Sodium Pyruvate Ameliorates Influenza A Virus Infection In Vivo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Welfare
2.2. Virus Production
2.3. In Vivo Infection and NaPyr Treatments
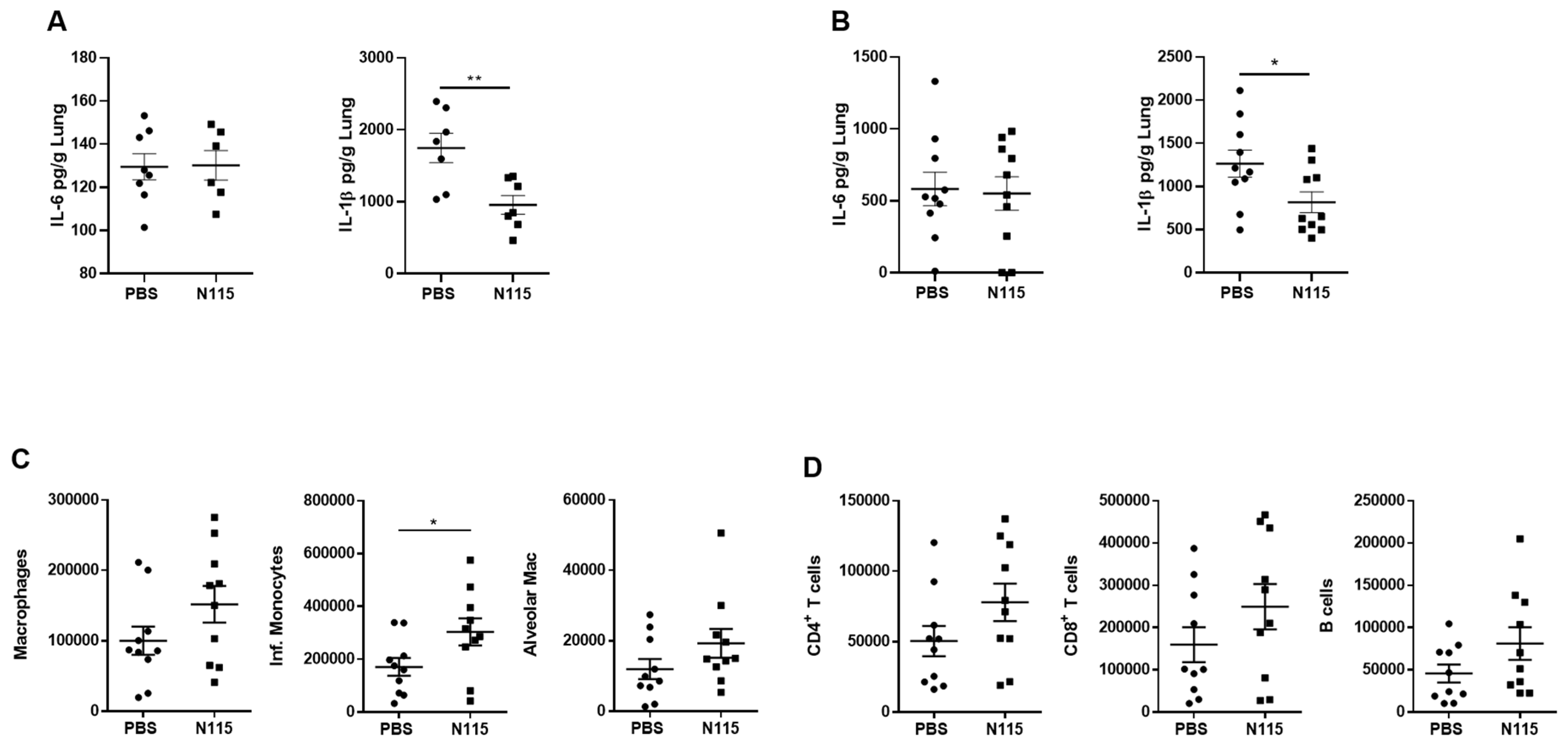
2.4. Tissue Collection and Processing
2.5. Flow Cytometry for Innate and Adaptive Immune Cells
2.6. Viral Plaque Assay
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Statistical Analysis
3. Results
3.1. NaPyr Is Not Toxic In Vivo
3.2. Nebulized NaPyr Improves Weight Loss in IAV Infected Mice
3.3. N115 Decreases Weight Loss and Increases Chow Intake during IAV Infection
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prevention CfDCa. Estimated Influenza Illnesses, Medical Visits, Hospitalizations, and Deaths in the United States—2019–2020 Influenza Season. Retrieved 11-23-2020. 2020. Available online: https://www.cdc.gov/flu/about/burden/2019-2020.html (accessed on 11 December 2020).
- Simonsen, L.; Spreeuwenberg, P.; Lustig, R.; Taylor, R.J.; Fleming, D.M.; Kroneman, M.; Van Kerkhove, M.D.; Mounts, A.W.; Paget, W.J.; G.L.C. Teams. Global mortality estimates for the 2009 Influenza Pandemic from the GLaMOR project: A modeling study. PLoS Med. 2013, 10, e1001558. [Google Scholar] [CrossRef] [Green Version]
- Uchide, N.; Toyoda, H. Antioxidant therapy as a potential approach to severe influenza-associated complications. Molecules 2011, 16, 2032–2052. [Google Scholar] [CrossRef] [Green Version]
- Kohio, H.P.; Adamson, A.L. Glycolytic control of vacuolar-type ATPase activity: A mechanism to regulate influenza viral infection. Virology 2013, 444, 301–309. [Google Scholar] [CrossRef] [Green Version]
- Smallwood, H.S.; Duan, S.; Morfouace, M.; Rezinciuc, S.; Shulkin, B.L.; Shelat, A.; Zink, E.E.; Milasta, S.; Bajracharya, R.; Oluwaseum, A.J.; et al. Targeting Metabolic Reprogramming by Influenza Infection for Therapeutic Intervention. Cell Rep. 2017, 19, 1640–1653. [Google Scholar] [CrossRef] [Green Version]
- Halestrap, A.P.; Price, N.T. The proton-linked monocarboxylate transporter (MCT) family: Structure, function and regulation. Biochem. J. 1999, 343, 281–299. [Google Scholar] [CrossRef]
- Schell, J.C.; Rutter, J. The long and winding road to the mitochondrial pyruvate carrier. Cancer Metab. 2013, 1, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heiden, M.G.V.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, L.; Chen, X.; Wang, L.; Chen, S. The sweet trap in tumors: Aerobic glycolysis and potential targets for therapy. Oncotarget 2016, 7, 38908–38926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, J.; Cao, R.; Zhang, Y.; Xia, Y.; Zheng, Y.; Li, X.; Wang, L.; Yang, J.L.R.C.Y.Z.W.; Lu, Y.X.Y.Z.X.L.Z. PKM2 dephosphorylation by Cdc25A promotes the Warburg effect and tumorigenesis. Nat. Commun. 2016, 7, 12431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dijkstra, U.; Gabreels, F.; Joosten, E.; Wevers, R.; Lamers, K.; Doesburg, W.; Renier, W. Friedreich’s ataxia: Intravenous pyruvate load to demonstrate a defect in pyruvate metabolism. Neurology 1984, 34, 1493–1497. [Google Scholar] [CrossRef]
- Sharma, P.; Mongan, P.D. Hypertonic sodium pyruvate solution is more effective than Ringer’s ethyl pyruvate in the treatment of hemorrhagic shock. Shock 2010, 33, 532–540. [Google Scholar] [CrossRef]
- Cherry, B.H.; Nguyen, A.Q.; Hollrah, R.A.; Williams, A.G.; Hoxha, B.; Olivencia-Yurvati, A.H.; Mallet, R.T. Pyruvate stabilizes electrocardiographic and hemodynamic function in pigs recovering from cardiac arrest. Exp. Biol. Med. 2015, 240, 1774–1784. [Google Scholar] [CrossRef] [Green Version]
- DeBoer, L.W.; Bekx, P.A.; Han, L.; Steinke, L. Pyruvate enhances recovery of rat hearts after ischemia and reperfusion by preventing free radical generation. Am. J. Physiol. 1993, 265, H1571–H1576. [Google Scholar] [CrossRef]
- Kang, Y.-H.; Chung, S.-J.; Kang, I.-J.; Park, J.H.Y.; Bünger, R. Intramitochondrial pyruvate attenuates hydrogen peroxide-induced apoptosis in bovine pulmonary artery endothelium. Mol. Cell Biochem. 2001, 216, 37–46. [Google Scholar] [CrossRef]
- Desagher, S.; Glowinski, J.; Prémont, J. Pyruvate protects neurons against hydrogen peroxide-induced toxicity. J. Neurosci. 1997, 17, 9060–9067. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, M.; Lee, S.M.; Moro, N.; Hovda, D.A.; Sutton, R.L. Metabolic and histologic effects of sodium pyruvate treatment in the rat after cortical contusion injury. J. Neurotrauma 2009, 26, 1095–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.F.; Cynader, M.S. Pyruvate released by astrocytes protects neurons from copper-catalyzed cysteine neurotoxicity. J. Neurosci. 2001, 21, 3322–3331. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, H.; Leinonen, H.; Puurula, M.; Hafez, H.S.; Barrera, G.A.; Stridh, M.H.; Waagepetersen, H.S.; Tiainen, M.; Soininen, P.; Zilberter, Y.; et al. Chronic Pyruvate Supplementation Increases Exploratory Activity and Brain Energy Reserves in Young and Middle-Aged Mice. Front Aging Neurosci. 2016, 8, 41. [Google Scholar] [CrossRef]
- Fink, M.P. Reactive oxygen species as mediators of organ dysfunction caused by sepsis, acute respiratory distress syndrome, or hemorrhagic shock: Potential benefits of resuscitation with Ringer’s ethyl pyruvate solution. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 167–174. [Google Scholar] [CrossRef]
- Huang, C.; Kuo, W.; Huang, C.; Lee, T.; Chen, C.; Peng, W.; Lu, K.; Yang, C.; Yu, L.C. Distinct cytoprotective roles of pyruvate and ATP by glucose metabolism on epithelial necroptosis and crypt proliferation in ischaemic gut. J. Physiol. 2017, 595, 505–521. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuijs-Moeke, G.J.; Pischke, S.E.; Berger, S.P.; Sanders, J.S.F.; Pol, R.A.; Struys, M.M.R.F.; Ploeg, R.J.; Leuvenink, H.G.D. Ischemia and Reperfusion Injury in Kidney Transplantation: Relevant Mechanisms in Injury and Repair. J. Clin. Med. 2020, 9, 253. [Google Scholar] [CrossRef] [Green Version]
- Peltz, M.; Hamilton, T.T.; He, T.-T.; Adams, G.A.; Koshy, S.; Burgess, S.C.; Chao, R.Y.; Jessen, M.E.; Meyer, D.M. Lung preservation solution substrate composition affects rat lung oxidative metabolism during hypothermic storage. Res. Physiol. Neurobiol. 2005, 148, 275–283. [Google Scholar] [CrossRef]
- Cobert, M.L.; Peltz, M.; West, L.M.; Merritt, M.E.; Jessen, M.E. Glucose is an Ineffective Substrate for Preservation of Machine Perfused Donor Hearts. J. Surg. Res. 2012, 173, 198–205. [Google Scholar] [CrossRef]
- Peltz, M.; Milchgrub, S.; Jessen, M.E.; Meyer, D.M. Effect of pyruvate and HEPES on rat lung allograft acidosis and cell death after long-term hypothermic storage. Transpl. Proc. 2010, 42, 2771–2776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peltz, M.; He, T.-T.; Adams, G.A.; Chao, R.Y.; Jessen, M.E.; Meyer, D.M. Pyruvate-modified perfadex improves lung function after long-term hypothermic storage. J. Heart Lung Transpl. 2005, 24, 896–903. [Google Scholar] [CrossRef]
- Xia, S.; Chen, G.; Wang, B.; Yin, Y.; Sun, Z.; Zhao, J.; Li, P.; Zhao, L.; Zhou, H. Addition of Sodium Pyruvate to Stored Red Blood Cells Attenuates Liver Injury in a Murine Transfusion. Model. Med. Inflamm. 2016, 2016, 3549207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawson, R.B.; Hershey, R.T.; Myers, C.S. Blood preservation. XXIX. Pyruvate maintains normal red cell 2,3-DPG for six weeks of storage in CPD-adenine. Transfusion 1980, 20, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Rastogi, S.; Prakash, J.; Joshi, S.; Gupta, Y.K.; Awor, L.; Verma, S.D. Anti-inflammatory activity of sodium pyruvate--a physiological antioxidant. Indian J. Physiol. Pharmacol. 2000, 44, 101–104. [Google Scholar]
- Jung, S.M.; Lee, J.; Baek, S.Y.; Lee, J.; Jang, S.G.; Hong, S.-M.; Park, J.-S.; Cho, M.-L.; Park, S.-H.; Kwok, S.-K. Ethyl pyruvate ameliorates inflammatory arthritis in mice. Int. Immunopharmacol. 2017, 52, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Abusalamah, H.; Reel, J.M.; Lupfer, C.R. Pyruvate affects inflammatory responses of macrophages during influenza A virus infection. Virus Res. 2020, 286, 198088. [Google Scholar] [CrossRef]
- Sheridan, J.; Kern, E.; Martin, A.; Booth, A. Evaluation of antioxidant healing formulations in topical therapy of experimental cutaneous and genital herpes simplex virus infections. Antivir. Res. 1997, 36, 157–166. [Google Scholar] [CrossRef]
- Lupfer, C.; Stein, D.A.; Mourich, D.V.; Tepper, S.E.; Iversen, P.L.; Pastey, M. Inhibition of influenza A H3N8 virus infections in mice by morpholino oligomers. Arch. Virol. 2008, 153, 929–937. [Google Scholar] [CrossRef]
- Votto, J.J.; Bowen, J.B.; Barton, R.W.; Thrall, R.S. Inhaled sodium pyruvate improved FEV1 and decreased expired breath levels of nitric oxide in patients with chronic obstructive pulmonary disease. J. Aerosol. Med. Pulm. Drug Deliv. 2008, 21, 329–334. [Google Scholar] [CrossRef]
- Leiva-Juarez, M.M.; Kirkpatrick, C.T.; Gilbert, B.E.; Scott, B.; Tuvim, M.J.; Dickey, B.F.; Evans, S.E.; Markesich, D. Combined aerosolized Toll-like receptor ligands are an effective therapeutic agent against influenza pneumonia when co-administered with oseltamivir. Eur. J. Pharmacol. 2018, 818, 191–197. [Google Scholar] [CrossRef]
- Cao, Z.; Zhou, Y.; Zhu, S.; Feng, J.; Chen, X.; Liu, S.; Peng, N.; Yang, X.; Xu, G.; Zhu, Y. Pyruvate Carboxylase Activates the RIG-I-like Receptor-Mediated Antiviral Immune Response by Targeting the MAVS signalosome. Sci. Rep. 2016, 6, 22002. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell-Tormey, J.; Nathan, C.F.; Lanks, K.; DeBoer, C.J.; De La Harpe, J. Secretion of pyruvate. An antioxidant defense of mammalian cells. J. Exp. Med. 1987, 165, 500–514. [Google Scholar] [CrossRef]
- Duwe, S. Influenza viruses—Antiviral therapy and resistance. GMS Infect Dis. 2017, 5, Doc04. [Google Scholar]
- Prevention CfDCa. Influenza (flu)—Young Children. Retrieved 11-23-2020. 2020. Available online: https://www.cdc.gov/flu/highrisk/children.htm (accessed on 23 November 2020).
- Leeb, R.T.; Price, S.; Sliwa, S.; Kimball, A.; Szucs, L.; Caruso, E.; Godfred-Cato, S.; Lozier, M. COVID-19 Trends Among School-Aged Children—United States, March 1–September 19, 2020. MMWR Morb. Mortal Wkly Rep. 2020, 69, 1410–1415. [Google Scholar] [CrossRef]



| Fluorophore | Monocyte Stain | Cat# | Lymphocyte Stain | Cat# |
|---|---|---|---|---|
| FITC | CD11c | 35-0114-U100 | CD4 | 35-0042-U100 |
| PE | Gr1 | 50-5931-U100 | CD8 | 100707 |
| PerCP 5.5 | CD3ε | 65-0031-U100 | CD3ε | 65-0031-U100 |
| APC | CD11b | 20-0112-U100 | CD19 | 115511 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reel, J.M.; Lupfer, C.R. Sodium Pyruvate Ameliorates Influenza A Virus Infection In Vivo. Microbiol. Res. 2021, 12, 258-267. https://doi.org/10.3390/microbiolres12020018
Reel JM, Lupfer CR. Sodium Pyruvate Ameliorates Influenza A Virus Infection In Vivo. Microbiology Research. 2021; 12(2):258-267. https://doi.org/10.3390/microbiolres12020018
Chicago/Turabian StyleReel, Jessica M., and Christopher R. Lupfer. 2021. "Sodium Pyruvate Ameliorates Influenza A Virus Infection In Vivo" Microbiology Research 12, no. 2: 258-267. https://doi.org/10.3390/microbiolres12020018
APA StyleReel, J. M., & Lupfer, C. R. (2021). Sodium Pyruvate Ameliorates Influenza A Virus Infection In Vivo. Microbiology Research, 12(2), 258-267. https://doi.org/10.3390/microbiolres12020018







