The Regulation of Non-Specific Membrane Permeability Transition in Yeast Mitochondria under Oxidative Stress
Abstract
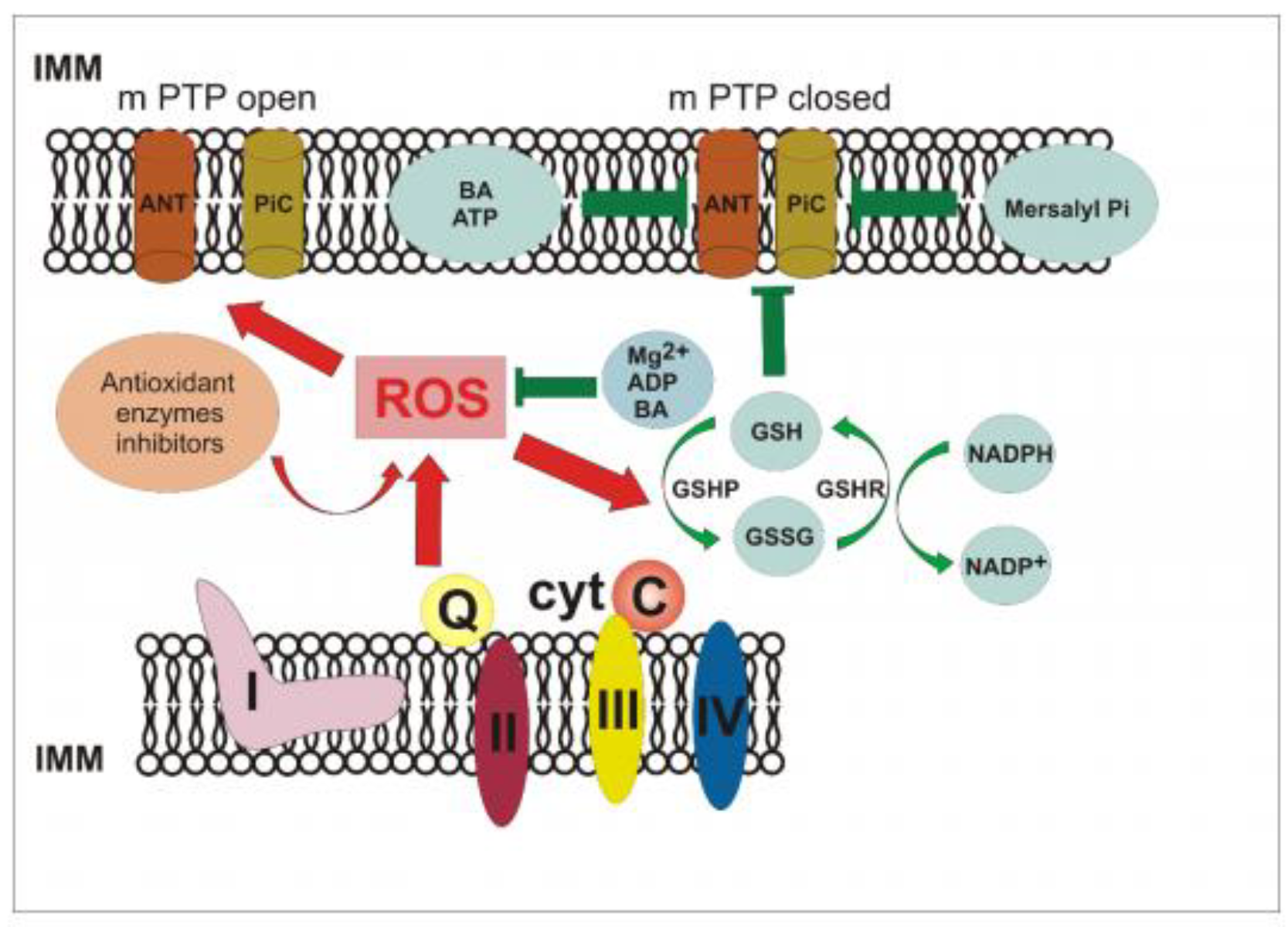
:1. Introduction
2. Materials and Methods
2.1. Yeast Strain and Culture Conditions
2.2. Respiration Assessment
2.3. Potential-Dependent Staining
2.4. Isolation of Mitochondria
2.5. Mitochondrial Membrane Potential Assay
2.6. Transmission Electron Microscopy (TEM)
2.7. ROS Generation Assay
2.8. Statistical Analysis
3. Results
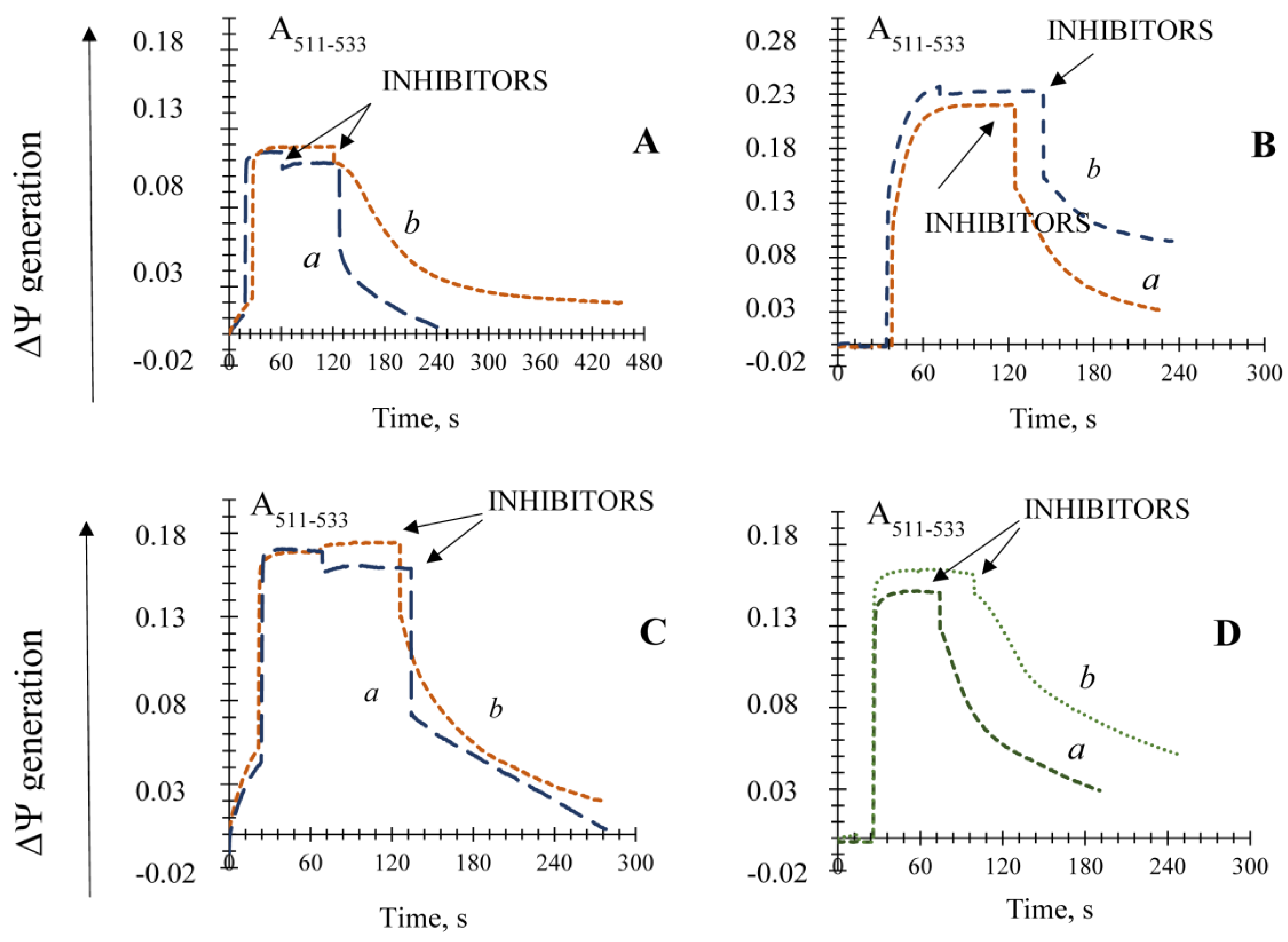
3.1. Influence of the Key Antioxidant Enzyme Inhibitors on Mitochondria Coupling Features
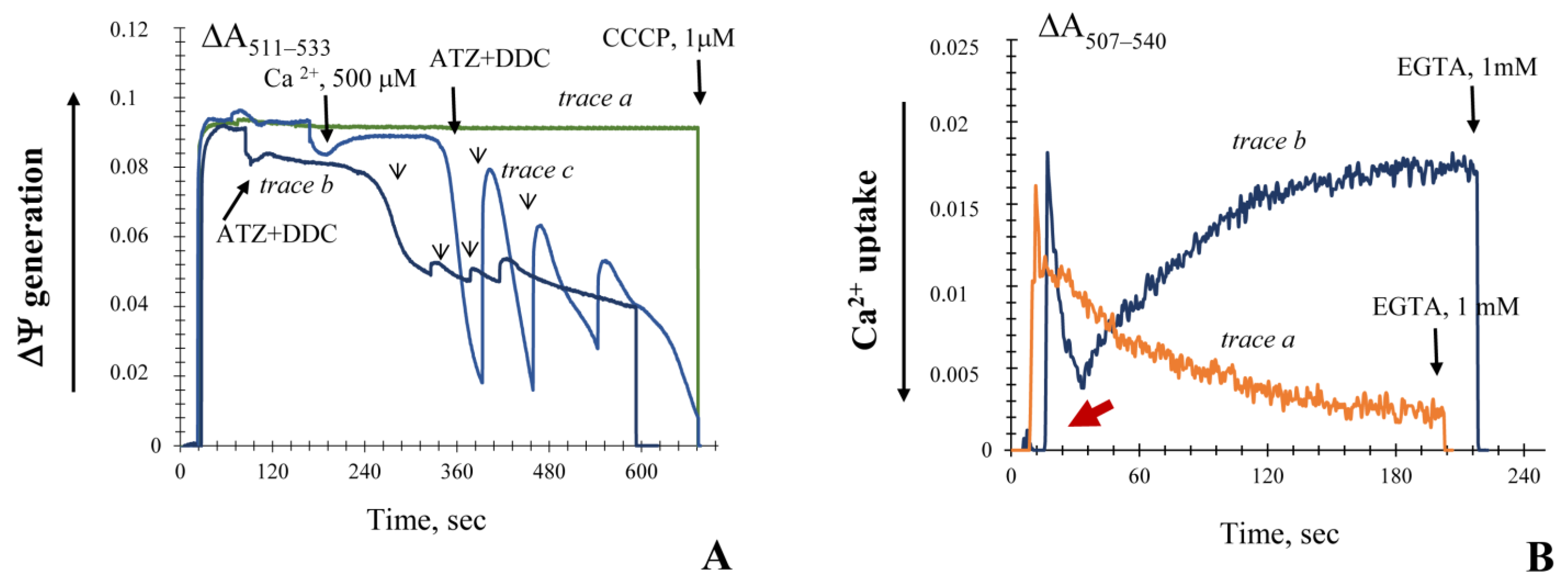
3.2. Generation upon Blocking Antioxidant Enzyme Activities and Influence of Ca2+ Ions on Non-Specific Changes in Yeast Mitochondria Permeability
3.3. The Non-Specific Changes of Permeability Transition in the Yeast Mitochondria upon Using Different Respiratory Substrates
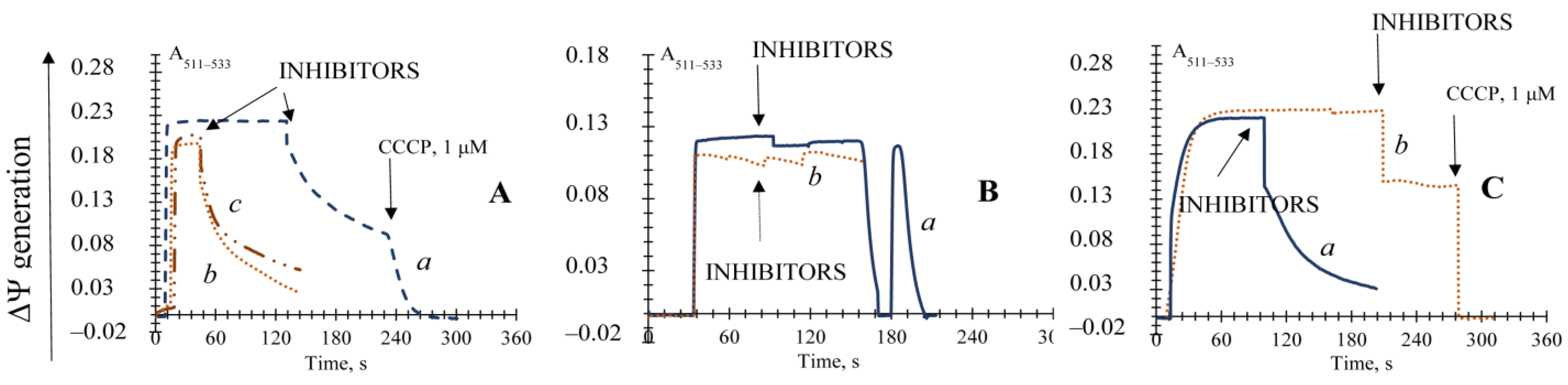
3.4. The Effect of Pi and Mersalyl on the Non-Specific Mitochondrial Permeability Transition in the E. magnusii Yeast
3.5. The Effect of Different Known Modulators of the mPTP on the Non-Specific Mitochondrial Permeability Transition in the Yeast
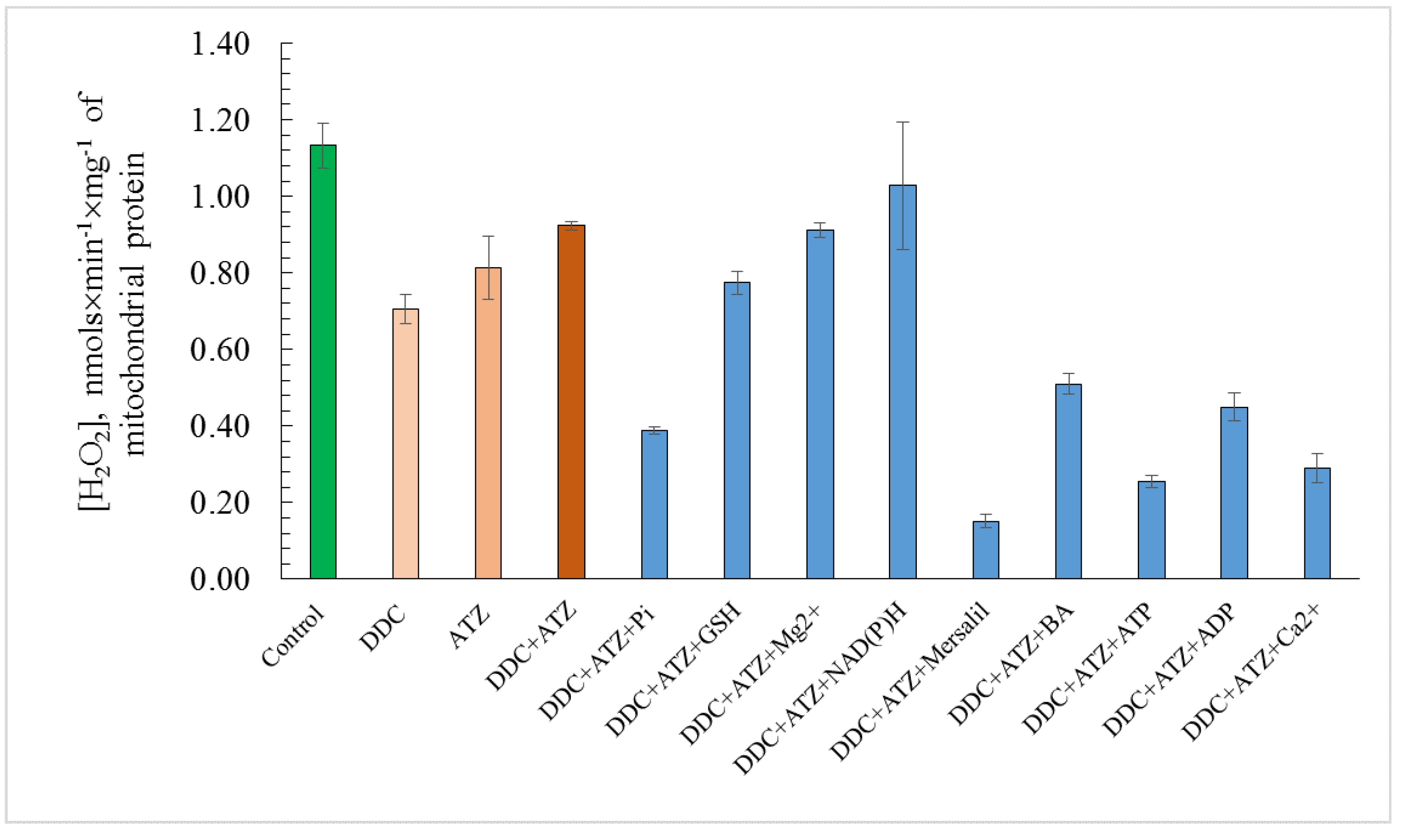
3.6. ROS Generation in the Presence of the Effectors of the Non-Specific Mitochondrial Permeability Transition in the Yeast
3.7. The Influence of the Inhibitors of the Non-Specific Permeability Transition on Ultra-Structural Features of the Yeast Mitochondria
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Di Lisa, F.; Bernardi, P. Modulation of Mitochondrial Permeability Transition in Ischemia-Reperfusion Injury of the Heart. Advantages and Limitations. Curr. Med. Chem. 2015, 22, 2480–2487. [Google Scholar] [CrossRef]
- Briston, T.; Selwood, D.L.; Szabadkai, G.; Duchen, M.R. Mitochondrial Permeability Transition: A Molecular Lesion with Multiple Drug Targets. Trends Pharmacol. Sci. 2019, 40, 50–70. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.R.; Haworth, R.A. The Ca2+-induced membrane transition in mitochondria. I. The protective mechanisms. Arch. Biochem. Biophys. 1979, 195, 453–459. [Google Scholar] [CrossRef]
- Haworth, R.A.; Hunter, D.R. The Ca2+-induced membrane transition in mitochondria. II. Nature of the Ca2+ trigger site. Arch. Biochem. Biophys. 1979, 195, 460–467. [Google Scholar] [CrossRef]
- Hunter, D.R.; Haworth, R.A. The Ca2+-induced membrane transition in mitochondria. III. Transitional Ca2+ release. Arch. Biochem. Biophys. 1979, 195, 468–477. [Google Scholar] [CrossRef]
- Halestrap, A.P.; Richardson, A.P. The mitochondrial permeability transition: A current perspective on its identity and role in ischaemia/reperfusion. J. Mol. Cell Cardiol. 2015, 78, 129–141. [Google Scholar] [CrossRef]
- Venditti, P.; Di Meo, S. The Role of Reactive Oxygen Species in the Life Cycle of the Mitochondrion. Int. J. Mol. Sci. 2020, 21, 2173. [Google Scholar] [CrossRef] [Green Version]
- Bernardi, P. Mitochondrial transport of cations: Channels, exchangers, and permeability transition. Physiol. Rev. 1999, 79, 1127–1155. [Google Scholar] [CrossRef]
- Carraro, M.; Bernardi, P. Calcium and reactive oxygen species in regulation of the mitochondrial permeability transition and of programmed cell death in yeast. Cell Calcium 2016, 60, 102–107. [Google Scholar] [CrossRef] [Green Version]
- Fournier, N.; Ducet, G.; Crevat, A. Action of cyclosporine on mitochondrial calcium fluxes. J. Bioenerg. Biomembr. 1987, 19, 297–303. [Google Scholar] [CrossRef]
- Halestrap, A.P.; Davidson, A.M. Inhibition of Ca2(+)-induced large-amplitude swelling of liver and heart mitochondria by cyclosporin is probably caused by the inhibitor binding to mitochondrial-matrix peptidyl-prolyl cis-trans isomerase and preventing it interacting with the adenine nucleotide translocase. Biochem. J. 1990, 268, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Halestrap, A.P.; Clarke, S.J.; Javadov, S.A. Mitochondrial permeability transition pore opening during myocardial reperfusion–a target for cardioprotection. Cardiovasc. Res. 2004, 61, 372–385. [Google Scholar] [CrossRef] [Green Version]
- Halestrap, A.P. Calcium, mitochondria and reperfusion injury: A pore way to die. Biochem. Soc. Trans. 2006, 34, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Halestrap, A.P.; Brenner, C. The adenine nucleotide translocase: A central component of the mitochondrial permeability transition pore and key player in cell death. Curr. Med. Chem. 2003, 10, 1507–1525. [Google Scholar] [CrossRef] [Green Version]
- Halestrap, A.P. A pore way to die: The role of mitochondria in reperfusion injury and cardioprotection. Biochem. Soc. Trans. 2010, 38, 841–860. [Google Scholar] [CrossRef] [Green Version]
- Pavlov, E.; Zakharian, E.; Bladen, C.; Diao, C.T.; Grimbly, C.; Reusch, R.N.; French, R.J. A large, voltage-dependent channel, isolated from mitochondria by water-free chloroform extraction. Biophys. J. 2005, 88, 2614–2625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sultan, A.; Sokolove, P.M. Palmitic acid opens a novel cyclosporin A-insensitive pore in the inner mitochondrial membrane. Arch. Biochem. Biophys. 2001, 386, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Nesci, S. The mitochondrial permeability transition pore in cell death: A promising drug binding bioarchitecture. Med. Res. Rev. 2020, 40, 811–817. [Google Scholar] [CrossRef]
- Baines, C.P.; Gutiérrez-Aguilar, M. The still uncertain identity of the channel-forming unit(s) of the mitochondrial permeability transition pore. Cell Calcium 2018, 73, 121–130. [Google Scholar] [CrossRef]
- Karch, J.M.; Bround, M.J.; Khalil, H.; Sargent, M.A.; Latchman, N.; Terada, N.; Peixoto, P.M.; Molkentin, J.D. Inhibition of mitochondrial permeability transition by deletion of the ANT family and CypD. Sci. Adv. 2019, 5, eaaw4597. [Google Scholar] [CrossRef] [Green Version]
- Leung, A.W.; Varanyuwatana, P.; Halestrap, A.P. The mitochondrial phosphate carrier interacts with cyclophilin D and may play a key role in the permeability transition. J. Biol. Chem. 2008, 283, 26312–26323. [Google Scholar] [CrossRef] [Green Version]
- Kwong, J.Q.; Davis, J.; Baines, C.P.; Sargent, M.A.; Karch, J.; Wang, X.; Huang, T.; Molkentin, J.D. Genetic deletion of the mitochondrial phosphate carrier desensitizes the mitochondrial permeability transition pore and causes cardiomyopathy. Cell Death Differ. 2014, 21, 1209–1217. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Lemasters, J.J. Regulated and unregulated mitochondrial permeability transition pores: A new paradigm of pore structure and function? FEBS Lett. 2002, 512, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Carraro, M.; Checchetto, V.; Szabó, I.; Bernardi, P. F-ATP synthase and the permeability transition pore: Fewer doubts, more certainties. FEBS Lett. 2019, 593, 1542–1553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giorgio, V.; von Stockum, S.; Antoniel, M.; Fabbro, A.; Fogolari, F.; Forte, M.; Glick, G.D.; Petronilli, V.; Zoratti, M.; Szabó, I.; et al. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc. Natl. Acad. Sci. USA 2013, 110, 5887–5892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerle, C. Mitochondrial F-ATP synthase as the permeability transition pore. Pharmacol Res. 2020, 160, 105081. [Google Scholar] [CrossRef] [PubMed]
- Winquist, R.J.; Gribkoff, V.K. Targeting putative components of the mitochondrial permeability transition pore for novel therapeutics. Biochem. Pharmacol. 2020, 177, 113995. [Google Scholar] [CrossRef] [PubMed]
- Bonora, M.; Patergnani, S.; Ramaccini, D.; Morciano, G.; Pedriali, G.; Kahsay, A.E.; Bouhamida, E.; Giorgi, C.; Wieckowski, M.R.; Pinton, P. Physiopathology of the Permeability Transition Pore: Molecular Mechanisms in Human Pathology. Biomolecules 2020, 10, 998. [Google Scholar] [CrossRef]
- Jung, D.W.; Bradshaw, P.C.; Pfeiffer, D.R. Properties of a cyclosporin-insensitive permeability transition pore in yeast mitochondria. J. Biol. Chem. 1997, 272, 21104–22112. [Google Scholar] [CrossRef] [Green Version]
- Prieto, S.; Bouillaud, F.; Ricquier, D.; Rial, E. Activation by ATP of a proton-conducting pathway in yeast mitochondria. Eur. J. Biochem. 1992, 208, 487–491. [Google Scholar] [CrossRef]
- Roucou, X.; Manon, S.; Guérin, M. Conditions allowing different states of ATP- and GDP-induced permeability in mitochondria from different strains of Saccharomyces cerevisiae. Biochim. Biophys. Acta 1997, 1324, 120–132. [Google Scholar] [CrossRef] [Green Version]
- McStay, G.P.; Clarke, S.J.; Halestrap, A.P. Role of critical thiol groups on the matrix surface of the adenine nucleotide translocase in the mechanism of the mitochondrial permeability transition pore. Biochem. J. 2002, 367, 541–548. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Vázquez, V.; Saavedra-Molina, A.; Uribe, S. In Saccharomyces cerevisiae, cations control the fate of the energy derived from oxidative metabolism through the opening and closing of the yeast mitochondrial unselective channel. J. Bioenerg. Biomembr. 2003, 35, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Yamada, A.; Yamamoto, T.; Yoshimura, Y.; Gouda, S.; Kawashima, S.; Yamazaki, N.; Yamashita, K.; Kataoka, M.; Nagata, T.; Terada, H.; et al. Ca2+-induced permeability transition can be observed even in yeast mitochondria under optimized experimental conditions. Biochim. Biophys. Acta 2009, 1787, 1486–1491. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Aguilar, M.; Pérez-Martínez, X.; Chávez, E.; Uribe-Carvajal, S. Saccharomyces cerevisiae, the phosphate carrier is a component of the mitochondrial unselective channel. Arch. Biochem. Biophys. 2010, 494, 184–191. [Google Scholar] [CrossRef]
- Deryabina, Y.I.; Isakova, E.P.; Shurubor, E.I.; Zvyagilskaya, R.A. Calcium-dependent nonspecific permeability of the inner mitochondrial membrane is not induced in mitochondria of the yeast Endomyces magnusii. Biochemistry 2004, 69, 1025–1033. [Google Scholar] [CrossRef]
- Bazhenova, E.N.; Saris, N.E.; Zvyagilskaya, R.A. Stimulation of the yeast mitochondrial calcium uniporter by hypotonicity and by ruthenium red Biochim. Biophys. Acta 1998, 1371, 96–100. [Google Scholar] [CrossRef] [Green Version]
- Deryabina, Y.I.; Bazhenova, E.N.; Saris, N.E.; Zvyagilskaya, R.A. Ca(2+) efflux in mitochondria from the yeast Endomyces magnusii. J. Biol. Chem. 2001, 276, 47801–47806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deryabina, Y.; Isakova, E.; Antipov, A.; Saris, N.E. The inhibitors of antioxidant cell enzymes induce permeability transition in yeast mitochondria. J. Bioenerg. Biomembr. 2013, 45, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Deryabina, Y.; Isakova, E.; Sekova, V.; Antipov, A.; Saris, N.E. Inhibition of free radical scavenging enzymes affects mitochondrial membrane permeability transition during growth and aging of yeast cells. J. Bioenerg. Biomembr. 2014, 46, 479–492. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Åkerman, K.E.O.; Wikström, M.K.F. Safranine as a probe of the mitochondrial membrane potential. FEBS Lett. 1976, 68, 191–197. [Google Scholar] [CrossRef] [Green Version]
- Bazhenova, E.N.; Deryabina, Y.I.; Eriksson, O.; Zvyagilskaya, R.A.; Saris, N.E. Characterization of a high capacity calcium transport system in mitochondria of the yeast Endomyces magnusii. J. Biol. Chem. 1998, 273, 4372–4377. [Google Scholar] [CrossRef] [Green Version]
- Wang, E.; Erdahl, W.L.; Hamidinia, S.A.; Chapman, C.J.; Taylor, R.W.; Pfeiffer, D.R. Transport properties of the calcium ionophore ETH-129. Biophys. J. 2001, 81, 3275–3284. [Google Scholar] [CrossRef] [Green Version]
- Tyler, D.D. Evidence of a phosphate-transporter system in the inner membrane of isolated mitochondria. Biochem. J. 1969, 111, 665–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabrera-Orefice, A.; Ibarra-García-Padilla, R.; Maldonado-Guzmán, R.; Guerrero-Castillo, S.; Luévano-Martínez, L.A.; Pérez-Vázquez, V.; Gutiérrez-Aguilar, M.; Uribe-Carvajal, S. The Saccharomyces cerevisiae mitochondrial unselective channel behaves as a physiological uncoupling system regulated by Ca2+, Mg2+, phosphate and ATP. J. Bioenerg. Biomembr. 2015, 47, 77–91. [Google Scholar] [CrossRef]
- Beatrice, M.C.; Stiers, D.L.; Pfeiffer, D.R. Increased permeability of mitochondria during Ca2+ release induced by t-butyl hydroperoxide or oxalacetate. The effect of ruthenium red. J. Biol. Chem. 1982, 257, 7161–7171. [Google Scholar] [PubMed]
- Petronilli, V.; Cola, C.; Massari, S.; Colonna, R.; Bernardi, P. Physiological effectors modify voltage sensing by the cyclosporin A-sensitive permeability transition pore of mitochondria. J. Biol. Chem. 1993, 268, 21939–21945. [Google Scholar] [CrossRef]
- Han, D.; Williams, E.; Cadenas, E. Mitochondrial respiratory chain-dependent generation of superoxide anion and its release into the intermembrane space. Biochem. J. 2001, 353, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Handy, D.E.; Loscalzo, J. Redox regulation of mitochondrial function. Antioxid. Redox Signal. 2012, 16, 1323–1367. [Google Scholar] [CrossRef]
- Wang, C.H.; Wu, S.B.; Wu, Y.T.; Wei, Y.H. Oxidative stress response elicited by mitochondrial dysfunction: Implication in the pathophysiology of aging. Exp. Biol. Med. 2013, 238, 450–460. [Google Scholar] [CrossRef]
- Chauvin, C.; De Oliveira, F.; Ronot, X.; Mousseau, M.; Leverve, X.; Fontaine, E. Rotenone inhibits the mitochondrial permeability transition-induced cell death in U937 and KB cells. J. Biol. Chem. 2001, 276, 41394–41398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briston, T.; Roberts, M.; Lewis, S.; Powney, B.; Staddon, J.M.; Szabadkai, G.; Duchen, M.R. Mitochondrial permeability transition pore: Sensitivity to opening and mechanistic dependence on substrate availability. Sci. Rep. 2017, 7, 10492. [Google Scholar] [CrossRef]
- Overkamp, K.M.; Bakker, B.M.; Kötter, P.; van Tuijl, A.; de Vries, S.; van Dijken, J.P.; Pronk, J.T. In vivo analysis of the mechanisms for oxidation of cytosolic NADH by Saccharomyces cerevisiae mitochondria. J. Bacteriol. 2000, 182, 2823–2830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, A.W.; Halestrap, A.P. Recent progress in elucidating the molecular mechanism of the mitochondrial permeability transition pore. Biochim. Biophys. Acta 2008, 1777, 946–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (RoS) and RoS-induced RoS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Wang, W.; Xiong, Z.; Kong, J.; Qiu, Y.; Shen, F.; Huang, Z. Activation of SiRT3 attenuates triptolide-induced toxicity through closing mitochondrial permeability transition pore in cardiomyocytes. Toxicol. In Vitro 2016, 34, 128–137. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Wu, Q.; Xu, B.; Wang, X.; Wu, J.; Huang, L.; Cheng, J. Suppression of Stim1 reduced intracellular calcium concentration and attenuated hypoxia/reoxygenation induced apoptosis in h9C2 cells. Biosci. Rep. 2017, 37, BSR20171249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrero, E.; Ros, J.; Bel, G.; Cabiscol, E. Redox control and oxidative stress in yeast cells. Biochim. Biophys. Acta 2008, 1780, 1217–1235. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, P. The mitochondrial permeability transition pore: A mystery solved? Front. Physiol. 2013, 4, 95. [Google Scholar] [CrossRef] [Green Version]
- Bernardi, P.; Di Lisa, F. The mitochondrial permeability transition pore: Molecular nature and role as a target in cardioprotection. J. Mol. Cell Cardiol. 2015, 78, 100–106. [Google Scholar] [CrossRef]
- Halestrap, A.P.; Woodfield, K.Y.; Connern, C.P. Oxidative stress, thiol reagents, andmembrane potential modulate the mitochondrial permeability transition byaffecting nucleotide binding to the adenine nucleotide translocase. J. Biol. Chem. 1997, 272, 3346–3354. [Google Scholar] [CrossRef] [Green Version]
- Houstĕk, J.; Pedersen, P.L. Adenine nucleotide and phosphate transport systems of mitochondria. Relative location of sulfhydryl groups based on the use of the novel fluorescent probe eosin-5-maleimide. J. Biol. Chem 1985, 260, 6288–6295. [Google Scholar] [CrossRef]
- Kowaltowski, A.J.; Vercesi, A.E.; Castilho, R.F. Mitochondrial membrane protein thiol reactivity with N-ethylmaleimide or mersalyl is modified by Ca2þ: Correlation with mitochondrial permeability transition. Biochim. Biophys. Acta 1997, 1318, 395e402. [Google Scholar] [CrossRef] [Green Version]
- De Palma, A.; Scalera, V.; Bisaccia, F.; Prezioso, G. Citrate uniport by the mitochondrial tricarboxylate carrier: A basis for a new hypothesis for the transport mechanism. J. Bioenerg. Biomembr. 2003, 35, 133–140. [Google Scholar] [CrossRef] [PubMed]
- García, N.; Martínez-Abundis, E.; Pavon, N.; Chavez, E. On the opening of an insensitive cyclosporin A non-specific pore by phenylarsine plus mersalyl. Cell. Biochem. Biophys. 2007, 49, 84–90. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Ford, H.C.; Carroll, J.; Ding, S.; Fearnley, I.M.; Walker, J.E. Persistence of the mitochondrial permeability transition in the absence of subunit c of human ATP synthase. Proc. Natl. Acad. Sci. USA 2017, 114, 3409–3414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, J.E.; Carroll, J.; He, J. Reply to Bernardi: The mitochondrial permeability transition pore and the ATP synthase. Proc. Natl. Acad. Sci. USA 2020, 117, 2745–2746. [Google Scholar] [CrossRef] [PubMed]




| Mitochondrial Respiration Stage | Respiration Rate Control | Respiration Rate (+4 mM ATZ) | Respiration Rate (+4 mM DDC) |
|---|---|---|---|
| State 4L | 19.6 ± 0.63 | 19.56 ± 1.39 | 20.78 ± 1.04 |
| State 3 | 42.04 ± 0.08 | 42.81 ± 1.76 | 44.25 ± 2.02 |
| State 4Ch | 16.95 ± 0.77 | 16.93 ± 0.69 | 15.64 ± 1.02 |
| RCL | 2.17 ± 0.12 | 2.23 ± 0.12 | 2.13 ± 0.08 |
| RCCh | 2.48 ± 0.08 | 2.53 ± 0.17 | 2.83 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isakova, E.P.; Klein, O.I.; Deryabina, Y.I. The Regulation of Non-Specific Membrane Permeability Transition in Yeast Mitochondria under Oxidative Stress. Microbiol. Res. 2021, 12, 419-439. https://doi.org/10.3390/microbiolres12020029
Isakova EP, Klein OI, Deryabina YI. The Regulation of Non-Specific Membrane Permeability Transition in Yeast Mitochondria under Oxidative Stress. Microbiology Research. 2021; 12(2):419-439. https://doi.org/10.3390/microbiolres12020029
Chicago/Turabian StyleIsakova, Elena P., Olga I. Klein, and Yulia I. Deryabina. 2021. "The Regulation of Non-Specific Membrane Permeability Transition in Yeast Mitochondria under Oxidative Stress" Microbiology Research 12, no. 2: 419-439. https://doi.org/10.3390/microbiolres12020029
APA StyleIsakova, E. P., Klein, O. I., & Deryabina, Y. I. (2021). The Regulation of Non-Specific Membrane Permeability Transition in Yeast Mitochondria under Oxidative Stress. Microbiology Research, 12(2), 419-439. https://doi.org/10.3390/microbiolres12020029





