266, 35–60. [Google Scholar] [CrossRef] [PubMed]
- Farhang-Azad, A.; Traub, R.; Baqar, S. Transovarial transmission of murine typhus rickettsiae in Xenopsylla cheopis fleas. Science 1985, 227, 543–545. [Google Scholar] [CrossRef] [PubMed]
- Farhang Azad, A.; Traub, R. Transmission of murine typhus rickettsiae by Xenopsylla cheopis, with notes on experimental infection and effects of temperature. Am. J. Trop. Med. Hyg. 1985, 34, 555–563. [Google Scholar] [CrossRef]
- Pratt, H.D. The changing picture of murine typhus in the United States. Ann. N.Y. Acad. Sci. 1958, 70, 516–527. [Google Scholar] [CrossRef]
- Russell, E.P. The strange career of DDT: Experts, federal capacity, and environmentalism in World War II. Technol. Cult. 1999, 40, 770–796. [Google Scholar] [CrossRef]
- Wiley, J.S. Recent developments in murine typhus fever control. Am. J. Public. Health Nations Health 1946, 36, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Hill, E.L.; Morlan, H.B. Evaluation of county-wide DDT dusting operations in murine typhus control. Public Health Rep. 1948, 63, 1635–1653. [Google Scholar] [CrossRef] [PubMed]
- Strandtmann, R.W.; Eben, D.J. A survey of typhus in rats and rat ectoparasites in Galveston, Texas. Tex. Rep. Biol. Med. 1953, 11, 144–151. [Google Scholar] [PubMed]
- Older, J.J. The epidemiology of murine typhus in Texas, 1969. JAMA 1970, 214, 2011–2017. [Google Scholar] [CrossRef] [PubMed]
- Adams, W.H.; Emmons, R.W.; Brooks, J.E. The changing ecology of murine (endemic) typhus in Southern California. Am. J. Trop. Med. Hyg. 1970, 19, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Adjemian, J.; Parks, S.; McElroy, K.; Campbell, J.; Eremeeva, M.E.; Nicholson, W.L.; McQuiston, J.; Taylor, J. Murine typhus in Austin, Texas, USA, 2008. Emerg. Infect. Dis. 2010, 16, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Blanton, L.S.; Idowu, B.M.; Tatsch, T.N.; Henderson, J.M.; Bouyer, D.H.; Walker, D.H. Opossums and cat fleas: New insights in the ecology of murine typhus in Galveston, Texas. Am. J. Trop. Med. Hyg. 2016, 95, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Maina, A.N.; Fogarty, C.; Krueger, L.; Macaluso, K.R.; Odhiambo, A.; Nguyen, K.; Farris, C.M.; Luce-Fedrow, A.; Bennett, S.; Jiang, J.; et al. Rickettsial infections among Ctenocephalides felis and host animals during a flea-borne rickettsioses outbreak in Orange County, California. PLoS ONE 2016, 11, e0160604. [Google Scholar] [CrossRef]
- Santoyo-Colin, V.; Sanchez-Montes, S.; Salceda-Sanchez, B.; Huerta-Jimenez, H.; Alcantara-Rodriguez, V.; Becker, I.; Gual-Sill, F.; Lopez-Perez, A.M. Urban foci of murine typhus involving cat fleas (Ctenocephalides felis felis) collected from opossums in Mexico City. Zoonoses Public Health 2021, 68, 1–7. [Google Scholar] [CrossRef]
- Brigham, G.D. Susceptibility of the opossum (Didelphis virginiana) to the virus of endemic typhus fever. Public Health Rep. 1936, 51, 333–337. [Google Scholar]
- Blanton, L.S.; Quade, B.R.; Ramirez-Hernandez, A.; Mendell, N.L.; Villasante-Tezanos, A.; Bouyer, D.H.; VandeBerg, J.L.; Walker, D.H. Experimental Rickettsia typhi infection in Monodelphis domestica: Implications for opossums as an amplifying host in the suburban cycle of murine typhus. Am. J. Trop. Med. Hyg. 2022, 107, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Farhang-Azad, A.; Traub, R.; Sofi, M.; Wisseman, C.L., Jr. Experimental murine typhus infection in the cat flea, Ctenocephalides felis (Siphonaptera: Pulicidae). J. Med. Entomol. 1984, 21, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Irons, J.V.; Bohls, S.W.; Thurman, D.C.; McGregor, T. Probable role of the cat flea, Ctenocephalides felis, in transmission of murine typhus. Am. J. Trop. Med. 1944, 24, 359–362. [Google Scholar] [CrossRef]
- Keaton, R.; Nash, B.J.; Murphy, J.N., Jr.; Irons, J.V. Complement fixation tests for murine typhus on small mammals. Public Health Rep. 1953, 68, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Rust, M.K.; Dryden, M.W. The biology, ecology, and management of the cat flea. Annu. Rev. Entomol. 1997, 42, 451–473. [Google Scholar] [CrossRef] [PubMed]
- Lepine, P.; Lorando, N. Etude d’un foyer de typhus endemique d’origine murine. Bull. Soc. Pathol. Exot. Ses Fil. 1936, 29, 285–295. [Google Scholar]
- Mullins, K.E.; Maina, A.N.; Krueger, L.; Jiang, J.; Cummings, R.; Drusys, A.; Williams, G.; Dhillon, M.; Richards, A.L. Rickettsial infections among cats and cat fleas in Riverside County, California. Am. J. Trop. Med. Hyg. 2018, 99, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.; Maina, A.N.; Brisco, A.; Foo, C.; Croker, C.; Ngo, V.; Civen, R.; Richards, A.L.; Fujioka, K.; Wekesa, J.W. A 2015 outbreak of flea-borne rickettsiosis in San Gabriel Valley, Los Angeles County, California. PLoS Negl. Trop. Dis. 2018, 12, e0006385. [Google Scholar] [CrossRef]
- Blanton, L.S.; Vohra, R.F.; Fistein, L.; Quade, B.; Walker, D.H.; Bouyer, D.H. Rickettsiae within the fleas of feral cats in Galveston, Texas. Vector Borne Zoonotic Dis. 2019, 19, 647–651. [Google Scholar] [CrossRef]
- Kuo, C.C.; Wardrop, N.; Chang, C.T.; Wang, H.C.; Atkinson, P.M. Significance of major international seaports in the distribution of murine typhus in Taiwan. PLoS Negl. Trop. Dis. 2017, 11, e0005430. [Google Scholar]
- Blanton, L.S.; Vohra, R.F.; Bouyer, D.H.; Walker, D.H. Reemergence of murine typhus in Galveston, Texas, USA, 2013. Emerg. Infect. Dis. 2015, 21, 484–486. [Google Scholar] [CrossRef]
- Acuna-Soto, R.; Calderon-Romero, L.; Romero-Lopez, D.; Bravo-Lindoro, A. Murine typhus in Mexico City. Trans. R. Soc. Trop. Med. Hyg. 2000, 94, 45. [Google Scholar] [CrossRef]
- Hidalgo, M.; Montoya, V.; Martinez, A.; Mercado, M.; De la Ossa, A.; Velez, C.; Estrada, G.; Perez, J.E.; Faccini-Martinez, A.A.; Labruna, M.B.; et al. Flea-borne rickettsioses in the north of Caldas province, Colombia. Vector Borne Zoonotic Dis. 2013, 13, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Aita, T.; Sando, E.; Katoh, S.; Hamaguchi, S.; Fujita, H.; Kurita, N. Nonnegligible seroprevalence and predictors of murine typhus, Japan. Emerg. Infect. Dis. 2023, 29, 1438–1442. [Google Scholar] [CrossRef] [PubMed]
- Gikas, A.; Doukakis, S.; Pediaditis, J.; Kastanakis, S.; Psaroulaki, A.; Tselentis, Y. Murine typhus in Greece: Epidemiological, clinical, and therapeutic data from 83 cases. Trans. R. Soc. Trop. Med. Hyg. 2002, 96, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu-Wittel, M.; Pachon, J.; Alarcon, A.; Lopez-Cortes, L.F.; Viciana, P.; Jimenez-Mejias, M.E.; Villanueva, J.L.; Torronteras, R.; Caballero-Granado, F.J. Murine typhus as a common cause of fever of intermediate duration: A 17-year study in the south of Spain. Arch. Intern. Med. 1999, 159, 872–876. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.H.; Dong, T.; Zhang, H.L.; Wang, S.W.; Yu, H.L.; Zhang, Y.Z.; Liu, Y.H.; Yin, Z.L.; Feng, Y.; Qu, Z.Y.; et al. Murine typhus in drug detoxification facility, Yunnan Province, China, 2010. Emerg. Infect. Dis. 2012, 18, 1388–1390. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.; Salguero, E.; de la Ossa, A.; Sanchez, R.; Vesga, J.F.; Orejuela, L.; Valbuena, G. Murine typhus in Caldas, Colombia. Am. J. Trop. Med. Hyg. 2008, 78, 321–322. [Google Scholar] [CrossRef]
- Bhengsri, S.; Baggett, H.C.; Edouard, S.; Dowell, S.F.; Dasch, G.A.; Fisk, T.L.; Raoult, D.; Parola, P. Sennetsu neorickettsiosis, spotted fever group, and typhus group rickettsioses in three provinces in Thailand. Am. J. Trop. Med. Hyg. 2016, 95, 43–49. [Google Scholar] [CrossRef]
- Mayxay, M.; Sengvilaipaseuth, O.; Chanthongthip, A.; Dubot-Peres, A.; Rolain, J.M.; Parola, P.; Craig, S.B.; Tulsiani, S.; Burns, M.A.; Khanthavong, M.; et al. Causes of fever in rural southern Laos. Am. J. Trop. Med. Hyg. 2015, 93, 517–520. [Google Scholar] [CrossRef]
- Roberts, T.; Parker, D.M.; Bulterys, P.L.; Rattanavong, S.; Elliott, I.; Phommasone, K.; Mayxay, M.; Chansamouth, V.; Robinson, M.T.; Blacksell, S.D.; et al. A spatio-temporal analysis of scrub typhus and murine typhus in Laos; implications from changing landscapes and climate. PLoS Negl. Trop. Dis. 2021, 15, e0009685. [Google Scholar] [CrossRef] [PubMed]
- Riswari, S.F.; Prodjosoewojo, S.; Mony, S.R.; Megantara, I.; Iskandar, S.; Mayasari, W.; Heryaman, H.; Mast, Q.; der Ven, A.V.; Kosasih, H.; et al. Murine typhus is a common cause of acute febrile illness in Bandung, Indonesia. PLoS ONE 2023, 18, e0283135. [Google Scholar] [CrossRef] [PubMed]
- Trung, N.V.; Hoi, L.T.; Hoa, T.M.; Huong, D.T.; Huyen, M.T.; Tien, V.Q.; Mai, D.T.T.; Ha, N.T.T.; Kinh, N.V.; Farris, C.M.; et al. Systematic surveillance of rickettsial diseases in 27 hospitals from 26 provinces throughout Vietnam. Trop. Med. Infect. Dis. 2022, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, R.; Shrestha, U.; Gautam, S.C.; Thorson, S.; Shrestha, K.; Yadav, B.K.; Kelly, D.F.; Adhikari, N.; Pollard, A.J.; Murdoch, D.R. Bloodstream infection among children presenting to a general hospital outpatient clinic in urban Nepal. PLoS ONE 2012, 7, e47531. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Rahman, M.Z.; Banu, S.; Rahman, M.; Chisti, M.J.; Chowdhury, F.; Akhtar, Z.; Palit, A.; Martin, D.W.; Anwar, M.U.; et al. Acute febrile illness among outpatients seeking health care in Bangladeshi hospitals prior to the COVID-19 pandemic. PLoS ONE 2022, 17, e0273902. [Google Scholar] [CrossRef] [PubMed]
- Devamani, C.S.; Schmidt, W.P.; Ariyoshi, K.; Anitha, A.; Kalaimani, S.; Prakash, J.A.J. Risk factors for scrub typhus, murine typhus, and spotted fever seropositivity in urban areas, rural plains, and peri-forest hill villages in South India: A Cross-Sectional Study. Am. J. Trop. Med. Hyg. 2020, 103, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Alonso, B.; Almeida, H.; Alonso-Sardon, M.; Velasco-Tirado, V.; Robaina Bordon, J.M.; Carranza Rodriguez, C.; Perez Arellano, J.L.; Belhassen-Garcia, M. Murine typhus. How does it affect us in the 21st century? The epidemiology of inpatients in Spain (1997–2015). Int. J. Infect. Dis. 2020, 96, 165–171. [Google Scholar] [CrossRef]
- Rogozin, E.; Lazarovitch, T.; Weinberger, M. High morbidity due to murine typhus upsurge in urban neighborhoods in Central Israel. Am. J. Trop. Med. Hyg. 2019, 100, 952–956. [Google Scholar] [CrossRef]
- Faccini-Martinez, A.A.; Walker, D.H.; Blanton, L.S. Murine typhus in Latin America: Perspectives of a once recognized but now neglected vector-borne disease. Am. J. Trop. Med. Hyg. 2022, 107, 740–746. [Google Scholar] [CrossRef]
- Dzul-Rosado, K.; Gonzalez-Martinez, P.; Peniche-Lara, G.; Zavala-Velazquez, J.; Zavala-Castro, J. Murine typhus in humans, Yucatan, Mexico. Emerg. Infect. Dis. 2013, 19, 1021–1022. [Google Scholar] [CrossRef]
- Sanchez-Montes, S.; Colunga-Salas, P.; Fernandez-Figueroa, E.A.; Medel, M.L.H.; Benitez, C.R.; Becker, I. Murine typhus in Mexico City: Report of an imported case. Rev. Inst. Med. Trop. Sao Paulo 2019, 61, e16. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease, C. Prevention, Murine typhus--Hawaii, 2002. MMWR Morb Mortal Wkly Rep 2003, 52, 1224–1226. [Google Scholar]
- Eremeeva, M.E.; Karpathy, S.E.; Krueger, L.; Hayes, E.K.; Williams, A.M.; Zaldivar, Y.; Bennett, S.; Cummings, R.; Tilzer, A.; Velten, R.K.; et al. Two pathogens and one disease: Detection and identification of flea-borne rickettsiae in areas endemic for murine typhus in California. J. Med. Entomol. 2012, 49, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.O.; Evert, N.; Mayes, B.; Fonken, E.; Erickson, T.; Garcia, M.N.; Sidwa, T. Typhus group rickettsiosis, Texas, USA, 2003–2013. Emerg. Infect. Dis. 2017, 23, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Blanton, L.S.; Caravedo Martinez, M.A.; Mendell, N.; Villasante-Tezanos, A.; Walker, D.H.; Bouyer, D. Increased seroprevalence of typhus group rickettsiosis, Galveston County, Texas, USA. Emerg. Infect. Dis. 2023, 29, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Jensenius, M.; Han, P.V.; Schlagenhauf, P.; Schwartz, E.; Parola, P.; Castelli, F.; von Sonnenburg, F.; Loutan, L.; Leder, K.; Freedman, D.O. Acute and potentially life-threatening tropical diseases in western travelers—A GeoSentinel multicenter study, 1996–2011. Am. J. Trop. Med. Hyg. 2013, 88, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Walter, G.; Botelho-Nevers, E.; Socolovschi, C.; Raoult, D.; Parola, P. Murine typhus in returned travelers: A report of thirty-two cases. Am. J. Trop. Med. Hyg. 2012, 86, 1049–1053. [Google Scholar] [CrossRef]
- Raby, E.; Dyer, J.R. Endemic (murine) typhus in returned travelers from Asia, a case series: Clues to early diagnosis and comparison with dengue. Am. J. Trop. Med. Hyg. 2013, 88, 701–703. [Google Scholar] [CrossRef]
- Mashru, J.S.; Bogoch, I.I. Murine typhus in returned travelers to Toronto, Canada. Travel. Med. Infect. Dis. 2023, 53, 102587. [Google Scholar] [CrossRef]
- Warrell, C.E.; Osborne, J.; Nabarro, L.; Gibney, B.; Carter, D.P.; Warner, J.; Houlihan, C.F.; Brooks, T.J.G.; Rampling, T. Imported rickettsial infections to the United Kingdom, 2015–2020. J. Infect. 2023, 86, 446–452. [Google Scholar] [CrossRef]
- Miller, E.S.; Beeson, P.B. Murine typhus fever. Medicine 1946, 25, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Stuart, B.M.; Pullen, R.L. Endemic (murine) typhus fever: Clinical observations of 180 cases. Ann. Intern. Med. 1945, 23, 17. [Google Scholar]
- Kaplowitz, L.G.; Robertson, G.L. Hyponatremia in Rocky Mountain spotted fever: Role of antidiuretic hormone. Ann. Intern. Med. 1983, 98, 334–335. [Google Scholar] [CrossRef] [PubMed]
- Afzal, Z.; Kallumadanda, S.; Wang, F.; Hemmige, V.; Musher, D. Acute febrile illness and complications due to murine typhus, Texas, USA. Emerg. Infect. Dis. 2017, 23, 1268–1273. [Google Scholar] [CrossRef] [PubMed]
- Lin, I.F.; Lin, J.N.; Tsai, C.T.; Wu, Y.Y.; Chen, Y.H.; Lai, C.H. Serum C-reactive protein and procalcitonin values in acute Q fever, scrub typhus, and murine typhus. BMC Infect. Dis. 2020, 20, 334. [Google Scholar] [CrossRef] [PubMed]
- Shaked, Y.; Samra, Y.; Maeir, M.K.; Rubinstein, E. Murine typhus and spotted fever in Israel in the eighties: Retrospective analysis. Infection 1988, 16, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Hernandez Cabrera, M.; Angel-Moreno, A.; Santana, E.; Bolanos, M.; Frances, A.; Martin-Sanchez, M.S.; Perez-Arellano, J.L. Murine typhus with renal involvement in Canary Islands, Spain. Emerg. Infect. Dis. 2004, 10, 740–743. [Google Scholar] [CrossRef]
- Blanton, L.S.; Berman, M.A.; Afrouzian, M. Case report: Renal failure due to focal segmental glomerulosclerosis in a patient with murine typhus. Am. J. Trop. Med. Hyg. 2020, 103, 1017–1019. [Google Scholar] [CrossRef]
- van der Vaart, T.W.; van Thiel, P.P.; Juffermans, N.P.; van Vugt, M.; Geerlings, S.E.; Grobusch, M.P.; Goorhuis, A. Severe murine typhus with pulmonary system involvement. Emerg. Infect. Dis. 2014, 20, 1375–1377. [Google Scholar] [CrossRef]
- Whelton, A.; Donadio, J.V., Jr.; Elisberg, B.L. Acute renal failure complicating rickettsial infections in glucose-6-phosphate dehydrogenase-deficient individuals. Ann. Intern. Med. 1968, 69, 323–328. [Google Scholar] [CrossRef]
- Walker, D.H. The role of host factors in the severity of spotted fever and typhus rickettsioses. Ann. N.Y. Acad. Sci. 1990, 590, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Doppler, J.F.; Newton, P.N. A systematic review of the untreated mortality of murine typhus. PLoS Negl. Trop. Dis. 2020, 14, e0008641. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, S.; Rattanavong, S.; Lee, S.J.; Panyanivong, P.; Craig, S.B.; Tulsiani, S.M.; Blacksell, S.D.; Dance, D.A.; Dubot-Peres, A.; Sengduangphachanh, A.; et al. Orientia, rickettsia, and leptospira pathogens as causes of CNS infections in Laos: A prospective study. Lancet Glob. Health 2015, 3, e104–e112. [Google Scholar] [CrossRef] [PubMed]
- Talhelm, C.F.; Helms, J.L.; Tran, L.T.; Contreras, B.X.; Stevens, M.L.; Sierra-Hoffman, M.; Castro-Lainez, M.T.; Deliz, R.J. Rickettsia typhi central nervous system infection. IDCases 2020, 21, e00852. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, S.; Sunyakumthorn, P.; Rattanavong, S.; Phetsouvanh, R.; Panyanivong, P.; Sengduangphachanh, A.; Phouminh, P.; Anantatat, T.; Chanthongthip, A.; Lee, S.J.; et al. Blood-brain barrier function and biomarkers of central nervous system injury in rickettsial versus other neurological infections in Laos. Am. J. Trop. Med. Hyg. 2015, 93, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Silpapojakul, K.; Ukkachoke, C.; Krisanapan, S.; Silpapojakul, K. Rickettsial meningitis and encephalitis. Arch. Intern. Med. 1991, 151, 1753–1757. [Google Scholar] [CrossRef] [PubMed]
- Vander, T.; Medvedovsky, M.; Valdman, S.; Herishanu, Y. Facial paralysis and meningitis caused by Rickettsia typhi infection. Scand. J. Infect. Dis. 2003, 35, 886–887. [Google Scholar] [CrossRef]
- Moy, W.L.; Ooi, S.T. Abducens nerve palsy and meningitis by Rickettsia typhi. Am. J. Trop. Med. Hyg. 2015, 92, 620–624. [Google Scholar] [CrossRef]
- Simon, N.G.; Cremer, P.D.; Graves, S.R. Murine typhus returns to New South Wales: A case of isolated meningoencephalitis with raised intracranial pressure. Med. J. Aust. 2011, 194, 652–654. [Google Scholar] [CrossRef]
- Shafi, H.; Hipolito, L.G. Murine typhus presenting with status epilepticus. Int. J. Infect. Dis. 2019, 83, 145–147. [Google Scholar] [CrossRef]
- Rosenblum, M.J.; Masland, R.L.; Harrell, G.T. Residual effects of rickettsial disease on the central nervous system; results of neurologic examinations and electroencephalograms following Rocky Mountain spotted fever. AMA Arch. Intern. Med. 1952, 90, 444–455. [Google Scholar] [CrossRef] [PubMed]
- Gorman, R.J.; Saxon, S.; Snead, O.C., 3rd. Neurologic sequelae of Rocky Mountain spotted fever. Pediatrics 1981, 67, 354–357. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, M.J.; Byrge, K.C.; Ivey, K.S.; Pruthi, S.; Bloch, K.C. Meningoencephalitis due to spotted fever rickettsioses, including Rocky Mountain spotted fever. Clin. Infect. Dis. 2020, 71, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Carr, S.B.; Bergamo, D.F.; Emmanuel, P.J.; Ferreira, J.A. Murine typhus as a cause of cognitive impairment: Case report and a review of the literature. Pediatr. Neurol. 2014, 50, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Lew, H.L.; Lane, B.; Zeiner, H. Neuroimaging and clinical manifestations of bilateral temporal encephalopathy secondary to murine typhus infection. Am. J. Phys. Med. Rehabil. 2005, 84, 310–311. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Samra, Y.; Shaked, Y.; Maier, M.K. Delayed neurologic display in murine typhus. Report of two cases. Arch. Intern. Med. 1989, 149, 949–951. [Google Scholar] [CrossRef] [PubMed]
- Hudson, H.L.; Thach, A.B.; Lopez, P.F. Retinal manifestations of acute murine typhus. Int. Ophthalmol. 1997, 21, 121–126. [Google Scholar] [CrossRef]
- Shukla, K.; Fergie, J. Murine typhus associated with Parinaud’s oculoglandular syndrome in 2 children. Pediatr. Infect. Dis. J. 2014, 33, 1195–1196. [Google Scholar] [CrossRef]
- Dixon, M.K.; Dayton, C.L.; Anstead, G.M. Parinaud’s oculoglandular syndrome: A case in an adult with flea-borne typhus and a review. Trop. Med. Infect. Dis. 2020, 5, 126. [Google Scholar] [CrossRef]
- Biggs, H.M.; Behravesh, C.B.; Bradley, K.K.; Dahlgren, F.S.; Drexler, N.A.; Dumler, J.S.; Folk, S.M.; Kato, C.Y.; Lash, R.R.; Levin, M.L.; et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis—United States. MMWR Recomm. Rep. 2016, 65, 1–44. [Google Scholar] [CrossRef]
- Blanton, L.S.; Walker, D.H.; Bouyer, D.H. Rickettsia and Orientia In Manual of Clinical Microbiology, 12th ed.; Carroll, K.C., Pfaller, M.A., Landry, M.L., McAdam, A.J., Patel, R., Richter, S.S., Warnock, D.W., Eds.; ASM Press: Washington, DC, USA, 2019; Volume 1, pp. 1149–1162. [Google Scholar]
- Paris, D.H.; Dumler, J.S. State of the art of diagnosis of rickettsial diseases: The use of blood specimens for diagnosis of scrub typhus, spotted fever group rickettsiosis, and murine typhus. Curr. Opin. Infect. Dis. 2016, 29, 433–439. [Google Scholar] [CrossRef]
- Phakhounthong, K.; Mukaka, M.; Dittrich, S.; Tanganuchitcharnchai, A.; Day, N.P.J.; White, L.J.; Newton, P.N.; Blacksell, S.D. The temporal dynamics of humoral immunity to Rickettsia typhi infection in murine typhus patients. Clin. Microbiol. Infect. 2020, 26, 781.e9–781.e16. [Google Scholar] [CrossRef] [PubMed]
- Lokida, D.; Sudarmono, P.; Kosasih, H.; Butar-Butar, D.P.; Salim, G.; Antonjaya, U.; Sari, R.A.; Aman, A.T.; Parwati, I.; Arif, M.; et al. Comparison of commercial enzyme-linked immunosorbent assay and immunofluorescence assay for diagnosis of acute Rickettsia typhi infections. Vector Borne Zoonotic Dis. 2020, 20, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Hechemy, K.E.; Stevens, R.W.; Sasowski, S.; Michaelson, E.E.; Casper, E.A.; Philip, R.N. Discrepancies in Weil-Felix and microimmunofluorescence test results for Rocky Mountain spotted fever. J. Clin. Microbiol. 1979, 9, 292–293. [Google Scholar] [CrossRef] [PubMed]
- La Scola, B.; Rydkina, L.; Ndihokubwayo, J.B.; Vene, S.; Raoult, D. Serological differentiation of murine typhus and epidemic typhus using cross-adsorption and Western blotting. Clin. Diagn. Lab. Immunol. 2000, 7, 612–616. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chao, C.C.; Belinskaya, T.; Zhang, Z.; Ching, W.M. Development of recombinase polymerase amplification assays for detection of Orientia tsutsugamushi or Rickettsia typhi. PLoS Negl. Trop. Dis. 2015, 9, e0003884. [Google Scholar] [CrossRef]
- Dittrich, S.; Castonguay-Vanier, J.; Moore, C.E.; Thongyoo, N.; Newton, P.N.; Paris, D.H. Loop-mediated isothermal amplification for Rickettsia typhi (the causal agent of murine typhus): Problems with diagnosis at the limit of detection. J. Clin. Microbiol. 2014, 52, 832–838. [Google Scholar] [CrossRef]
- Chung, I.H.; Robinson, L.K.; Stewart-Juba, J.J.; Dasch, G.A.; Kato, C.Y. Analytically sensitive Rickettsia species detection for laboratory diagnosis. Am. J. Trop. Med. Hyg. 2022, 106, 1352–1357. [Google Scholar] [CrossRef]
- Centeno, F.H.; Lasco, T.; Ahmed, A.A.; Al Mohajer, M. Characteristics of Rickettsia typhi infections detected with next-generation sequencing of microbial cell-free deoxyribonucleic acid in a tertiary care hospital. Open Forum Infect. Dis. 2021, 8, ofab147. [Google Scholar] [CrossRef]
- Stafford, I.A.; Centeno, F.H.; Al Mohajer, M.; Parkerson, G.; Woc-Colburn, L.; Burgos-Lee, A.J.; Rac, M.; Dunn, J.; Muldrew, K. Successful detection of unrecognized Rickettsia typhi in pregnancy using cell-free next-generation sequencing. Case Rep. Obstet. Gynecol. 2020, 2020, 6767351. [Google Scholar] [CrossRef]
- Qian, P.; He, X.; Yang, M.; Wei, L.; Zhang, L.; Xing, X. Detection of severe murine typhus by nanopore targeted sequencing, China. Emerg. Infect. Dis. 2023, 29, 1275–1277. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.H.; Feng, H.M.; Ladner, S.; Billings, A.N.; Zaki, S.R.; Wear, D.J.; Hightower, B. Immunohistochemical diagnosis of typhus rickettsioses using an anti-lipopolysaccharide monoclonal antibody. Mod. Pathol. 1997, 10, 1038–1042. [Google Scholar] [PubMed]
- Newton, P.N.; Keolouangkhot, V.; Lee, S.J.; Choumlivong, K.; Sisouphone, S.; Choumlivong, K.; Vongsouvath, M.; Mayxay, M.; Chansamouth, V.; Davong, V.; et al. A prospective, open-label, randomized trial of doxycycline versus azithromycin for the treatment of uncomplicated murine typhus. Clin. Infect. Dis. 2019, 68, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Howard, A.; Fergie, J. Murine typhus in South Texas children: An 18-Year Review. Pediatr. Infect. Dis. J. 2018, 37, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Caravedo Martinez, M.A.; Ramirez-Hernandez, A.; Blanton, L.S. Manifestations and management of flea-borne rickettsioses. Res. Rep. Trop. Med. 2021, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rolain, J.M.; Maurin, M.; Vestris, G.; Raoult, D. In vitro susceptibilities of 27 rickettsiae to 13 antimicrobials. Antimicrob. Agents Chemother. 1998, 42, 1537–1541. [Google Scholar] [CrossRef] [PubMed]
- Dumler, J.S. Clincial Disease: Current Treatment and New Challenges. In Intracellular Pathogens II: Rickettsiales; Palmer, G.H., Azad, A.F., Eds.; ASM Press: Washington, DC, USA, 2012; pp. 1–39. [Google Scholar]
- Moffa, M.; Brook, I. Tetracyclines, glycyclines, and chloramphenicol. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 9th ed.; Bennett, J.E., Dolin, R., Blaser, M.J., Eds.; Elsevier: Philadelphia, PA, USA, 2020; Volume 1, pp. 318–336. [Google Scholar]
- Quade, B.R.; Ramirez-Hernandez, A.; Blanton, L.S. In vitro susceptibility of Rickettsia species to eravacycline, omadacycline, and tigecycline. Antimicrob. Agents Chemother. 2021, 65, e0066521. [Google Scholar] [CrossRef] [PubMed]
- Luciani, F.; Cione, E.; Corsonello, A.; Guido, F.; De Santis, S.; Cannataro, R.; Perri, M.; Caroleo, M.C.; Cannataro, A.M. Spotted fever from Rickettsia typhi in an older woman: A case report from a geographic area where it would not be expected. Int. J. Infect. Dis. 2014, 27, 10–12. [Google Scholar] [CrossRef]
- Wilson, P.A.; Tierney, L.; Lai, K.; Graves, S. Queensland tick typhus: Three cases with unusual clinical features. Intern. Med. J. 2013, 43, 823–825. [Google Scholar] [CrossRef]
- Mastroianni, A.; Greco, S.; Urso, F.; Mauro, M.V.; Vangeli, V. Does tigecycline have a place in therapy for rickettsial infection of the central nervous system? Infect. Chemother. 2022, 54, 165–172. [Google Scholar] [CrossRef]
- Gikas, A.; Doukakis, S.; Pediaditis, J.; Kastanakis, S.; Manios, A.; Tselentis, Y. Comparison of the effectiveness of five different antibiotic regimens on infection with Rickettsia typhi: Therapeutic data from 87 cases. Am. J. Trop. Med. Hyg. 2004, 70, 576–579. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Laferl, H.; Fournier, P.E.; Seiberl, G.; Pichler, H.; Raoult, D. Murine typhus poorly responsive to ciprofloxacin: A case report. J. Travel. Med. 2002, 9, 103–104. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Beltran, R.; Herrero Herrero, J.I. Deleterious effect of trimethoprim-sulfamethoxazole in Mediterranean spotted fever. Antimicrob. Agents Chemother. 1992, 36, 1342–1343. [Google Scholar] [CrossRef] [PubMed]
- Kimberil, D.W.; Brady, M.T.; Jackson, M.A.; Long, S.S. Endemic typhus (murine typhus). In Red Book 2018: Report of the Committee on Infectious Diseases, 31st ed.; American Academy of Pediatrics: Elk Grove Village, IL, USA, 2018; pp. 864–865. [Google Scholar]
- Grossman, E.R.; Walchek, A.; Freedman, H. Tetracyclines and permanent teeth: The relation between dose and tooth color. Pediatrics 1971, 47, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Todd, S.R.; Dahlgren, F.S.; Traeger, M.S.; Beltran-Aguilar, E.D.; Marianos, D.W.; Hamilton, C.; McQuiston, J.H.; Regan, J.J. No visible dental staining in children treated with doxycycline for suspected Rocky Mountain spotted fever. J. Pediatr. 2015, 166, 1246–1251. [Google Scholar] [CrossRef] [PubMed]
- Lochary, M.E.; Lockhart, P.B.; Williams, W.T., Jr. Doxycycline and staining of permanent teeth. Pediatr. Infect. Dis. J. 1998, 17, 429–431. [Google Scholar] [CrossRef] [PubMed]
- McGready, R.; Prakash, J.A.; Benjamin, S.J.; Watthanaworawit, W.; Anantatat, T.; Tanganuchitcharnchai, A.; Ling, C.L.; Tan, S.O.; Ashley, E.A.; Pimanpanarak, M.; et al. Pregnancy outcome in relation to treatment of murine typhus and scrub typhus infection: A fever cohort and a case series analysis. PLoS Negl. Trop. Dis. 2014, 8, e3327. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, M.B.; Blanton, L.S.; La Rosa, M.; Webb, C.M. Murine typhus infection in pregnancy: Case series and literature review. Pathogens 2021, 10, 219. [Google Scholar] [CrossRef]
- Cross, R.; Ling, C.; Day, N.P.; McGready, R.; Paris, D.H. Revisiting doxycycline in pregnancy and early childhood--time to rebuild its reputation? Expert. Opin. Drug Saf. 2016, 15, 367–382. [Google Scholar] [CrossRef]
- Kaundinnyayana, S.; Kamath, A. Doxycycline use and adverse pregnancy or neonatal outcomes: A descriptive study using the United States Food and Drug Administration Adverse Event Reporting System database. Health Sci. Rep. 2022, 5, e931. [Google Scholar] [CrossRef]
- Silva, L.J.; Papaiordanou, P.M. Murine (endemic) typhus in Brazil: Case report and review. Rev. Inst. Med. Trop. Sao Paulo 2004, 46, 283–285. [Google Scholar] [CrossRef] [PubMed]
- Mohr, C.O.; Morlan, H.B. The nature of parasitism of the opossum by fleas in southwestern Georgia. J. Parasitol. 1959, 45, 233–237. [Google Scholar] [CrossRef]
- Vohra, R.F.; Walker, D.H.; Blanton, L.S. Analysis of health-care charges in murine typhus: Need for improved clinical recognition and diagnostics for acute disease. Am. J. Trop. Med. Hyg. 2018, 98, 1594. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanton, L.S. Murine Typhus: A Review of a Reemerging Flea-Borne Rickettsiosis with Potential for Neurologic Manifestations and Sequalae. Infect. Dis. Rep. 2023, 15, 700-716. https://doi.org/10.3390/idr15060063
Blanton LS. Murine Typhus: A Review of a Reemerging Flea-Borne Rickettsiosis with Potential for Neurologic Manifestations and Sequalae. Infectious Disease Reports. 2023; 15(6):700-716. https://doi.org/10.3390/idr15060063
Chicago/Turabian StyleBlanton, Lucas S. 2023. "Murine Typhus: A Review of a Reemerging Flea-Borne Rickettsiosis with Potential for Neurologic Manifestations and Sequalae" Infectious Disease Reports 15, no. 6: 700-716. https://doi.org/10.3390/idr15060063
APA StyleBlanton, L. S. (2023). Murine Typhus: A Review of a Reemerging Flea-Borne Rickettsiosis with Potential for Neurologic Manifestations and Sequalae. Infectious Disease Reports, 15(6), 700-716. https://doi.org/10.3390/idr15060063




