Clinical Ramifications of Bacterial Aggregation in Pleural Fluid
Abstract
1. Introduction
2. Materials and Methods
2.1. Pleural Fluid and Bacteria
2.2. Bacterial Aggregation in Pleural Fluid under Different Conditions and Visualization of Aggregates
2.3. Quantifying Bacteria Concentrations before and after Tissue Plasminogen Activator and Antibiotics
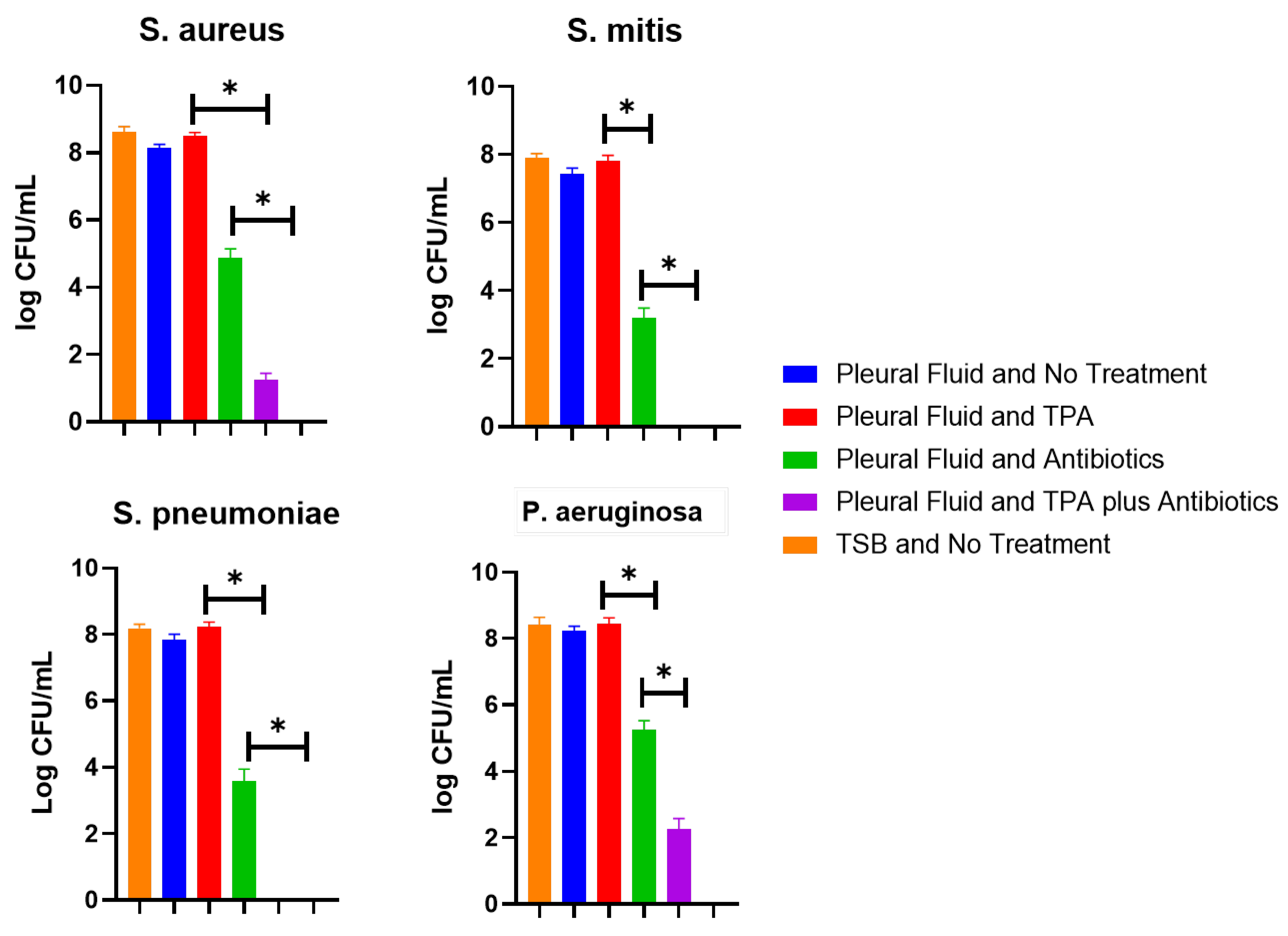
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Staats, A.; Burback, P.W.; Eltobgy, M.; Parker, D.M.; Amer, A.O.; Wozniak, D.J.; Wang, S.-H.; Stevenson, K.B.; Urish, K.L.; Stoodley, P. Synovial fluid induced aggregation occurs across Staphylococcus aureus clinical isolates and is mechanistically independent of attached biofilm formation. Microbiol. Spectr. 2021, 9, e0026721. [Google Scholar] [CrossRef] [PubMed]
- Gilbertie, J.M.; Schnabel, L.V.; Hickok, N.J.; Jacob, M.E.; Conlon, B.P.; Shapiro, I.M.; Parvizi, J.; Schaer, T.P. Equine or porcine synovial fluid as a novel ex vivo model for the study of bacterial free-floating biofilms that form in human joint infections. PLoS ONE 2019, 14, e0221012. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, H.; Jasiak, A.; Doub, J.B. Do All Prosthetic Joint Infection Clinical Isolates Form Aggregates in Synovial Fluid That Are Resistant to Antibiotic Agents? Surg. Infect. 2024, 25, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Doub, J.B.; Mao, Y.; Kjellerup, B.V. Do bacteriophages have activity in synovial fluid and against synovial fluid induced bacterial aggregates? J. Orthop. Res. 2024, 42, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Staats, A.; Burback, P.W.; Schwieters, A.; Li, D.; Sullivan, A.; Horswill, A.R.; Stoodley, P. Rapid aggregation of Staphylococcus aureus in synovial fluid is influenced by synovial fluid concentration, viscosity, and fluid dynamics, with evidence of polymer bridging. mBio 2022, 13, e0023622. [Google Scholar] [CrossRef] [PubMed]
- Psallidas, I.; Corcoran, J.P.; Rahman, N.M. Management of parapneumonic effusions and empyema. Semin. Respir. Crit. Care Med. 2014, 35, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Rahman, N.M.; Maskell, N.A.; West, A.; Teoh, R.; Arnold, A.; Mackinlay, C.; Peckham, D.; Davies, C.W.; Ali, N.; Kinnear, W.; et al. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N. Engl. J. Med. 2011, 365, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Majid, A.; Kheir, F.; Folch, A.; Fernandez-Bussy, S.; Chatterji, S.; Maskey, A.; Fashjian, M.; Cheng, G.; Ochoa, S.; Alape, D.; et al. Concurrent Intrapleural Instillation of Tissue Plasminogen Activator and DNase for Pleural Infection. A Single-Center Experience. Ann. Am. Thorac. Soc. 2016, 13, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Porcel, J.M.; Light, R.W. Pleural Fluid Analysis: Are Light’s Criteria Still Relevant After Half a Century? Clin. Chest. Med. 2021, 42, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Secor, P.R.; Michaels, L.A.; Ratjen, A.; Jennings, L.K.; Singh, P.K. Entropically driven aggregation of bacteria by host polymers promotes antibiotic tolerance in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2018, 115, 10780–10785. [Google Scholar] [CrossRef] [PubMed]
- Herman-Bausier, P.; Labate, C.; Towell, A.M.; Derclaye, S.; Geoghegan, J.A.; Dufrêne, Y.F. Staphylococcus aureus clumping factor A is a force-sensitive molecular switch that activates bacterial adhesion. Proc. Natl. Acad. Sci. USA 2018, 115, 5564–5569. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Gupta, T.T.; Patel, K.; Stoodley, P. The Influence of Patterned Surface Features on the Accumulation of Bovine Synovial Fluid-Induced Aggregates of Staphylococcus aureus. Appl. Environ. Microbiol. 2022, 88, e0121722. [Google Scholar] [CrossRef] [PubMed]
- Charalampidis, C.; Youroukou, A.; Lazaridis, G.; Baka, S.; Mpoukovinas, I.; Karavasilis, V.; Kioumis, I.; Pitsiou, G.; Papaiwannou, A.; Karavergou, A.; et al. Physiology of the pleural space. J. Thorac. Dis. 2015, 7 (Suppl. 1), S33–S37. [Google Scholar] [CrossRef] [PubMed]
- Merchant, N.; Liu, C. Thoracic empyema: Aetiology, diagnosis, treatment, and prevention. Curr. Opin. Pulm. Med. 2024, 30, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Davies, H.E.; Davies, R.J.; Davies, C.W. Management of pleural infection in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010, 65 (Suppl. 2), ii41–ii53. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-F.; Chen, C.-M.; Chen, Y.-L.; Cheng, C.-Y.; Huang, C.-L.; Hung, W.-H.; Wang, B.-Y. The outcomes of thoracoscopic decortication between fungal empyema and bacterial empyema. BMC Infect. Dis. 2023, 23, 8. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Li, S.; Luo, W. Comparative analysis of antibiotic susceptibility patterns and clinical features of mucoid and non-mucoid Pseudomonas aeruginosa infections: A retrospective study. Front. Public Health 2024, 12, 1333477. [Google Scholar] [CrossRef] [PubMed]
- Nasser, A.; Jahanbakhshi, S.; Soltan Dallal, M.M.; Banar, M.; Sattari-Maraji, A.; Azimi, T. Staphylococcus aureus Dormancy: Waiting for Insurgency. Curr. Pharm. Biotechnol. 2023, 24, 1898–1915. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Cargill, T.; Harriss, E.; Asciak, R.; Mercer, R.M.; Bedawi, E.O.; McCracken, D.J.; Psallidas, I.; Corcoran, J.P.; Rahman, N.M. The microbiology of pleural infection in adults: A systematic review. Eur. Respir. J. 2019, 54, 1900542. [Google Scholar] [CrossRef] [PubMed]

| High Shaking (120 RPM) | Low Shaking (30 RPM) | Static | |
|---|---|---|---|
| Tryptic soy broth | No aggregation | No aggregation | No aggregation |
| Transudative | Aggregation | No aggregation | No aggregation |
| Low-protein exudate | Aggregation | No aggregation | No aggregation |
| High-protein exudate | Aggregation | Aggregation | No aggregation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doub, J.B.; Putnam, N. Clinical Ramifications of Bacterial Aggregation in Pleural Fluid. Infect. Dis. Rep. 2024, 16, 608-614. https://doi.org/10.3390/idr16040046
Doub JB, Putnam N. Clinical Ramifications of Bacterial Aggregation in Pleural Fluid. Infectious Disease Reports. 2024; 16(4):608-614. https://doi.org/10.3390/idr16040046
Chicago/Turabian StyleDoub, James B., and Nicole Putnam. 2024. "Clinical Ramifications of Bacterial Aggregation in Pleural Fluid" Infectious Disease Reports 16, no. 4: 608-614. https://doi.org/10.3390/idr16040046
APA StyleDoub, J. B., & Putnam, N. (2024). Clinical Ramifications of Bacterial Aggregation in Pleural Fluid. Infectious Disease Reports, 16(4), 608-614. https://doi.org/10.3390/idr16040046






