Global Landscape of Infection-Induced Pulmonary Hypertension
Abstract
1. Introduction
2. Evolution of Pulmonary Hypertension Definition and Classification
3. Epidemiological Challenges and Advancements
4. Pulmonary Arterial Hypertension: A Global Health Challenge
5. The Role of Helminthic Infection
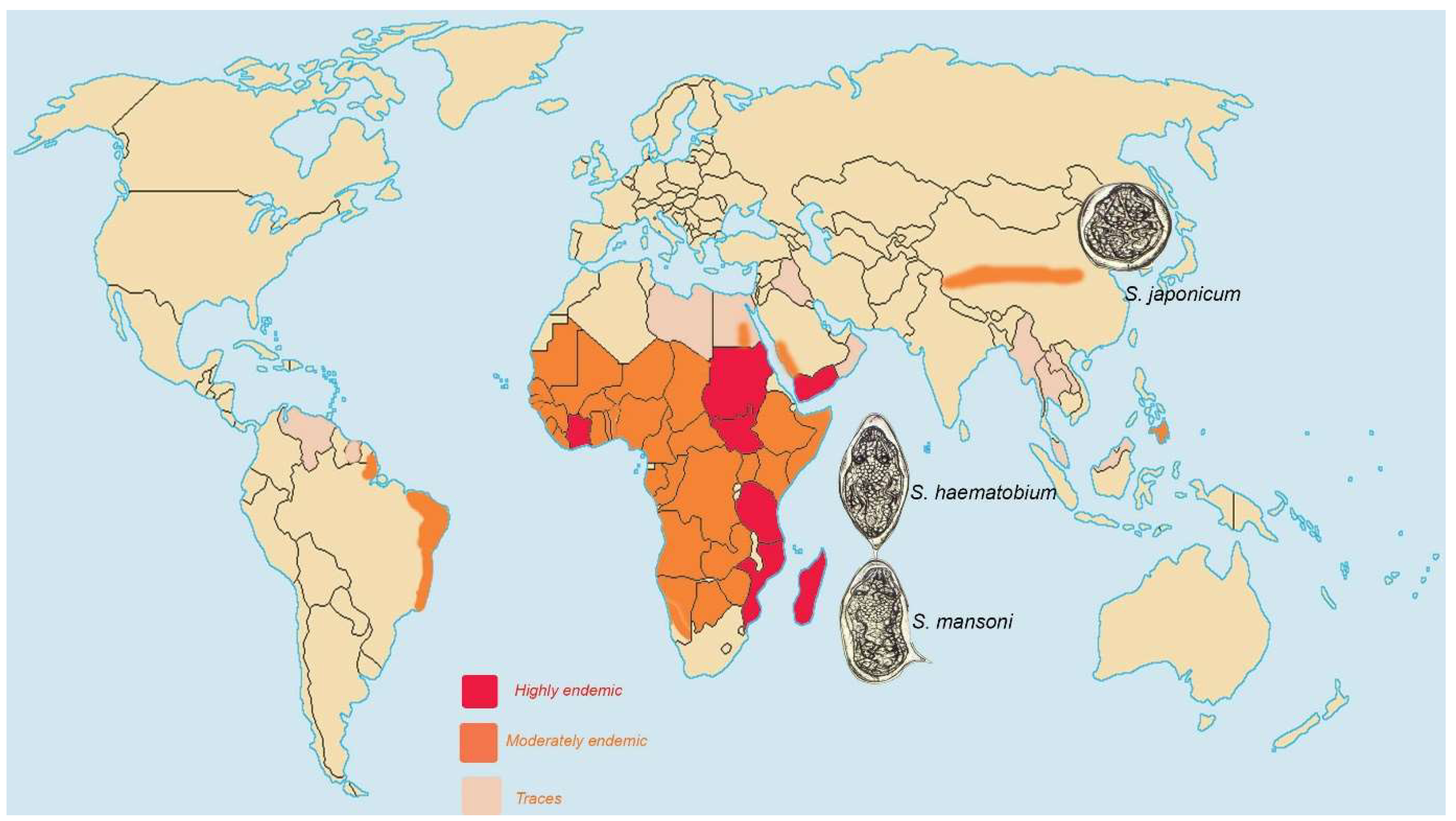
5.1. Schistosomiasis
5.2. Lymphatic filariasis
5.3. Chinese Liver Fluke
5.4. Hydatid Diseases
6. The Role of Bacterial Infection
6.1. Whooping Cough
6.2. Tuberculosis
7. The Role of Fungal Infection
8. The Role of Viral Infection
The Human Immunodeficiency Virus (HIV)
9. The Role of the Microbiome
10. Conclusions and Call to Action
- Investigate how parasitic infections lead to pulmonary hypertension, with a focus on the role of inflammation in pulmonary vascular remodeling.
- Conduct extensive studies in endemic regions to better understand the prevalence and risk factors for pulmonary hypertension in populations affected by parasitic infections.
- Explore how co-infection (polyparasitism) and opportunistic parasitic infections influence the development and progression of pulmonary vascular pathology.
- Develop and validate novel diagnostic methods, such as biomarkers or advanced imaging techniques, to enable the timely identification of pulmonary hypertension in patients with parasitic infections.
- Investigate targeted treatments that address infectious agents and the associated pulmonary vascular damage. This includes evaluating antiparasitic therapies alongside interventions aimed at mitigating vascular remodeling.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weatherald, J.; Hemnes, A.R.; Maron, B.A.; Mielniczuk, L.M.; Gerges, C.; Price, L.C.; Hoeper, M.M.; Humbert, M. Phenotypes in pulmonary hypertension. Eur. Respir. J. 2024, 64, 2301633. [Google Scholar] [CrossRef] [PubMed]
- Mocumbi, A.; Humbert, M.; Saxena, A.; Jing, Z.-C.; Sliwa, K.; Thienemann, F.; Archer, S.L.; Stewart, S. Pulmonary hypertension. Nat. Rev. Dis. Prim. 2024, 10, 1. [Google Scholar] [CrossRef]
- Oliveira, S.D.; Almodóvar, S.; Butrous, G.; Perez, V.D.J.; Fabro, A.; Graham, B.B.; Mocumbi, A.; Nyasulu, P.S.; Tura-Ceide, O.; Oliveira, R.K.F.; et al. Infection and pulmonary vascular diseases consortium: United against a global health challenge. Pulm. Circ. 2024, 14, e70003. [Google Scholar] [CrossRef]
- Al-Tawfiq, J.A.; Kim, H.; Memish, Z.A. Parasitic lung diseases. Eur. Respir. Rev. 2022, 31, 220093. [Google Scholar] [CrossRef]
- Butrous, G.; Mathie, A. Infection in pulmonary vascular diseases: Would another consortium really be the way to go? Glob. Cardiol. Sci. Pr. 2019, 2019, 1. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.; Bartolome, S.; Denton, C.P.; Gatzoulis, M.A.; Gu, S.; Khanna, D.; Badesch, D.; Montani, D. Definition, classification and diagnosis of pulmonary hypertension. Eur. Respir. J. 2024, 64, 2401324. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Galiè, N.; Rubin, L.J.; Simonneau, G.; McLaughlin, V.V. The Seventh World Symposium on Pulmonary Hypertension: Our journey to Barcelona. Eur. Respir. J. 2024, 64, 2401222. [Google Scholar] [CrossRef]
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Humbert, M.; Souza, R.; Idrees, M.; Kawut, S.M.; Sliwa-Hahnle, K.; Jing, Z.-C.; Gibbs, J.S.R. A global view of pulmonary hypertension. Lancet Respir. Med. 2016, 4, 306–322. [Google Scholar] [CrossRef]
- Swinnen, K.; Quarck, R.; Godinas, L.; Belge, C.; Delcroix, M. Learning from registries in pulmonary arterial hypertension: Pitfalls and recommendations. Eur. Respir. Rev. 2019, 28, 190050. [Google Scholar] [CrossRef]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Global Burden of Disease (GBD). Available online: https://www.healthdata.org/research-analysis/gbd-research-library (accessed on 13 January 2025).
- Leary, P.J.; Lindstrom, M.; Johnson, C.O.; Emmons-Bell, S.; Rich, S.; Corris, P.A.; DuBrock, H.M.; Ventetuolo, C.E.; Abate, Y.H.; Abdelmasseh, M.; et al. Global, regional, and national burden of pulmonary arterial hypertension, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Respir. Med. 2025, 13, 69–79. [Google Scholar] [CrossRef]
- Deng, Y.; Dong, X.; Qian, J.; Zhao, J.; Xu, D.; Wang, Y.; Li, M.; Zeng, X.; Wang, Q. Global, Regional, and National Burden of Pulmonary Arterial Hypertension, 1990–2021: A Systematic Analysis of the Global Burden of Disease Study 2021. medRxiv 2024. [Google Scholar] [CrossRef]
- Butrous, G. Pulmonary hypertension: From an orphan disease to a global epidemic. Glob. Cardiol. Sci. Pr. 2020, 2020, e202005. [Google Scholar] [CrossRef] [PubMed]
- Butrous, G. The Global Challenge of Pulmonary Vascular Diseases and its Forgotten Impact in the Developing World. Adv. Pulm. Hypertens. 2012, 11, 117–118. [Google Scholar] [CrossRef]
- Fling, C.; De Marco, T.; Kime, N.A.; Lammi, M.R.; Oppegard, L.J.; Ryan, J.J.; Ventetuolo, C.E.; White, R.J.; Zamanian, R.T.; Leary, P.J. Regional Variation in Pulmonary Arterial Hypertension in the United States: The Pulmonary Hypertension Association Registry. Ann. Am. Thorac. Soc. 2023, 20, 1718–1725. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, C.; Cai, J.; Kong, R.; Wang, Y.; Wang, Y.; Li, S.; Zhan, J.; Liu, Y. Trends and levels of the global, regional, and national burden of pulmonary arterial hypertension from 1990 to 2021: Findings from the global burden of disease study 2021. Front. Med. 2024, 11, 1515961. [Google Scholar] [CrossRef]
- Butrous, G. Pulmonary Vascular Diseases Secondary to Schistosomiasis. Adv. Pulm. Hypertens. 2017, 15, 144–148. [Google Scholar] [CrossRef]
- Butrous, G. Schistosome infection and its effect on pulmonary circulation. Glob. Cardiol. Sci. Pr. 2019, 2019, 5. [Google Scholar] [CrossRef]
- Sibomana, J.P.; Campeche, A.; Carvalho-Filho, R.J.; Correa, R.A.; Duani, H.; Pacheco Guimaraes, V.; Hilton, J.F.; Kassa, B.; Kumar, R.; Lee, M.H.; et al. Schistosomiasis Pulmonary Arterial Hypertension. Front. Immunol. 2020, 11, 608883. [Google Scholar] [CrossRef]
- Graham, B.B. Schistosomiasis-Associated Pulmonary Arterial Hypertension. Adv. Pulm. Hypertens. 2022, 21, 109–114. [Google Scholar] [CrossRef]
- de Cleva, R.; de Cleva, P.H.R.; Rodrigues, G. Fathal pulmonary hypertension after distal splenorenal shunt in schistosomal portal hypertension. World J. Gastroenterol. 2004, 10, 1836–1837. [Google Scholar] [CrossRef] [PubMed]
- Lambertucci, J.R.; Serufo, J.; Gerspacher-Lara, R.; Rayes, A.A.; Teixeira, R.; Nobre, V.; Antunes, C.M. Schistosoma mansoni: Assessment of morbidity before and after control. Acta Trop. 2000, 77, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Mendes, A.A.; Roncal, C.G.P.; de Oliveira, F.R.A.; de Albuquerque, E.S.; Góes, G.H.B.; Piscoya, I.C.D.V.; Filho, D.C.S. Demographic and clinical characteristics of pulmonary arterial hypertension caused by schistosomiasis are indistinguishable from other etiologies. Rev. Soc. Bras. Med. Trop. 2020, 53, e20190418. [Google Scholar] [CrossRef]
- Papamatheakis, D.G.; Mocumbi, A.O.H.; Kim, N.H.; Mandel, J. Schistosomiasis-associated pulmonary hypertension. Pulm. Circ. 2014, 4, 596–611. [Google Scholar] [CrossRef]
- Knafl, D.; Gerges, C.; King, C.H.; Humbert, M.; Bustinduy, A.L. Schistosomiasis-associated pulmonary arterial hypertension: A systematic review. Eur. Respir. Rev. 2020, 29, 190089. [Google Scholar] [CrossRef]
- Kolosionek, E.; King, J.; Rollinson, D.; Schermuly, R.T.; Grimminger, F.; Graham, B.B.; Morrell, N.; Butrous, G. Schistosomiasis causes remodeling of pulmonary vessels in the lung in a heterogeneous localized manner: Detailed Study. Pulm. Circ. 2013, 3, 356–362. [Google Scholar] [CrossRef]
- Adenowo, A.F.; Oyinloye, B.E.; Ogunyinka, B.I.; Kappo, A.P. Impact of human schistosomiasis in sub-Saharan Africa. Braz. J. Infect. Dis. 2015, 19, 196–205. [Google Scholar] [CrossRef]
- Aula, O.P.; McManus, D.P.; Jones, M.K.; Gordon, C.A. Schistosomiasis with a Focus on Africa. Trop. Med. Infect. Dis. 2021, 6, 109. [Google Scholar] [CrossRef]
- Thienemann, F.; Katoto, P.D.M.C.; Azibani, F.; Kodogo, V.; Mukasa, S.L.; Sani, M.U.; Karaye, K.M.; Mbanze, I.; Mocumbi, A.O.; Dzudie, A.; et al. Long-Term Follow-up of Human Immunodeficiency Virus-Associated Pulmonary Hypertension: Clinical Features and Survival Outcomes of the Pan Africa Pulmonary Hypertension Cohort (PAPUCO). Open Forum Infect. Dis. 2022, 9, ofac604. [Google Scholar] [CrossRef]
- Butrous, G. Human Immunodeficiency Virus-Associated Pulmonary Arterial Hypertension Considerations for Pulmonary Vascular Diseases in the Developing World. Circulation 2015, 131, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Colombe, S.; Machemba, R.; Mtenga, B.; Lutonja, P.; Kalluvya, S.E.; de Dood, C.J.; Hoekstra, P.T.; van Dam, G.J.; Corstjens, P.L.A.M.; Urassa, M.; et al. Impact of schistosome infection on long-term HIV/AIDS outcomes. PLoS Negl. Trop. Dis. 2018, 12, e0006613. [Google Scholar] [CrossRef] [PubMed]
- Bustinduy, A.; King, C.; Scott, J.; Appleton, S.; Sousa-Figueiredo, J.C.; Betson, M.; Stothard, J.R. HIV and schistosomiasis co-infection in African children. Lancet Infect. Dis. 2014, 14, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Noya, O.; Katz, N.; Pointier, J.P.; Théron, A.; Alarcón de Noya, B. Schistosomiasis in America. In Neglected Tropical Diseases—Latin America and the Caribbean, Neglected Tropical Diseases; Franco-Paredes, C., Santos-Preciado, J.I., Eds.; Springer: Vienna, Austria, 2015; pp. 11–43. [Google Scholar] [CrossRef]
- Lapa, M.; Dias, B.; Jardim, C.; Fernandes, C.J.; Dourado, P.M.; Figueiredo, M.; Farias, A.; Tsutsui, J.; Terra-Filho, M.; Humbert, M.; et al. Cardiopulmonary Manifestations of Hepatosplenic Schistosomiasis. Circulation 2009, 119, 1518–1523. [Google Scholar] [CrossRef]
- Fernandes, C.J.; Jardim, C.; Souza, R. Pulmonary Hypertension Complicating Schistosomiasis. In Pulmonary Hypertension; CRC Press: Boca Raton, FL, USA, 2009; Volume 214. [Google Scholar]
- Barbosa, M.M.; Lamounier, J.A.; Oliveira, E.C.; Souza, M.V.; Marques, D.S.; Silva, A.A.; Lambertucci, J. Pulmonary hypertension in schistosomiasis mansoni. Trans. R. Soc. Trop. Med. Hyg. 1996, 90, 663–665. [Google Scholar] [CrossRef]
- Ferreira, R.C.S.; Domingues, A.L.C.; Bandeira, Â.P.; Filho, B.M.; Filho, E.S.A.; de Araújo, A.C.C.C.; Batista, L.J.B.; Markman, M.; Campelo, A.R.L. Prevalence of pulmonary hypertension in patients with schistosomal liver fibrosis. Ann. Trop. Med. Parasitol. 2009, 103, 129–143. [Google Scholar] [CrossRef]
- Gonçalves, E.C.; Fonseca, A.P.; Pittella, J.E. Frequency of schistosomiasis mansoni, of its clinicopathological forms and of the ectopic locations of the parasite in autopsies in Belo Horizonte, Brazil. J. Trop. Med. Hyg. 1995, 98, 289–295. [Google Scholar]
- Zeng, X.; Huang, X.; Rathinasabapathy, A.; Xu, Z.; Li, K.; Liu, N.; Chen, H.; Jiang, Y.; Zha, L.; Yu, Z. Prevalence of Schistosoma japonicum-associated Pulmonary Hypertension in China: An Echocardiography-based Assessment. Ann. Am. Thorac. Soc. 2021, 18, 2095–2098. [Google Scholar] [CrossRef]
- Valverde, A.B.; Soares, J.M.; Viana, K.P.; Gomes, B.; Soares, C.; Souza, R. Pulmonary arterial hypertension in Latin America: Epidemiological data from local studies. BMC Pulm. Med. 2018, 18, 106. [Google Scholar] [CrossRef]
- Farrag, A.; El-Aroussy, W.; Zaghloul, S.; El-Guindy, M.; Yacoub, M. Prevalence and severity of pulmonary hypertension in asymptomatic rural residents with schistosomal infection in the Nile Delta. Trop. Med. Int. Health 2011, 17, 112–118. [Google Scholar] [CrossRef]
- Bedford, D.E.; Aidaros, S.M.; Girgis, B. Bilharzial Heart Disease in Egypt: Cor Pulmonale due to Bilharzial Pulmonary Endarteritis. Heart 1946, 8, 87–95. [Google Scholar] [CrossRef]
- Shaw, A.F.B.; Ghareeb, A.A. The Pathogenesis of Pulmonary Sehistosomiasis in Egypt with Special Reference to Ayerza’s Disease. J. Pathol. Bacteriol. 1938, 46, 401–424. [Google Scholar] [CrossRef]
- Gordon, C.A.; Williams, G.M.; Gray, D.J.; Clements, A.C.A.; Zhou, X.-N.; Li, Y.; Utzinger, J.; Kurscheid, J.; Forsyth, S.; Alene, K.A.; et al. Schistosomiasis in the People’s Republic of China—Down but not out. Parasitology 2021, 149, 218–233. [Google Scholar] [CrossRef] [PubMed]
- Alene, K.A.; Gordon, C.A.; Clements, A.C.A.; Williams, G.M.; Gray, D.J.; Zhou, X.-N.; Li, Y.; Utzinger, J.; Kurscheid, J.; Forsyth, S.; et al. Spatial Analysis of Schistosomiasis in Hunan and Jiangxi Provinces in the People’s Republic of China. Diseases 2022, 10, 93. [Google Scholar] [CrossRef]
- McManus, D.P.; Gray, D.J.; Ross, A.G.; Williams, G.M.; He, H.-B.; Li, Y.-S. Schistosomiasis Research in the Dongting Lake Region and Its Impact on Local and National Treatment and Control in China. PLoS Neglected Trop. Dis. 2011, 5, e1053. [Google Scholar] [CrossRef]
- Ross, A.G.P.; Sleigh, A.C.; Li, Y.; Davis, G.M.; Williams, G.M.; Jiang, Z.; Feng, Z.; McManus, D.P. Schistosomiasis in the People’s Republic of China: Prospects and Challenges for the 21st Century. Clin. Microbiol. Rev. 2001, 14, 270–295. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-S.; Raso, G.; Zhao, Z.-Y.; He, Y.-K.; Ellis, M.K.; McManus, D.P. Large water management projects and schistosomiasis control, Dongting Lake region, China. Emerg. Infect. Dis. 2007, 13, 973. [Google Scholar] [CrossRef]
- Bigna, J.J.; Noubiap, J.J.; Nansseu, J.R.; Aminde, L.N. Prevalence, incidence and aetiologies of pulmonary hypertension in Africa: A systematic review and meta-analysis protocol. BMJ Open 2017, 7, e014768. [Google Scholar] [CrossRef]
- Loureiro, C.M.C.; Scheibler Filho, A.L.; Menezes, V.M.A.S.; Correa, R.A.; Oliveira, R.K.F.; Mickael, C.; Hilton, J.F.; Graham, B.B. Clinical, Functional, and Hemodynamic Profile of Schistosomiasis-Associated Pulmonary Arterial Hypertension Patients in Brazil: Systematic Review and Meta-Analysis. Infect. Dis. Rep. 2025, 17, 22. [Google Scholar] [CrossRef]
- Tsanglao, W.R.; Nandan, D.; Chandelia, S.; Arya, N.K.; Sharma, A. Filarial tropical pulmonary eosinophilia: A condition masquerading asthma, a series of 12 cases. J. Asthma 2018, 56, 791–798. [Google Scholar] [CrossRef]
- Ottesen, E.A.; Nutman, T.B. Tropical Pulmonary Eosinophilia. Annu. Rev. Med. 1992, 43, 417–424. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mukherjee, S.; Maiti, T.K.; Bhattacharya, S.; Babu, S.P.S. A Novel Ligand of Toll-like Receptor 4 From the Sheath of Wuchereria bancrofti Microfilaria Induces Proinflammatory Response in Macrophages. J. Infect. Dis. 2017, 215, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Walloopillai, N. Primary Pulmonary Hypertension, an Unexplained Epidemic in Sri Lanka. Pathobiology 1975, 43, 248–250. [Google Scholar] [CrossRef]
- Clonorchiasis—Infectious Diseases. MSD Manual. Professional Version. Available online: https://www.msdmanuals.com/professional/infectious-diseases/trematodes-flukes/clonorchiasis (accessed on 12 February 2025).
- Gao, Y.; Li, Y.; Liu, X.; Zhang, T.; Yu, G.; Wang, Y.; Shi, Y.; Chi, X.; Wang, X.; Gao, X.; et al. High prevalence of Clonorchis sinensis infections and coinfection with hepatitis virus in riverside villages in northeast China. Sci. Rep. 2020, 10, 1174. [Google Scholar] [CrossRef]
- Reddy, A.K.; Chakrabarty, M.; Liu, Y.; Cohen, S.H.; Maniar, A.H. Case Report: Clonorchis sinensis Infection Associated with Eosinophilic Pneumonia. Am. J. Trop. Med. Hyg. 2021, 104, 2065–2068. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.S.; McFadzean, A.J.; Yeung, R. Microembolic pulmonary hypertension in pyogenic cholangitis. BMJ 1968, 1, 22–24. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Vuitton, L.; Tuxun, T.; Li, J.; Vuitton, D.A.; Zhang, W.; McManus, D.P. Echinococcosis: Advances in the 21st Century. Clin. Microbiol. Rev. 2019, 32, e00075-18. [Google Scholar] [CrossRef]
- Bulman, W.; Coyle, C.M.; Brentjens, T.E.; Horn, E.M.; Dickstein, M.L.; Wilt, J.S.; Emond, J.; Kawut, S.M. Severe pulmonary hypertension due to chronic echinococcal pulmonary emboli treated with targeted pulmonary vascular therapy and hepatic resection. Chest 2007, 132, 1356–1358. [Google Scholar] [CrossRef]
- Camporrotondo, M.; Vrancic, M.; Piccinini, F.; Navia, D. Surgical treatment of pulmonary hypertension caused by echinococcosis disease. J. Thorac. Cardiovasc. Surg. 2014, 147, e15–e16. [Google Scholar] [CrossRef]
- Ogul, H.; Topcu, S.; Aydin, Y.; Ulas, A.B.; Kantarci, M.; Eroglu, A. An Unusual Cause of Pulmonary Arterial Hypertension: Hydatid Cyst. Ann. Thorac. Surg. 2020, 109, E267–E269. [Google Scholar] [CrossRef]
- Scanlon, K.M.; Chen, L.; Carbonetti, N.H. Pertussis Toxin Promotes Pulmonary Hypertension in an Infant Mouse Model of Bordetella pertussis Infection. J. Infect. Dis. 2021, 225, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Paddock, C.D.; Sanden, G.N.; Cherry, J.D.; Gal, A.A.; Langston, C.; Tatti, K.M.; Wu, K.; Goldsmith, C.S.; Greer, P.W.; Montague, J.L.; et al. Pathology and Pathogenesis of Fatal Bordetella pertussis Infection in Infants. Clin. Infect. Dis. 2008, 47, 328–338. [Google Scholar] [CrossRef]
- Peters, M.J.; Pierce, C.M.; Klein, N.J. Mechanisms of pulmonary hypertension in Bordetella pertussis. Arch. Dis. Child. 2003, 88, 92–93. [Google Scholar] [CrossRef]
- Scanlon, K.; Skerry, C.; Carbonetti, N. Association of Pertussis Toxin with Severe Pertussis Disease. Toxins 2019, 11, 373. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.E.H.; Ibrahim, A.S.; Elshafie, S.M. Pulmonary Hypertension in Patients with Treated Pulmonary Tuberculosis: Analysis of 14 Consecutive Cases. Clin. Med. Insights Circ. Respir. Pulm. Med. 2011, 5, CCRPM-S6437. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Saha, D.; Bhattacherjee, P.D.; Das, S.K.; Dey, R. Tuberculosis associated pulmonary hypertension: The revelation of a clinical observation. Lung India 2016, 33, 135–139. [Google Scholar] [CrossRef]
- van Heerden, J.K.; Louw, E.H.; Thienemann, F.; Engel, M.E.; Allwood, B.W. The prevalence of pulmonary hypertension in post-tuberculosis and active tuberculosis populations: A systematic review and meta-analysis. Eur. Respir. Rev. 2024, 33, 230154. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M.; Gami, S.P. Pulmonary Hypertension in Pulmonary tuberculosis—A Prognostic Indicator. Eur. Respir. J. 2017, 50, PA2432. [Google Scholar]
- Chatterjee, R.; Ghosh, P.; Sarkar, K.; Samajdar, S.S.; Mukherjee, S.; George, A.; Pramanik, N. Prevalence of pulmonary arterial hypertension in post-tuberculosis lung fibrosis patients: A cross-sectional observational study. J. Assoc. Chest Physicians 2023, 11, 198–201. [Google Scholar] [CrossRef]
- Louw, E.; Baines, N.; Maarman, G.; Osman, M.; Sigwadhi, L.; Irusen, E.; Koegelenberg, C.; Doubell, A.; Nathan, S.; Channick, R.; et al. The prevalence of pulmonary hypertension after successful tuberculosis treatment in a community sample of adult patients. Pulm. Circ. 2023, 13, e12184. [Google Scholar] [CrossRef]
- Jumaar, C.; Jacobs, S.; Payne, C.; Sanni, O.; Louw, E.; Baines, N.; Maree, D.; Botha, B.; Feyasa, M.B.; Strijdom, H.; et al. Endothelial Dysfunction Markers Correlate with the Time Since Completion of Tuberculosis Treatment and the Number of Previous Tuberculosis Episodes. Infect. Dis. Rep. 2025, 17, 21. [Google Scholar] [CrossRef] [PubMed]
- de Queiroz-Telles, F.V.; Pietrobom, P.M.P.; Júnior, M.R.; Baptista, R.d.M.; Peçanha, P.M. New Insights on Pulmonary Paracoccidioidomycosis. Semin. Respir. Crit. Care Med. 2020, 41, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Borges-Walmsley, M.; Chen, D.; Shu, X.; Walmsley, A.R. The pathobiology of Paracoccidioides brasiliensis. Trends Microbiol. 2002, 10, 80–87. [Google Scholar] [CrossRef]
- Batah, S.; dos Santos Leao, P.; Veronez, J.; Veronez, D.; Ignacio de Padua, A.; Baddini Martinez, J.A.; Salgado, H.C.; Capelozzi, V.L.; Achcar, R.D.; Fabro, A.T.; et al. Pulmonary Hypertension Due to the Human Paracoccidioidomycosis. Am. J. Respir. Crit. Care Med. 2018, 197, A4378. [Google Scholar]
- Gaspar, G.G.; Cocio, T.A.; Guioti-Puga, F.; Nascimento, E.; Fabro, A.T.; Kress, M.R.V.Z.; Bagagli, E.; Martinez, R. Paracoccidioidomycosis due to Paracoccidioides lutzii complicated with adrenal injury and pulmonary arterial hypertension. Rev. Inst. Med. Trop. Sao Paulo 2020, 62, e89. [Google Scholar] [CrossRef] [PubMed]
- Batah, S.S.; Alda, M.A.; Machado-Rugulo, J.R.; Felix, R.G.; Nascimento, E.; Martinez, R.; de Pádua, A.I.; Bagagli, E.; Hrycyk, M.F.; Salgado, H.C.; et al. Pulmonary paracoccidioidomycosis-induced pulmonary hypertension. Clin. Transl. Med. 2020, 10, e213. [Google Scholar] [CrossRef]
- Aberg, J.A. Cardiovascular Complications in HIV Management: Past, Present, and Future. Am. J. Ther. 2009, 50, 54–64. [Google Scholar] [CrossRef]
- Basyal, B.; Jarrett, H.; Barnett, C.F. Pulmonary Hypertension in HIV. Can. J. Cardiol. 2019, 35, 288–298. [Google Scholar] [CrossRef]
- Prakash, P.; Vetha, B.S.S.; Chakraborty, R.; Wenegieme, T.-Y.; Masenga, S.K.; Muthian, G.; Balasubramaniam, M.; Wanjalla, C.N.; Hinton, A.O.; Kirabo, A.; et al. HIV-Associated Hypertension: Risks, Mechanisms, and Knowledge Gaps. Circ. Res. 2024, 134, e150–e175. [Google Scholar] [CrossRef]
- Almodovar, S. The Complexity of HIV Persistence and Pathogenesis in the Lung Under Antiretroviral Therapy: Challenges Beyond AIDS. Viral Immunol. 2014, 27, 186–199. [Google Scholar] [CrossRef]
- Cribbs, S.K.; Crothers, K.; Morris, A. Pathogenesis of HIV-Related Lung Disease: Immunity, Infection, and Inflammation. Physiol. Rev. 2020, 100, 603–632. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, L.; Kress, T.; Kennard, S.; de Chantemele, E.B. HIV-derived proteins contribute to the development of pulmonary arterial hypertension in mice. Physiology 2023, 38, 5732706. [Google Scholar] [CrossRef]
- Garcia, A.K.; Almodovar, S. The Intersection of HIV and Pulmonary Vascular Health: From HIV Evolution to Vascular Cell Types to Disease Mechanisms. J. Vasc. Dis. 2024, 3, 174–200. [Google Scholar] [CrossRef]
- Duncan, M.S.; Alcorn, C.W.; Freiberg, M.S.; So-Armah, K.; Patterson, O.V.; DuVall, S.L.; Crothers, K.A.; Re, V.L.; Butt, A.A.; Lim, J.K.; et al. Association between HIV and incident pulmonary hypertension in US Veterans: A retrospective cohort study. Am. J. Med. Sci. 2021, 2, e417–e425. [Google Scholar] [CrossRef]
- Brittain, E.L.; Duncan, M.S.; Chang, J.; Patterson, O.V.; DuVall, S.L.; Brandt, C.A.; So-Armah, K.A.; Goetz, M.; Akgun, K.; Crothers, K.; et al. Increased Echocardiographic Pulmonary Pressure in HIV-infected and -uninfected Individuals in the Veterans Aging Cohort Study. Am. J. Respir. Crit. Care Med. 2018, 197, 923–932. [Google Scholar] [CrossRef]
- Freyhaus, H.T.; Vogel, D.; Lehmann, C.; Kümmerle, T.; Wyen, C.; Fätkenheuer, G.; Rosenkranz, S. Echocardiographic screening for pulmonary arterial hypertension in HIV-positive patients. Infection 2014, 42, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Youssef, M.; Salama, M.B.; Rehman, N.; Hanna, C.; Waniss, M.R.; Mbuagbaw, L. Pulmonary hypertension survival and hospitalisations in people living with HIV: A systematic review and meta-analysis. BMJ Open Respir. Res. 2024, 11, e002318. [Google Scholar] [CrossRef]
- Ding, Y.; He, N. HIV and pulmonary hypertension: CD4 and viral load matter. The Lancet Healthy Longev. 2021, 2, e389–e390. [Google Scholar] [CrossRef]
- Alaiti, M.A.; Goud, A.; Ramani, G.; Bagchi, S.; Al-Kindi, S.; Sawicki, S.; Longenecker, C.; Jenkins, T.; Pauza, D.; Park, M.; et al. Design of the exercise MRI evaluation of HIV-pulmonary arterial hypertension longitudinal determinants (EXALTED) trial. J. Cardiovasc. Med. 2017, 18, 888–896. [Google Scholar] [CrossRef]
- Bigna, J.J.; Nansseu, J.R.; Noubiap, J.J. Pulmonary hypertension in the global population of adolescents and adults living with HIV: A systematic review and meta-analysis. Sci. Rep. 2019, 9, 7837. [Google Scholar] [CrossRef]
- Kumar, A.; Mahajan, A.; Salazar, E.A.; Pruitt, K.; Guzman, C.A.; Clauss, M.A.; Almodovar, S.; Dhillon, N.K. Impact of human immunodeficiency virus on pulmonary vascular disease. Glob. Cardiol. Sci. Pr. 2021, 2021, e202112. [Google Scholar] [CrossRef] [PubMed]
- Sitbon, O.; Lascoux-Combe, C.; Delfraissy, J.-F.; Yeni, P.G.; Raffi, F.; De Zuttere, D.; Gressin, V.; Clerson, P.; Sereni, D.; Simonneau, G. Prevalence of HIV-related Pulmonary Arterial Hypertension in the Current Antiretroviral Therapy Era. Am. J. Respir. Crit. Care Med. 2008, 177, 108–113. [Google Scholar] [CrossRef]
- Speich, R.; Jenni, R.; Opravil, M.; Pfab, M.; Russi, E.W. Primary Pulmonary Hypertension in HIV Infection. Chest 1991, 100, 1268–1271. [Google Scholar] [CrossRef]
- Kolaitis, N.A.; Lammi, M.; Mazimba, S.; Feldman, J.; McConnell, W.; Sager, J.S.; Raval, A.A.; Simon, M.A.; De Marco, T.; on behalf of the PHAR Investigators. HIV-associated Pulmonary Arterial Hypertension: A Report from the Pulmonary Hypertension Association Registry. Am. J. Respir. Crit. Care Med. 2022, 205, 1121–1124. [Google Scholar] [CrossRef]
- Katoto, P.D.M.C.; Mukasa, S.L.; Wolmarans, K.H.; Sani, M.U.; Karaye, K.M.; Mbanze, I.; Damasceno, A.; Mocumbi, A.O.; Dzudie, A.; Sliwa, K.; et al. Effects of post tuberculosis lung disease on survival in HIV-infected individuals with pulmonary hypertension: Insights from the Pan African Pulmonary Hypertension Cohort (PAPUCO) study. medRxiv 2023. medRxiv:23294338. [Google Scholar] [CrossRef]
- Medrano-Garcia, S.; Morales-Cano, D.; Barreira, B.; Vera-Zambrano, A.; Kumar, R.; Kosanovic, D.; Schermuly, R.T.; Graham, B.B.; Perez-Vizcaino, F.; Mathie, A.; et al. HIV and Schistosoma Co-Exposure Leads to Exacerbated Pulmonary Endothelial Remodeling and Dysfunction Associated with Altered Cytokine Landscape. Cells 2022, 11, 2414. [Google Scholar] [CrossRef] [PubMed]
- Degano, B.; Guillaume, M.; Savale, L.; Montani, D.; Jaïs, X.; Yaici, A.; Le Pavec, J.; Humbert, M.; Simonneau, G.; Sitbon, O. HIV-associated pulmonary arterial hypertension: Survival and prognostic factors in the modern therapeutic era. AIDS 2010, 24, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Nunes, H.; Humbert, M.; Sitbon, O.; Morse, J.H.; Deng, Z.; Knowles, J.A.; Le Gall, C.; Parent, F.; Garcia, G.; Hervé, P.; et al. Prognostic Factors for Survival in Human Immunodeficiency Virus-associated Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2003, 167, 1433–1439. [Google Scholar] [CrossRef]
- Ghofrani, H.; Friese, G.; Discher, T.; Olschewski, H.; Schermuly, R.; Weissmann, N.; Seeger, W.; Grimminger, F.; Lohmeyer, J. Inhaled iloprost is a potent acute pulmonary vasodilator in HIV-related severe pulmonary hypertension. Eur. Respir. J. 2004, 23, 321–326. [Google Scholar] [CrossRef]
- Zuber, J.; Calmy, A.; Evison, J.M.; Hasse, B.; Schiffer, V.; Wagels, T.; Nuesch, R.; Magenta, L.; Ledergerber, B.; Jenni, R.; et al. Pulmonary Arterial Hypertension Related to HIV Infection: Improved Hemodynamics and Survival Associated with Antiretroviral Therapy. Clin. Infect. Dis. 2004, 38, 1178–1185. [Google Scholar] [CrossRef]
- Mason, T.; Mukherjee, B.; Marino, P. Pulmonary Hypertension and the Gut Microbiome. Biomedicines 2024, 12, 169. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, H.; Liu, Y.; Long, Y. The Role of Gut and Airway Microbiota in Pulmonary Arterial Hypertension. Front. Microbiol. 2022, 13, 929752. [Google Scholar] [CrossRef]
- Oliveira, S.D. Cardiopulmonary Pathogenic Networks: Unveiling the Gut–Lung Microbiome Axis in Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2023, 207, 655–657. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Zhu, T.; Tan, Z.; Chen, S.; Fang, Z. Role of Gut Microbiota in Pulmonary Arterial Hypertension. Front. Cell. Infect. Microbiol. 2022, 12, 812303. [Google Scholar] [CrossRef]
- Kim, S.; Rigatto, K.; Gazzana, M.B.; Knorst, M.M.; Richards, E.M.; Pepine, C.J.; Raizada, M.K. Altered Gut Microbiome Profile in Patients With Pulmonary Arterial Hypertension. Hypertension 2020, 75, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Yuan, W.; Meng, L.-K.; Zhong, J.-C.; Liu, X.-Y. The Role and Mechanism of Gut Microbiota in Pulmonary Arterial Hypertension. Nutrients 2022, 14, 4278. [Google Scholar] [CrossRef]
- Suswał, K.; Tomaszewski, M.; Romaniuk, A.; Świechowska-Starek, P.; Zygmunt, W.; Styczeń, A.; Romaniuk-Suswał, M. Gut–Lung Axis in Focus: Deciphering the Impact of Gut Microbiota on Pulmonary Arterial Hypertension. J. Pers. Med. 2023, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Li, S.; Yan, M.; Yang, Y.; Zhong, C.; Hu, Y. Genetically determined gut microbiota associates with pulmonary arterial hypertension: A Mendelian randomization study. BMC Pulm. Med. 2024, 24, 235. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, D.; Miao, J.; Zhang, C.; Li, X.; Feng, H.; Xing, Y.; Zhang, Z.; Bao, C.; Lin, Z.; et al. Microbiome and metabolome dysbiosis of the gut-lung axis in pulmonary hypertension. Microbiol. Res. 2022, 265, 127205. [Google Scholar] [CrossRef]
- Marinho, Y.; Villarreal, E.S.; Aboagye, S.Y.; Williams, D.L.; Sun, J.; Silva, C.L.M.; Lutz, S.E.; Oliveira, S.D. Schistosomiasis-associated pulmonary hypertension unveils disrupted murine gut–lung microbiome and reduced endoprotective Caveolin-1/BMPR2 expression. Front. Immunol. 2023, 14, 1254762. [Google Scholar] [CrossRef]
- Oliveira, A.C.; Yang, T.; Li, J.; Sharma, R.K.; Karas, M.K.; Bryant, A.J.; de Kloet, A.D.; Krause, E.G.; Joe, B.; Richards, E.M.; et al. Fecal matter transplant from Ace2 overexpressing mice counteracts chronic hypoxia-induced pulmonary hypertension. Pulm. Circ. 2022, 12, e12015. [Google Scholar] [CrossRef]
- Prisco, S.Z.; Oliveira, S.D.S.; Weir, E.K.; Thenappan, T.; Al Ghouleh, I. Gut Microbiome in Pulmonary Arterial Hypertension—An Emerging Frontier. [CrossRef]

| Group 1: Pulmonary Arterial Hypertension |
| 1.1 Idiopathic |
| 1.1.1 Long-term responders to calcium channel blockers |
| 1.2 Heritable |
| 1.3 Associated with drugs and toxins |
| Note: patients with heritable pulmonary arterial hypertension or pulmonary arterial hypertension associated with drugs and toxins might be long-term responders to calcium channel blockers. |
| 1.4 Associated with: |
| 1.4.1 Connective Tissue Disease |
| 1.4.2 HIV infection |
| 1.4.3 Portal Hypertension |
| 1.4.4 Congenital Heart Disease |
| 1.4.5 Schistosomiasis |
| 1.5 Pulmonary Arterial Hypertension with features of venous/capillary involvement |
| (Pulmonary veno-occlusive disease or pulmonary capillary haemangiomatosis) |
| 1.6 Persistent PH of the newborn |
| Group 2: PH associated with left heart disease |
| 2.1 Heart failure: |
| 2.1.1 With preserved ejection fraction |
| 2.1.2 With reduced or mildly reduced ejection fraction |
| 2.1.3 Cardiomyopathies with specific aetiologies |
| (Hypertrophic, Amyloid, Fabry disease, and Chagas disease) |
| 2.2 Valvular heart disease: |
| 2.2.1 Aortic valve disease |
| 2.2.2 Mitral valve disease |
| 2.2.3 Mixed valvular disease |
| 2.3 Congenital/acquired cardiovascular conditions leading to post-capillary pulmonary hypertension group |
| Group 3: PH associated with lung diseases and/or hypoxia |
| 3.1 COPD and/or emphysema |
| 3.2 Interstitial lung disease |
| 3.3 Combined pulmonary fibrosis and emphysema |
| 3.4 Other parenchymal lung diseases |
| (Parenchymal lung diseases not included in the group) |
| 3.5 Nonparenchymal restrictive diseases: |
| 3.5.1 Hypoventilation syndromes |
| 3.5.2 Pneumonectomy |
| 3.6 Hypoxia without lung disease (e.g., high altitude) |
| 3.7 Developmental lung diseases |
| Group 4: PH associated with pulmonary artery obstructions |
| 4.1 Chronic thromboembolic pulmonary hypertension |
| 4.2 Other pulmonary artery obstructions |
| (Other causes of pulmonary artery obstructions include sarcomas (high- or intermediate-grade or angiosarcoma), other malignant tumors (e.g., renal carcinoma, uterine carcinoma, germ-cell tumors of the testis), nonmalignant tumours (e.g., uterine leiomyoma), arteritis without connective tissue disease, congenital pulmonary arterial stenoses and hydatidosis) |
| Group 5: PH with unclear and/or multifactorial mechanisms |
| 5.1 Hematological disorders |
| (including inherited and acquired chronic haemolytic anemia and chronic myeloproliferative disorders) |
| 5.2 Systemic disorders |
| (Sarcoidosis, pulmonary Langerhans cell histiocytosis, and neurofibromatosis type 1) |
| 5.3 Metabolic disorders |
| (Including glycogen storage diseases and Gaucher disease.) |
| 5.4 Chronic renal failure with or without haemodialysis |
| 5.5 Pulmonary tumor thrombotic microangiopathy |
| 5.6 Fibrosing mediastinitis |
| 5.7 Complex congenital heart disease |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butrous, G. Global Landscape of Infection-Induced Pulmonary Hypertension. Infect. Dis. Rep. 2025, 17, 35. https://doi.org/10.3390/idr17020035
Butrous G. Global Landscape of Infection-Induced Pulmonary Hypertension. Infectious Disease Reports. 2025; 17(2):35. https://doi.org/10.3390/idr17020035
Chicago/Turabian StyleButrous, Ghazwan. 2025. "Global Landscape of Infection-Induced Pulmonary Hypertension" Infectious Disease Reports 17, no. 2: 35. https://doi.org/10.3390/idr17020035
APA StyleButrous, G. (2025). Global Landscape of Infection-Induced Pulmonary Hypertension. Infectious Disease Reports, 17(2), 35. https://doi.org/10.3390/idr17020035






