Utility of Liver Biopsy in the Diagnosis and Management of Possible Drug-Induced Liver Injury in Patients Receiving Antituberculosis Therapy: A Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Tuberculosis Report. 2022. Available online: https://www.who.int/publications/i/item/9789240061729 (accessed on 8 July 2023).
- Rathi, C.; Pipaliya, N.; Patel, R.; Ingle, M.; Phadke, A.; Sawant, P. Drug Induced Liver Injury at a Tertiary Hospital in India: Etiology, Clinical Features and Predictors of Mortality. Ann. Hepatol. 2017, 16, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Abbara, A.; Chitty, S.; Roe, J.K.; Ghani, R.; Collin, S.M.; Ritchie, A.; Kon, O.M.; Dzvova, J.; Davidson, H.; Edwards, T.E.; et al. Drug-induced liver injury from antituberculous treatment: A retrospective study from a large TB centre in the UK. BMC Infect. Dis. 2017, 17, 231. [Google Scholar] [CrossRef] [PubMed]
- Zhong, T.; Fan, Y.; Dong, X.L.; Guo, X.; Wong, K.H.; Wong, W.T.; He, D.; Liu, S. An Investigation of the Risk Factors Associated with Anti-Tuberculosis Drug-Induced Liver Injury or Abnormal Liver Functioning in 757 Patients with Pulmonary Tuberculosis. Front. Pharmacol. 2021, 12, 708522. [Google Scholar] [CrossRef] [PubMed]
- Brennan, P.N.; Cartlidge, P.; Manship, T.; Dillon, J.F. Guideline review: EASL clinical practice guidelines: Drug-induced liver injury (DILI). Frontline Gastroenterol. 2021, 13, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Danan, G.; Benichou, C. Causality assessment of adverse reactions to drugs--I. A novel method based on the conclusions of international consensus meetings: Application to drug-induced liver injuries. J. Clin. Epidemiol. 1993, 46, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E. The pathology of drug-induced liver injury. Semin. Liver Dis. 2009, 29, 364–372. [Google Scholar] [CrossRef] [PubMed]
- WHO Consolidated Guidelines on Tuberculosis: Module 4: Treatment: Drug-Susceptible Tuberculosis Treatment. Available online: https://www.who.int/publications/i/item/9789240048126 (accessed on 8 July 2023).
- WHO Consolidated Guidelines on Tuberculosis: Module 4: Treatment: Drug-Resistant Tuberculosis Treatment. Available online: https://www.who.int/publications/i/item/9789240007048 (accessed on 8 July 2023).
- World Health Organization (WHO). Active Tuberculosis Drugsafety Monitoring and Management (aDSM). Framework for Implementation. (WHO/HTM/TB/2015.28). Geneva, WHO. 2015. Available online: http://apps.who.int/iris/bitstream/10665/204465/1/WHO_HTM_TB_2015.28_eng.pdf (accessed on 8 July 2023).
- Saukkonen, J.J.; Cohn, D.L.; Jasmer, R.M.; Schenker, S.; Jereb, J.A.; Nolan, C.M.; Peloquin, C.A.; Gordin, F.M.; Nunes, D.; Strader, D.B.; et al. ATS (American Thoracic Society) Hepatotoxicity of Antituberculosis Therapy Subcommittee. An official ATS statement: Hepatotoxicity of antituberculosis therapy. Am. J. Respir. Crit. Care Med. 2006, 174, 935–952. [Google Scholar] [CrossRef]
- Meeting Report of the WHO Expert Consultation on Drug-Resistant Tuberculosis Treatment Outcome Definitions. Available online: https://www.who.int/publications/i/item/9789240022195 (accessed on 8 May 2023).
- Gualano, G.; Mencarini, P.; Musso, M.; Mosti, S.; Santangelo, L.; Murachelli, S.; Cannas, A.; Di Caro, A.; Navarra, A.; Goletti, D.; et al. Putting in harm to cure: Drug related adverse events do not affect outcome of patients receiving treatment for multidrug-resistant Tuberculosis. Experience from a tertiary hospital in Italy. PLoS ONE 2019, 14, e0212948. [Google Scholar] [CrossRef]
- Hosford, J.D.; von Fricken, M.E.; Lauzardo, M.; Chang, M.; Dai, Y.; Lyon, J.A.; Shuster, J.; Fennelly, K.P. Hepatotoxicity from antituberculous therapy in the elderly: A systematic review. Tuberculosis 2015, 95, 112–122. [Google Scholar] [CrossRef]
- Gülbay, B.E.; Gürkan, O.U.; Yildiz, O.A.; Onen, Z.P.; Erkekol, F.O.; Baççioğlu, A.; Acican, T. Side effects due to primary antituberculosis drugs during the initial phase of therapy in 1149 hospitalized patients for tuberculosis. Respir. Med. 2006, 100, 1834–1842. [Google Scholar] [CrossRef]
- Gaude, G.S.; Chaudhury, A.; Hattiholi, J. Drug-induced hepatitis and the risk factors for liver injury in pulmonary tuberculosis patients. J. Fam. Med. Prim. Care. 2015, 4, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Tostmann, A.; Boeree, M.J.; Aarnoutse, R.E.; de Lange, W.C.; van der Ven, A.J.; Dekhuijzen, R. Antituberculosis drug-induced hepatotoxicity: Concise up-to-date review. J. Gastroenterol. Hepatol. 2008, 23, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Ramappa, V.; Aithal, G.P. Hepatotoxicity Related to Anti-tuberculosis Drugs: Mechanisms and Management. J. Clin. Exp. Hepatol. 2013, 3, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Lee, C.M.; Kim, T.H.; Kim, J.J.; Lee, J.M.; Kim, H.J.; Ha, C.Y.; Kim, H.J.; Jung, W.T.; Lee, O.J.; et al. Frequency and risk factors of drug-induced liver injury during treatment of multidrug-resistant tuberculosis. Int. J. Tuberc. Lung Dis. 2016, 20, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Aithal, G.P.; Watkins, P.B.; Andrade, R.J.; Larrey, D.; Molokhia, M.; Takikawa, H.; Hunt, C.M.; Wilke, R.A.; Avigan, M.; Kaplowitz, N.; et al. Case definition and phenotype standardization in drug-induced liver injury. Clin. Pharmacol. Ther. 2011, 89, 806–815. [Google Scholar] [CrossRef]
- Freitas, M.; Magalhães, J.; Marinho, C.; Cotter, J. Looking beyond appearances: When liver biopsy is the key for hepatic tuberculosis diagnosis. BMJ Case Rep. 2020, 13, e234491. [Google Scholar] [CrossRef] [PubMed]
- Clinton, J.W.; Kiparizoska, S.; Aggarwal, S.; Woo, S.; Davis, W.; Lewis, J.H. Drug-Induced Liver Injury: Highlights and Controversies in the Recent Literature. Drug Saf. 2021, 44, 1125–1149. [Google Scholar] [CrossRef] [PubMed]
- Costa-Moreira, P.; Gaspar, R.; Pereira, P.; Lopes, S.; Canão, P.; Lopes, J.; Carneiro, F.; Macedo, G. Role of liver biopsy in the era of clinical prediction scores for "drug-induced liver injury" (DILI): Experience of a tertiary referral hospital. Virchows Arch. 2020, 477, 517–525. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, W.L.; Zhu, Y.; Cheng, J.W.; Dong, J.; Li, M.X.; Yu, L.; Lv, Y.; Wang, B. Diagnosis and treatment of hepatic tuberculosis: Report of five cases and review of literature. Int. J. Clin. Exp. Med. 2013, 6, 845–850. [Google Scholar]
- Ungo, J.R.; Jones, D.; Ashkin, D.; Hollender, E.S.; Bernstein, D.; Albanese, A.P.; Pitchenik, A.E. Antituberculosis drug-induced hepatotoxicity. The role of hepatitis C virus and the human immunodeficiency virus. Am. J. Respir. Crit. Care Med. 1998, 157 Pt 1, 1871–1876. [Google Scholar] [CrossRef]
- Musso, M.; Mosti, S.; Gualano, G.; Mencarini, P.; Urso, R.; Ghirga, P.; Rianda, A.; Del Nonno, F.; Goletti, D.; Palmieri, F. Hepatitis C virus infection: A challenge in the complex management of two cases of multidrug-resistant tuberculosis. BMC Infect. Dis. 2019, 19, 882. [Google Scholar] [CrossRef] [PubMed]
- Tunesi, S.; Dû, D.L.; Gualano, G.; Millet, J.P.; Skrahin, A.; Bothamley, G.; Casas, X.; Goletti, D.; Lange, C.; Musso, M.; et al. TBnet, The ESGMYC, and The French MDR-TB Group. Co-administration of treatment for rifampicin-resistant TB and chronic HCV infection: A TBnet and ESGMYC study. J. Infect. 2022, 84, 834–872. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, Y.; Zhang, T.; Wang, Q.; Xie, W. Drug-Induced Liver Injury from Anti-Tuberculosis Treatment: A Retrospective Cohort Study. Med. Sci. Monit. 2020, 26, e920350. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Yan, H.; Liang, L.; Du, J.; Jin, S.; Yang, S.; Wang, H.; Hu, T.; Zhu, Y.; Wang, G.; et al. Incidence and risk factors of anti-tuberculosis drug induced liver injury (DILI): Large cohort study involving 4652 Chinese adult tuberculosis patients. Liver Int. 2021, 41, 1565–1575. [Google Scholar] [CrossRef] [PubMed]
- Gourishankar, A.; Navarro, F.; Debroy, A.N.; Smith, K.C. Isoniazid hepatotoxicity with clinical and histopathology correlate. Ann. Clin. Lab. Sci. 2014, 44, 87–90. [Google Scholar]
- Neuberger, J.; Cain, O. The Need for Alternatives to Liver Biopsies: Non-Invasive Analytics and Diagnostics. Hepat. Med. 2021, 13, 59–69. [Google Scholar] [CrossRef]
- Weber, S.; Benesic, A.; Rotter, I.; Gerbes, A.L. Early ALT response to corticosteroid treatment distinguishes idiosyncratic drug-induced liver injury from autoimmune hepatitis. Liver Int. 2019, 39, 1906–1917. [Google Scholar] [CrossRef]
| Pt. N. | Sex Age | Country of Birth | Comorbidities | Alcohol | HBV/HCV Infection | Diagnosis | Microbiology | Radiology | Treatment | Total Drugs | TD |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F 41 | Romania | uterine cancer | no | HCV | new | AFB+/culture-positive | Pulm; cavitation | Lfx, Cs, Etho, PAS, Lzd | 6 | 30 |
| 2 | M 66 | Italy | hypertension | yes | HCV | new | AFB+/culture-positive | Pulm; cavitation | E, Z, S, Lfx, Lzd | 6 | 330 |
| 3 | M 27 | Ukraine | none | no | neg | new | AFB-/culture-positive | Pulm; cavitation | E, Mfx, Amk, Cs | 4 | 240 |
| 4 | M 56 | Egypt | diabetes | yes | neg | new | AFB+/culture-positive | Pulm; cavitation | R, H, Z, E | 5 | 40 |
| 5 | M 19 | Cameron | none | no | neg | relapse | AFB+/culture-positive | Pulm; cavitation | R + H + E + Z | 4 | 60 |
| 6 | F 23 | Egypt | none | no | neg | relapse | AFB+/culture-positive | Pulm; cavitation | R, H, Z, E | 4 | 120 |
| 7 | M 36 | Eritrea | none | yes | neg | relapse | AFB+/culture-positive | Pulm + EPTB; cavitation | Lzd, Cfz, Cs, E, Amk, Bdq | 5 | 65 |
| 8 | F 42 | Philippines | valvular replacement, chronic atrial fibrillation | no | neg | new | AFB+/culture-positive | Pulm; cavitation | Bdq, Mfx, Amk, Cfz, Cs, Lzd | 6 | 60 |
| 9 | M 37 | Philippines | none | yes | neg | new | AFB-/culture-positive | EPTB | H, E, Lfx, Amk | 4 | 30 |
| 10 | M 36 | Romania | none | yes | neg | new | AFB+/culture-positive | Pulm + EPTB; cavitation | R, H, Z, E | 4 | 35 |
| Pat. N | Symptoms | Jaundice | Management AE | AST U/L | ALT U/L | ALP U/L | Severity Grading WHO | RUCAM Value | Pattern | DILI Causality Assessment | Hystology Findings | 4 DILI-Favoring Features | Diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | fatigue, nausea, lost of appetite | no | suspension | 316 | 749 | 156 | 4 | 12.5 | hepatocellular | 3, possible | Mild necroinflammatory activity, and portal fibrosis (Ishak Grade 5 and Stage 2) | 2 | Chronic viral hepatitis HCV correlated |
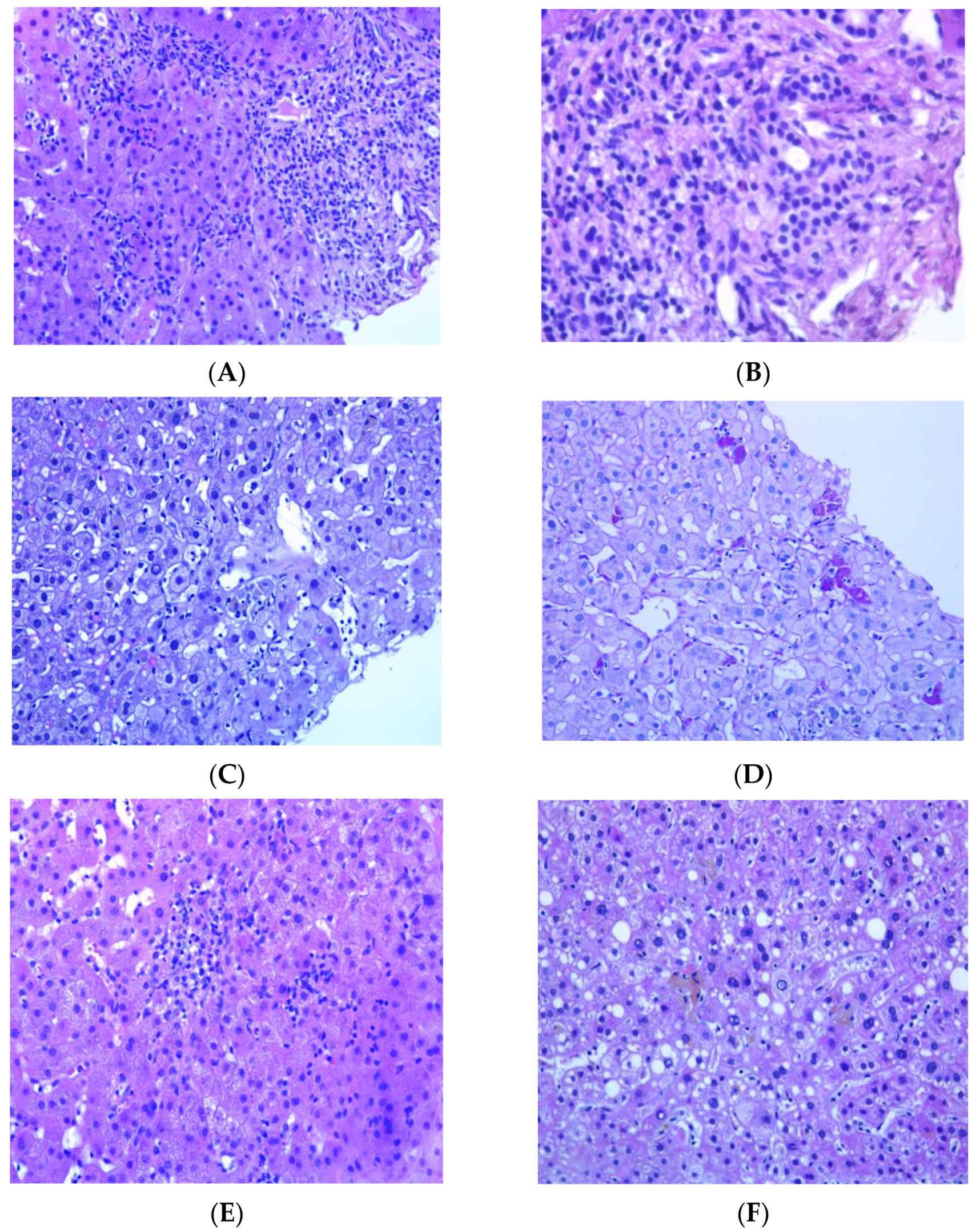
| 2 | fatigue, nausea, lost of appetite | no | suspension | 287 | 403 | 271 | 3 | 8.4 | hepatocellular | 4, possible | Portal tracts enlarged due to edema and fibrosis and containing inflammatory infiltrate of mild/moderate density, consisting mainly of lymphocytes, sometimes aggregated to form follicles, more than occasional plasma cells and some PASD+ macrophages. This inflammatory infiltrate sometimes surrounds the native bile duct with phenomena of lymphocytic cholangitis. The interlobular bile ducts demonstrate features of cholangiocyte senescence. In the acinar site there is an inflammatory intracinar lymphomonocytic infiltrate with spotty necrosis, some apoptotic bodies and hypertrophy of Küppfer cells with PASD+ pigment. Some areas appear regenerative in appearance. (Ishak Grade 9 Stage 4). | 3 | Chronic viral hepatitis, HCV correlated |
| 3 | fatigue, nausea, lost of appetite | no | suspension | 137 | 407 | 139 | 3 | 12.5 | hepatocellular | 1, unlikely | Portal tracts containing minimal inflammatory lymphocytic infiltrate with some PASD+ macrophages and occasional neutrophilic granulocytes. Interlobular bile ducts always visible. Diffuse steatosis with large and medium-sized vesicles is observed in the parenchymal area, greater than 66%. There is a mixed inflammatory infiltrate with foci of mediational and perivenular necrosis (spotty) and some lipogranulomas. Küppfer cells appear hypertrophic and contain PASD+ pigment. Iron deposit of the type with weak and diffuse staining (ferritin) of the type and of the small granule type at the level of the hepatocytes in zones 1 and 2 and of the non-confluent granule type at the level of the Küppfer cells. Minimal increase in collagen density in some portal tracts. | 0 | Non-alcoholic steatohepatitis (nash). Secondary iron overload |
| 4 | fatigue, nausea, lost of appetite | no | suspension | 440 | 426 | 152 | 3 | 8.4 | hepatocellular | 8, probable | inflammatory infiltrate consisting mainly of lymphocytes with some plasma cells neutrophils granulocytes and PASD+ macrophages. In the lobular area, the inflammatory lymphomonocytic infiltrate is moderate with spotty necrosis, sometimes confluent; in the lumen of the sinusoids the resident macrophages are hypertrophic/hyperplastic and several stellate cells (HSC) are also evident. | 4 | TB-DILI (acute) |
| 5 | fatigue, nausea, lost of appetite | yes | suspension | 2134 | 1180 | 542 | 4 | 11.6 | hepatocellular | 4, possible | Inflammatory infiltrate of mild to moderate density consisting predominantly of lymphocytes that shows no propensity to pass the lamina. In the parenchymal site various aspects of hepatocellular suffering are observed associated with pictures of dysarray of the acinus and lymphohistiocytic infiltration with the participation of neutrophils. Sinusoids reduced in amplitude also due to the presence of numerous macrophages. Mono- and polymorphonuclear inflammatory cells are dispersed in the sinusoids. | 3 | TB DILI (acute) |
| 6 | fatigue, nausea, lost of appetite | no | suspension | 472 | 480 | 161 | 3 | 8.2 | hepatocellular | 4, possible | Portal spaces containing mild inflammatory infiltrate consisting of lymphocytes, PASD+ macrophages, and occasional eosinophilic granulocytes. Minimal and focal involvement of the portoparenchymal limiting plate. The bile ducts are visible in most of the portal tracts, and rarely any lymphocytes permeate the basement membrane. At the periportal level, some intermediate immunophenotype cells are observed. The ductular reaction is absent. Spotty necrosis foci are observed in the parenchyma with a prevalence of macrophages with PASD+ pigment, often grouped in clusters. Minimal portal fibrosis. | 4 | TB-DILI (partial resolution) |
| 7 | fatigue, nausea, lost of appetite | no | suspension | 256 | 330 | 321 | 3 | 14.1 | hepatocellular | 5, possible | mild macrovesicular steatosis, focal microvesicular steatosis. With the aid of histochemical stains for the reticulum, PAS-D and Perls Iron staining, we also note: dilatation of the sinusoids with Küpffer cell hyperplasia with some inflammatory cells represented by lymphocytes and clusters of phagocytosing macrophages pigment partly referable to ceroid (PAS-D+) and partly refractive and slightly bluish with iron staining (Perls) as for hemosiderin. Mild biliary phenotype of perivenular and periportal hepatocytes is observed on immunohistochemical staining for cytokeratin 19. Mild perivenular and sinusoidal fibrosis | 3 | TB-DILI (partial resolution) |
| 8 | fatigue, nausea, lost of appetite | no | suspension | 189 | 220 | 115 | 3 | 4.6 | mixed | 5, possible | needle bioptic fragments of liver with preserved structure characterized by mild inflammatory infiltrate of the portal spaces consisting of rare lymphocytes and rare macrophages with PAS-D+ pigment, minimal macrovacuolar steatosis (<5% of the liver parenchyma). Rare acidophilic bodies, focus of spotty necrosis. Rare glycogenated nuclei in the lobule. No iron deposits are observed. Fibrous expansion of some portal spaces (F0-F1 sec Metavir) | 2 | minimal non-specific hepatitis |
| 9 | fatigue, nausea, lost of appetite | no | suspension | 152 | 270 | 469 | 3 | 1.2 | cholestatic | 8, probable | necrotizing granulomatous inflammation. A single bacillary formation ZN+ is found, which can be referred to an acid-fast bacillus | 1 | TB granulomatous hepatitis |
| 10 | fatigue, nausea, lost of appetite | no | suspension | 98 | 298 | 100 | 3 | 16.9 | hepatocellular | 4, possible | mild and focal portal inflammatory infiltrate consisting of neutrophil granulocytes, eosinophils, PASD+ macrophages and rare lymphocytes. ductular proliferation spotty necrosis, hepatocyte rosettes | 4 | TB-DILI |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gualano, G.; Zace, D.; Mosti, S.; Mencarini, P.; Musso, M.; Libertone, R.; Cerva, C.; Goletti, D.; Rianda, A.; Del Nonno, F.; et al. Utility of Liver Biopsy in the Diagnosis and Management of Possible Drug-Induced Liver Injury in Patients Receiving Antituberculosis Therapy: A Retrospective Study. Infect. Dis. Rep. 2023, 15, 735-746. https://doi.org/10.3390/idr15060066
Gualano G, Zace D, Mosti S, Mencarini P, Musso M, Libertone R, Cerva C, Goletti D, Rianda A, Del Nonno F, et al. Utility of Liver Biopsy in the Diagnosis and Management of Possible Drug-Induced Liver Injury in Patients Receiving Antituberculosis Therapy: A Retrospective Study. Infectious Disease Reports. 2023; 15(6):735-746. https://doi.org/10.3390/idr15060066
Chicago/Turabian StyleGualano, Gina, Drieda Zace, Silvia Mosti, Paola Mencarini, Maria Musso, Raffaella Libertone, Carlotta Cerva, Delia Goletti, Alessia Rianda, Franca Del Nonno, and et al. 2023. "Utility of Liver Biopsy in the Diagnosis and Management of Possible Drug-Induced Liver Injury in Patients Receiving Antituberculosis Therapy: A Retrospective Study" Infectious Disease Reports 15, no. 6: 735-746. https://doi.org/10.3390/idr15060066
APA StyleGualano, G., Zace, D., Mosti, S., Mencarini, P., Musso, M., Libertone, R., Cerva, C., Goletti, D., Rianda, A., Del Nonno, F., Falasca, L., & Palmieri, F. (2023). Utility of Liver Biopsy in the Diagnosis and Management of Possible Drug-Induced Liver Injury in Patients Receiving Antituberculosis Therapy: A Retrospective Study. Infectious Disease Reports, 15(6), 735-746. https://doi.org/10.3390/idr15060066









