Convergent Mechanisms in Virus-Induced Cancers: A Perspective on Classical Viruses, SARS-CoV-2, and AI-Driven Solutions
Abstract
:1. Introduction
2. Common Oncogenic Mechanisms: Classical Viruses and SARS-CoV-2
2.1. Cell Cycle Dysregulation
2.1.1. Classical Viral Mechanisms
2.1.2. SARS-CoV-2’S Interference with p53/pRb Pathways
2.1.3. Direct Comparison of Mechanisms
2.2. Inflammatory Signaling
2.2.1. Established Viral Inflammatory Pathways
2.2.2. SARS-CoV-2 Inflammatory Cascades
- Activation of NF-κB signaling pathways;
- Enhanced production of inflammatory mediators;
- Disruption of normal tissue homeostasis;
- Generation of oxidative stress.
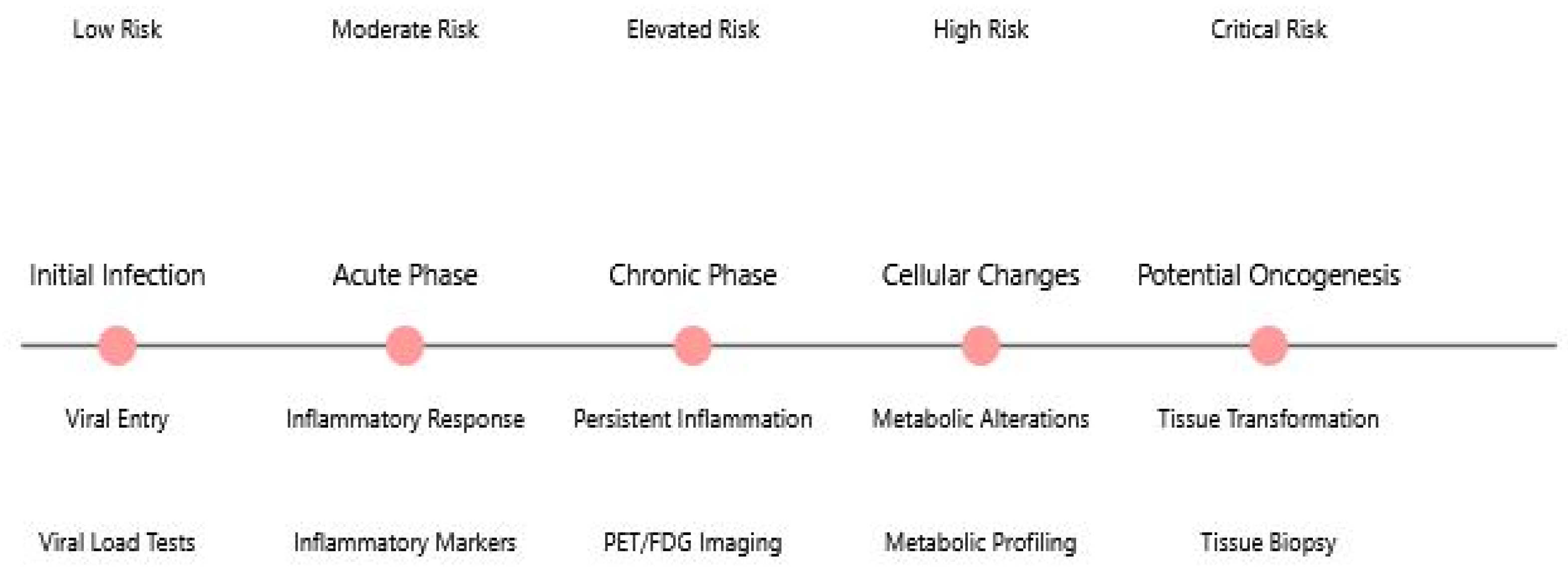
2.2.3. Chronic Inflammation in Long COVID
2.2.4. Neuroimaging Evidence
2.3. Immune Evasion Strategies
2.3.1. Classical Viral Strategies
2.3.2. SARS-CoV-2 Immune Modulation
2.3.3. Comparative Analysis
2.4. Metabolic Reprogramming
2.4.1. Established Viral Effects
2.4.2. SARS-CoV-2 Metabolic Changes
2.4.3. PET/FDG Imaging Findings
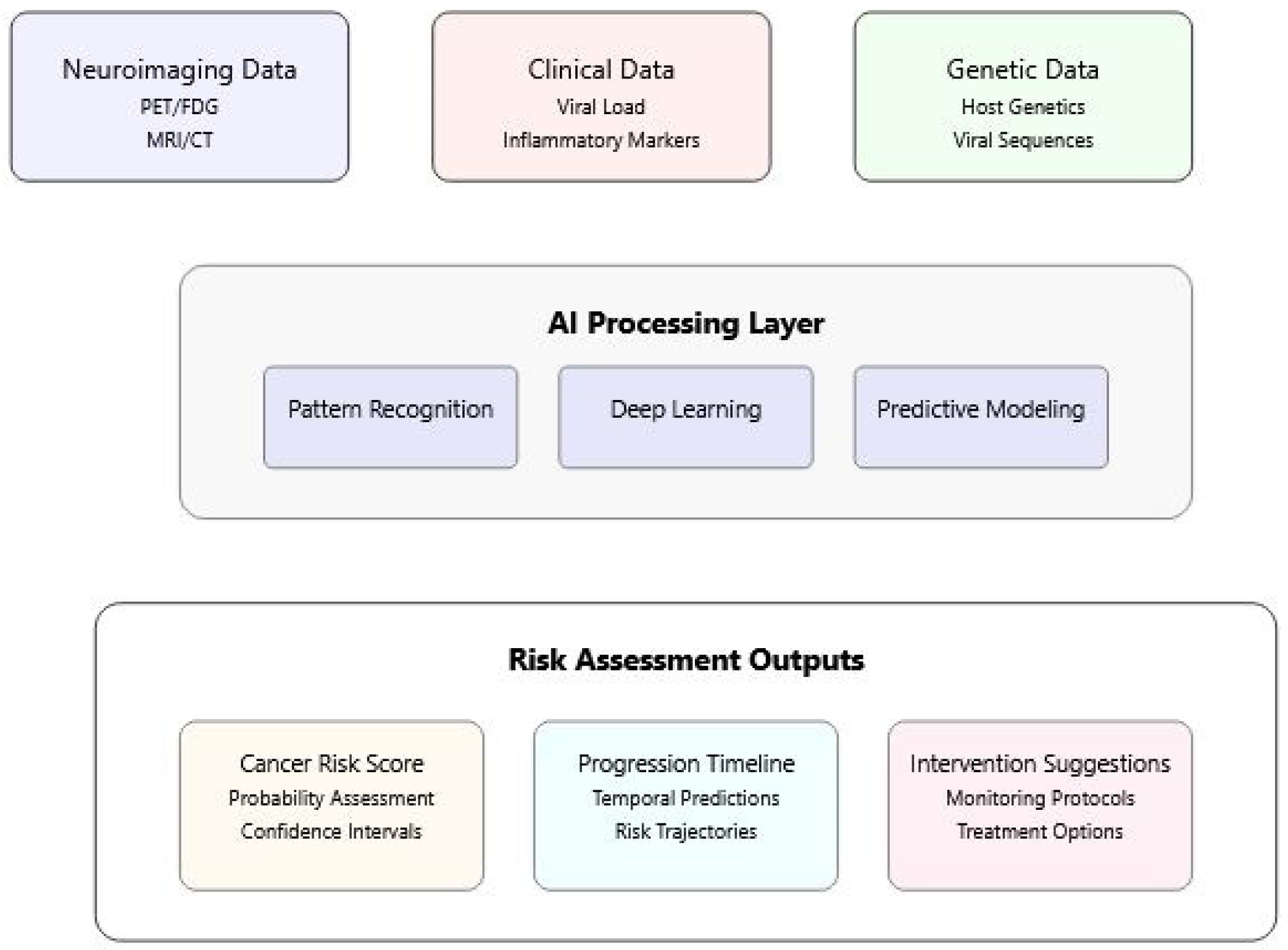
3. AI Applications in Understanding Viral Oncogenesis
3.1. Pattern Recognition in Virus–Host Interactions
3.2. Multi-Modal Data Integration
3.3. Risk Assessment and Prediction
4. Strengthening Forward-Looking Analysis
4.1. Novel Integration of Classical and Emerging Viral Threats
4.2. Hypotheses Regarding SARS-CoV-2 Oncogenic Mechanisms
4.3. Integration of Neuroimaging Evidence
4.4. Research Priorities and Future Directions
4.5. Implementation Strategy
5. Future Perspectives
5.1. Potential Oncogenic Implications of SARS-CoV-2
- Genomic instability: SARS-CoV-2 proteins (ORF6, NSP13, and N-protein) cause DNA damage and impair repair mechanisms by degrading CHK1 kinase and disrupting 53BP1 recruitment to damage sites [79].
- Cell cycle dysregulation: The virus induces G1 cell cycle arrest through both Smad3-dependent and p53-independent pathways, disrupting normal cell cycle control mechanisms [80,81]. Studies have shown that SARS-CoV-2 infection triggers redistribution of cyclin D1 and cyclin D3 from the nucleus to the cytoplasm, followed by proteasomal degradation, which can increase viral replication and potentially interfere with normal cell growth control [81].
- Cellular senescence: Infection triggers cellular senescence, creating a senescence-associated secretory phenotype (SASP) that promotes inflammation and potential tissue remodeling [82].
- Metabolic reprogramming: SARS-CoV-2 depletes dNTP pools and redirects cellular resources toward viral replication, potentially creating metabolic conditions favorable for cancer development. Additionally, evidence from coronavirus research shows that viral proteins like nsp13 can interact with DNA polymerase δ, inducing DNA replication stress and activating ATR-dependent DNA damage response pathways [83], which may further contribute to genomic instability in infected cells. In long COVID, persistent viral proteins or ongoing immune dysregulation could theoretically maintain these oncogenic-friendly cellular states. However, actual cancer development requires multiple additional steps, and epidemiological evidence linking COVID-19 to increased cancer incidence remains preliminary. Long-term studies tracking cancer in COVID-19 survivors will be essential to determine whether these theoretical mechanisms translate to actual cancer risk in humans.
5.2. Role of AI in Monitoring and Prediction
5.3. Research Directions
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Martel, C.; Ferlay, J.; Franceschi, S.; Vignat, J.; Bray, F.; Forman, D.; Plummer, M. Global burden of cancers attributable to infections in 2008: A review and synthetic analysis. Lancet Oncol. 2012, 13, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Plummer, M.; de Martel, C.; Vignat, J.; Ferlay, J.; Bray, F.; Franceschi, S. Global burden of cancers attributable to infections in 2012: A synthetic analysis. Lancet Glob. Health 2016, 4, e609–e616. [Google Scholar] [CrossRef] [PubMed]
- zur Hausen, H. The search for infectious causes of human cancers: Where and why (Nobel lecture). Angew. Chem. Int. Ed. 2009, 48, 5798–5808. [Google Scholar] [CrossRef]
- IARC Working Group. Biological agents. IARC Monogr. Eval. Carcinog. Risks Hum. 2012, 100B, 1–441. [Google Scholar]
- Tang, K.W.; Alaei-Mahabadi, B.; Samuelsson, T.; Lindh, M.; Larsson, E. The landscape of viral expression and host gene fusion and adaptation in human cancer. Nat. Commun. 2013, 4, 2513. [Google Scholar] [CrossRef]
- Krump, N.A.; You, J. Molecular mechanisms of viral oncogenesis in humans. Nat. Rev. Microbiol. 2018, 16, 684–698. [Google Scholar] [CrossRef]
- Policard, M.; Jain, S.; Rego, S.; Dakshanamurthy, S. Immune characterization and profiles of SARS-CoV-2 infected patients reveals potential host therapeutic targets and SARS-CoV-2 oncogenesis mechanism. Virus Res. 2021, 301, 198464. [Google Scholar] [CrossRef]
- Gómez-Carballa, A.; Martinón-Torres, F.; Salas, A. Is SARS-CoV-2 an oncogenic virus? J. Infect. 2022, 85, 573–607. [Google Scholar] [CrossRef]
- Rudroff, T. Long COVID in Brain Health Research: A Call to Action. Brain Sci. 2024, 14, 587. [Google Scholar] [CrossRef]
- Rudroff, T. Frontal-striatal glucose metabolism and fatigue in patients with multiple sclerosis, long COVID, and COVID-19 recovered controls. Exp. Brain Res. 2024, 242, 2125–2136. [Google Scholar] [CrossRef]
- Mesri, E.A.; Feitelson, M.A.; Munger, K. Human viral oncogenesis: A cancer hallmarks analysis. Cell Host Microbe 2014, 15, 266–282. [Google Scholar] [CrossRef]
- Moore, P.S.; Chang, Y. Why do viruses cause cancer? Highlights of the first century of human tumour virology. Nat. Rev. Cancer 2010, 10, 878–889. [Google Scholar] [CrossRef] [PubMed]
- Rous, P. A sarcoma of the fowl transmissible by an agent separable from the tumor cells. J. Exp. Med. 1911, 13, 397–411. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Lai, X.; Pan, Y.; Zheng, H.; Vera, J.; Liu, S.; Deng, S.; Zhang, L. Artificial intelligence in cancer target identification and drug discovery. Signal Transduct. Target. Ther. 2022, 7, 156. [Google Scholar] [CrossRef]
- Sharma, V.; Singh, A.; Chauhan, S.; Sharma, P.K.; Chaudhary, S.; Sharma, A.; Porwal, O.; Fuloria, N.K. Role of Artificial Intelligence in Drug Discovery and Target Identification in Cancer. Curr. Drug Deliv. 2024, 21, 870–886. [Google Scholar] [CrossRef]
- Mei, X.; Lee, H.C.; Diao, K.; Huang, M.; Lin, B.; Liu, C.; Xie, Z.; Ma, Y.; Robson, P.M.; Chung, M.; et al. Artificial intelligence-enabled rapid diagnosis of COVID-19 patients. medRxiv 2020. [Google Scholar] [CrossRef]
- Tang, K.W.; Larsson, E. Tumour virology in the era of high-throughput genomics. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160265. [Google Scholar] [CrossRef] [PubMed]
- Wainberg, M.; Merico, D.; Delong, A.; Frey, B.J. Deep learning in biomedicine. Nat. Biotechnol. 2018, 36, 829–838. [Google Scholar] [CrossRef]
- zur Hausen, H.; de Villiers, E.M. Cancer “causation” by infections—Individual contributions and synergistic networks. Semin. Oncol. 2014, 41, 860–875. [Google Scholar] [CrossRef]
- Lehoux, M.; D’Abramo, C.M.; Archambault, J. Molecular mechanisms of human papillomavirus-induced carcinogenesis. Public. Health Genom. 2009, 12, 268–280. [Google Scholar] [CrossRef]
- Parker, G.A.; Touitou, R.; Allday, M.J. Epstein-Barr virus EBNA3C can disrupt multiple cell cycle checkpoints and induce nuclear division divorced from cytokinesis. Oncogene 2000, 19, 700–709. [Google Scholar] [CrossRef]
- Esmaeili, A.; Awasthi, P.; Tabaee, S. Beyond immortality: Epstein-Barr virus and the intricate dance of programmed cell death in cancer development. Cancer Treat. Res. Commun. 2025, 43, 100880. [Google Scholar] [CrossRef] [PubMed]
- Agustiningsih, A.; Rasyak, M.R.; Turyadi; Jayanti, S.; Sukowati, C. The oncogenic role of hepatitis B virus X gene in hepatocarcinogenesis: Recent updates. Explor. Target. Antitumor Ther. 2024, 5, 120–134. [Google Scholar] [CrossRef]
- Justo Arevalo, S.; Castillo-Chávez, A.; Uribe Calampa, C.S.; Zapata Sifuentes, D.; Huallpa, C.J.; Landa Bianchi, G.; Garavito-Salini Casas, R.; Quiñones Aguilar, M.; Pineda Chavarría, R. What do we know about the function of SARS-CoV-2 proteins? Front. Immunol. 2023, 14, 1249607. [Google Scholar] [CrossRef] [PubMed]
- Murigneux, E.; Softic, L.; Aubé, C.; Grandi, C.; Judith, D.; Bruce, J.; Le Gall, M.; Guillonneau, F.; Schmitt, A.; Parissi, V.; et al. Proteomic analysis of SARS-CoV-2 particles unveils a key role of G3BP proteins in viral assembly. Nat. Commun. 2024, 15, 640. [Google Scholar] [CrossRef]
- Bruzzone, C.; Conde, R.; Embade, N.; Mato, J.M.; Millet, O. Metabolomics as a powerful tool for diagnostic, pronostic and drug intervention analysis in COVID-19. Front. Mol. Biosci. 2023, 10, 1111482. [Google Scholar] [CrossRef]
- Weeden, C.E.; Hill, W.; Lim, E.L.; Grönroos, E.; Swanton, C. Impact of risk factors on early cancer evolution. Cell 2023, 186, 1541–1563. [Google Scholar] [CrossRef]
- Mühlemann, B.; Wilks, S.H.; Baracco, L.; Bekliz, M.; Carreño, J.M.; Corman, V.M.; Davis-Gardner, M.E.; Dejnirattisai, W.; Diamond, M.S.; Douek, D.C.; et al. Comparative Analysis of SARS-CoV-2 Antigenicity across Assays and in Human and Animal Model Sera. bioRxiv 2023. [Google Scholar] [CrossRef]
- Torbati, E.; Krause, K.L.; Ussher, J.E. The Immune Response to SARS-CoV-2 and Variants of Concern. Viruses 2021, 13, 1911. [Google Scholar] [CrossRef]
- Jaiswal, A.; Shrivastav, S.; Kushwaha, H.R.; Chaturvedi, R.; Singh, R.P. Oncogenic potential of SARS-CoV-2-targeting hallmarks of cancer pathways. Cell Commun. Signal. 2024, 22, 447. [Google Scholar] [CrossRef]
- Ringelhan, M.; Pfister, D.; O’Connor, T.; Pikarsky, E.; Heikenwalder, M. The immunology of hepatocellular carcinoma. Nat. Immunol. 2018, 19, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Charostad, J.; Nakhaie, M.; Dehghani, A.; Faghihloo, E. The interplay between EBV and KSHV viral products and NF-κB pathway in oncogenesis. Infect. Agent. Cancer 2020, 15, 62. [Google Scholar] [CrossRef] [PubMed]
- Hibino, S.; Kawazoe, T.; Kasahara, H.; Itoh, S.; Ishimoto, T.; Sakata-Yanagimoto, M.; Taniguchi, K. Inflammation-Induced Tumorigenesis and Metastasis. Int. J. Mol. Sci. 2021, 22, 5421. [Google Scholar] [CrossRef]
- Singh, R.; Singh, P.K.; Kumar, R.; Kabir, M.T.; Kamal, M.A.; Rauf, A.; Albadrani, G.M.; Sayed, A.A.; Mousa, S.A.; Abdel-Daim, M.M.; et al. Multi-Omics Approach in the Identification of Potential Therapeutic Biomolecule for COVID-19. Front. Pharmacol. 2021, 12, 652335. [Google Scholar] [CrossRef]
- Mariggiò, G.; Koch, S.; Schulz, T.F. Kaposi sarcoma herpesvirus pathogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160275. [Google Scholar] [CrossRef] [PubMed]
- Nelemans, T.; Kikkert, M. Viral Innate Immune Evasion and the Pathogenesis of Emerging RNA Virus Infections. Viruses 2019, 11, 961. [Google Scholar] [CrossRef]
- Lei, X.; Dong, X.; Ma, R.; Wang, W.; Xiao, X.; Tian, Z.; Wang, C.; Wang, Y.; Li, L.; Ren, L.; et al. Activation and evasion of type I interferon responses by SARS-CoV-2. Nat. Commun. 2020, 11, 3810. [Google Scholar] [CrossRef]
- Yuen, C.K.; Lam, J.Y.; Wong, W.M.; Mak, L.F.; Wang, X.; Chu, H.; Cai, J.P.; Jin, D.Y.; To, K.K.; Chan, J.F.; et al. SARS-CoV-2 nsp13, nsp14, nsp15 and orf6 function as potent interferon antagonists. Emerg. Microbes Infect. 2020, 9, 1418–1428. [Google Scholar] [CrossRef]
- Thoms, M.; Buschauer, R.; Ameismeier, M.; Koepke, L.; Denk, T.; Hirschenberger, M.; Kratzat, H.; Hayn, M.; Mackens-Kiani, T.; Cheng, J.; et al. Structural basis for translational shutdown and immune evasion by the Nsp1 protein of SARS-CoV-2. Science 2020, 369, 1249–1255. [Google Scholar] [CrossRef]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.C.; Uhl, S.; Hoagland, D.; Møller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D.; et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 2020, 181, 1036–1045.e9. [Google Scholar] [CrossRef]
- Zheng, M.; Gao, Y.; Wang, G.; Song, G.; Liu, S.; Sun, D.; Xu, Y.; Tian, Z. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell. Mol. Immunol. 2020, 17, 533–535. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; To, K.K.; Wong, Y.C.; Liu, L.; Zhou, B.; Li, X.; Huang, H.; Mo, Y.; Luk, T.Y.; Lau, T.T.; et al. Acute SARS-CoV-2 infection impairs dendritic cell and T cell responses. Immunity 2020, 53, 864–877.e5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Y.; Li, Y.; Huang, F.; Luo, B.; Yuan, Y.; Xia, B.; Ma, X.; Yang, T.; Yu, F.; et al. The ORF8 protein of SARS-CoV-2 mediates immune evasion through down-regulating MHC-I. Proc. Natl. Acad. Sci. USA 2021, 118, e2024202118. [Google Scholar] [CrossRef]
- Xia, H.; Cao, Z.; Xie, X.; Zhang, X.; Chen, J.Y.; Wang, H.; Menachery, V.D.; Rajsbaum, R.; Shi, P.Y. Evasion of type I interferon by SARS-CoV-2. Cell Rep. 2020, 33, 108234. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; John Wherry, E. T cell responses in patients with COVID-19. Nat. Rev. Immunol. 2020, 20, 529–536. [Google Scholar] [CrossRef]
- Young, L.S.; Yap, L.F.; Murray, P.G. Epstein-Barr virus: More than 50 years old and still providing surprises. Nat. Rev. Cancer 2016, 16, 789–802. [Google Scholar] [CrossRef]
- Proal, A.D.; VanElzakker, M.B. Long COVID or Post-acute Sequelae of COVID-19 (PASC): An Overview of Biological Factors That May Contribute to Persistent Symptoms. Front. Microbiol. 2021, 12, 698169. [Google Scholar] [CrossRef]
- Sanchez, E.L.; Lagunoff, M. Viral activation of cellular metabolism. Virology 2015, 479–480, 609–618. [Google Scholar] [CrossRef]
- Thai, M.; Graham, N.A.; Braas, D.; Nehil, M.; Komisopoulou, E.; Kurdistani, S.K.; McCormick, F.; Graeber, T.G.; Christofk, H.R. Adenovirus E4ORF1-induced MYC activation promotes host cell anabolic glucose metabolism and virus replication. Cell Metab. 2014, 19, 694–701. [Google Scholar] [CrossRef]
- Thaker, S.K.; Ch’ng, J.; Christofk, H.R. Viral hijacking of cellular metabolism. BMC Biol. 2019, 17, 59. [Google Scholar] [CrossRef]
- Elbasir, A.; Ye, Y.; Schäffer, D.E.; Hao, X.; Wickramasinghe, J.; Tsingas, K.; Lieberman, P.M.; Long, Q.; Morris, Q.; Zhang, R.; et al. A deep learning approach reveals unexplored landscape of viral expression in cancer. Nat. Commun. 2023, 14, 785. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Zhang, Y.; Wu, M.; Peng, S.; Kwoh, C.K.; Luo, J.; Li, X. Heterogeneous graph attention networks for drug virus association prediction. Methods 2022, 198, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Y.; Zheng, X.L.; Coghi, P.S.; Chen, J.H.; Dong, B.J.; Fan, X.X. Revolutionizing adjuvant development: Harnessing AI for next-generation cancer vaccines. Front. Immunol. 2024, 15, 1438030. [Google Scholar] [CrossRef]
- Rudroff, T.; Rainio, O.; Klén, R. The untapped potential of dimension reduction in neuroimaging: AI-driven multimodal analysis of Long COVID fatigue. Brain Sci. 2024, 14, 1209. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Gu, D.; Xin, J.; Peters, U.; Song, M.; Cai, G.; Li, S.; Ben, S.; Meng, Y.; Chu, H.; et al. Integrated multi-omics approach to distinct molecular characterization and classification of early-onset colorectal cancer. Cell Rep. Med. 2023, 4, 100974. [Google Scholar] [CrossRef]
- Sosinsky, A.; Ambrose, J.; Cross, W.; Turnbull, C.; Henderson, S.; Jones, L.; Hamblin, A.; Arumugam, P.; Chan, G.; Chubb, D.; et al. Insights for precision oncology from the integration of genomic and clinical data of 13,880 tumors from the 100,000 Genomes Cancer Programme. Nat. Med. 2024, 30, 279–289. [Google Scholar] [CrossRef]
- Sufyan, M.; Shokat, Z.; Ashfaq, U.A. Artificial intelligence in cancer diagnosis and therapy: Current status and future perspective. Comput. Biol. Med. 2023, 165, 107356. [Google Scholar] [CrossRef]
- Chen, F.; Wang, L.; Hong, J.; Jiang, J.; Zhou, L. Unmasking bias in artificial intelligence: A systematic review of bias detection and mitigation strategies in electronic health record-based models. J. Am. Med. Inform. Assoc. 2024, 31, 1172–1183. [Google Scholar] [CrossRef]
- Deters, J.R.; Fietsam, A.C.; Gander, P.E.; Boles Ponto, L.L.; Rudroff, T. Effect of Post-COVID-19 on Brain Volume and Glucose Metabolism: Influence of Time Since Infection and Fatigue Status. Brain Sci. 2023, 13, 675. [Google Scholar] [CrossRef]
- Ogarek, N.; Oboza, P.; Olszanecka-Glinianowicz, M.; Kocelak, P. SARS-CoV-2 infection as a potential risk factor for the development of cancer. Front. Mol. Biosci. 2023, 10, 1260776. [Google Scholar] [CrossRef] [PubMed]
- Khalid, N.; Qayyum, A.; Bilal, M.; Al-Fuqha, A.; Qadir, J. Privacy-preserving artificial intelligence in healthcare: Techniques and applications. Comput. Biol. Med. 2023, 158, 106848. [Google Scholar] [CrossRef]
- Durant, T.J.S.; Knight, E.; Nelson, B.; Dudgeon, S.; Lee, S.J.; Walliman, D.; Young, H.P.; Ohno-Machado, L.; Schulz, W.L. A primer for quantum computing and its applications to healthcare and biomedical research. J. Am. Med. Inform. Assoc. 2024, 31, 1774–1784. [Google Scholar] [CrossRef] [PubMed]
- Felefly, T.; Roukoz, C.; Fares, G.; Achkar, S.; Yazbeck, S.; Meyer, P.; Kordahi, M.; Azoury, F.; Nasr, D.N.; Nasr, E.; et al. An Explainable MRI-Radiomic Quantum Neural Network to Differentiate Between Large Brain Metastases and High-Grade Glioma Using Quantum Annealing for Feature Selection. J. Digit. Imaging 2023, 36, 2335–2346. [Google Scholar] [CrossRef]
- Pal, S.; Bhattacharya, M.; Lee, S.S.; Chakraborty, C. Quantum Computing in the Next-Generation Computational Biology Landscape: From Protein Folding to Molecular Dynamics. Mol. Biotechnol. 2024, 66, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, M.; De Giglio, M.A.R.; Roviello, G.N. Deciphering the Relationship between SARS-CoV-2 and Cancer. Int. J. Mol. Sci. 2023, 24, 7803. [Google Scholar] [CrossRef]
- Lau, B.; Emani, P.S.; Chapman, J.; Yao, L.; Lam, T.; Merrill, P.; Warrell, J.; Gerstein, M.B.; Lam, H.Y.K. Insights from incorporating quantum computing into drug design workflows. Bioinformatics 2023, 39, btac789. [Google Scholar] [CrossRef]
- Ma-Lauer, Y.; Carbajo-Lozoya, J.; Hein, M.Y.; Müller, M.A.; Deng, W.; Lei, J.; Meyer, B.; Kusov, Y.; von Brunn, B.; Bairad, D.R.; et al. p53 down-regulates SARS coronavirus replication and is targeted by the SARS-unique domain and PLpro via E3 ubiquitin ligase RCHY1. Proc. Natl. Acad. Sci. USA 2016, 113, E5192–E5201. [Google Scholar] [CrossRef]
- Saini, G.; Aneja, R. Cancer as a prospective sequela of long COVID-19. Bioessays 2021, 43, e2000331. [Google Scholar] [CrossRef]
- Guedj, E.; Cionca, A.; Péron, J.A.; Ayubcha, C.; Assal, F.; Horowitz, T.; Alavi, A. Long Coronavirus Disease and the Brain: Molecular Neuroimaging Insights into Neurologic and Psychiatric Sequelae. PET Clin. 2025, 20, 39–55. [Google Scholar] [CrossRef]
- Horowitz, T.; Dudouet, P.; Campion, J.Y.; Kaphan, E.; Radulesco, T.; Gonzalez, S.; Cammilleri, S.; Ménard, A.; Guedj, E. Persistent brain metabolic impairment in long COVID patients with persistent clinical symptoms: A nine-month follow-up [18F]FDG-PET study. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 3215–3222. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Guo, Y.; Chen, H.; Sun, P.; Zhang, X.; Ye, T.; Li, L.; Pan, F.; Yang, L. Application and development of Deuterium Metabolic Imaging in tumor glucose metabolism: Visualization of different metabolic pathways. Front. Oncol. 2023, 13, 1285209. [Google Scholar] [CrossRef]
- Thompson, R.F.; Valdes, G.; Fuller, C.D.; Carpenter, C.M.; Morin, O.; Aneja, S.; Lindsay, W.D.; Aerts, H.J.W.L.; Agrimson, B.; Deville, C., Jr.; et al. Artificial intelligence in radiation oncology: A specialty-wide disruptive transformation? Radiother. Oncol. 2018, 129, 421–426. [Google Scholar] [CrossRef]
- Lotter, W.; Hassett, M.J.; Schultz, N.; Kehl, K.L.; Van Allen, E.M.; Cerami, E. Artificial Intelligence in Oncology: Current Landscape, Challenges, and Future Directions. Cancer Discov. 2024, 14, 711–726. [Google Scholar] [CrossRef] [PubMed]
- Chae, A.; Yao, M.S.; Sagreiya, H.; Goldberg, A.D.; Chatterjee, N.; MacLean, M.T.; Duda, J.; Elahi, A.; Borthakur, A.; Ritchie, M.D.; et al. Strategies for Implementing Machine Learning Algorithms in the Clinical Practice of Radiology. Radiology 2024, 310, e223170. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Traverso, A.; Niraula, D.; Zou, J.; Luo, Y.; Owen, D.; El Naqa, I.; Wei, L. Interpretable artificial intelligence in radiology and radiation oncology. Br. J. Radiol. 2023, 96, 20230142. [Google Scholar] [CrossRef]
- Hantel, A.; Clancy, D.D.; Kehl, K.L.; Marron, J.M.; Van Allen, E.M.; Abel, G.A. A Process Framework for Ethically Deploying Artificial Intelligence in Oncology. J. Clin. Oncol. 2022, 40, 3907–3911. [Google Scholar] [CrossRef]
- Bonmatí, L.M.; Miguel, A.; Suárez, A.; Aznar, M.; Beregi, J.P.; Fournier, L.; Neri, E.; Laghi, A.; França, M.; Sardanelli, F.; et al. CHAIMELEON Project: Creation of a Pan-European Repository of Health Imaging Data for the Development of AI-Powered Cancer Management Tools. Front. Oncol. 2022, 12, 742701. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Y.; Wang, Q.; Xing, Y.; Feng, L.; Kong, J.; Peng, C.; Zhang, L.; Yang, H.; Lu, M. Identification of proteasome and caspase inhibitors targeting SARS-CoV-2 M(pro). Signal Transduct. Target. Ther. 2021, 6, 214. [Google Scholar] [CrossRef]
- Gioia, M.; Ciaccio, C.; Calligari, P.; De Simone, G.; Sbardella, D.; Tundo, G.; Fasciglione, G.F.; Di Masi, A.; Di Pierro, D.; Bocedi, A.; et al. Role of proteolytic enzymes in the COVID-19 infection and promising therapeutic approaches. Biochem. Pharmacol. 2021, 182, 114225. [Google Scholar] [CrossRef]
- Gupta, R.K.; Mlcochova, P. Cyclin D3 restricts SARS-CoV-2 envelope incorporation into virions and interferes with viral spread. EMBO J. 2022, 41, e111653. [Google Scholar] [CrossRef] [PubMed]
- Husser, C.; Kwon, H.; Andersson, K.; Appelberg, S.; Montserrat, N.; Mirazimi, A.; Monteil, V.M. P53-Independent G1-Cell Cycle Arrest Increases SARS-CoV-2 RNA Replication. Microorganisms 2024, 12, 443. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.H.; Huang, M.; Fang, S.G.; Liu, D.X. Coronavirus infection induces DNA replication stress partly through interaction of its nonstructural protein 13 with the p125 subunit of DNA polymerase δ. J. Biol. Chem. 2011, 286, 39546–39559. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudroff, T. Convergent Mechanisms in Virus-Induced Cancers: A Perspective on Classical Viruses, SARS-CoV-2, and AI-Driven Solutions. Infect. Dis. Rep. 2025, 17, 33. https://doi.org/10.3390/idr17020033
Rudroff T. Convergent Mechanisms in Virus-Induced Cancers: A Perspective on Classical Viruses, SARS-CoV-2, and AI-Driven Solutions. Infectious Disease Reports. 2025; 17(2):33. https://doi.org/10.3390/idr17020033
Chicago/Turabian StyleRudroff, Thorsten. 2025. "Convergent Mechanisms in Virus-Induced Cancers: A Perspective on Classical Viruses, SARS-CoV-2, and AI-Driven Solutions" Infectious Disease Reports 17, no. 2: 33. https://doi.org/10.3390/idr17020033
APA StyleRudroff, T. (2025). Convergent Mechanisms in Virus-Induced Cancers: A Perspective on Classical Viruses, SARS-CoV-2, and AI-Driven Solutions. Infectious Disease Reports, 17(2), 33. https://doi.org/10.3390/idr17020033





