Culturable Endophytic Fungi from Glycyrrhiza inflata Distributed in Xinjiang, China with Antifungal Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Separation and Purification of G. inflata Endophytic Fungi
2.3. Taxonomic Identification of Endophytic Fungi
2.4. Preparation of the Crude Extraction of Endophytic Fungi
2.5. Antifungal Activity Determination
2.6. Data Analysis
3. Results and Discussion
3.1. Identification of the Endophytic Fungi in G. inflata Roots
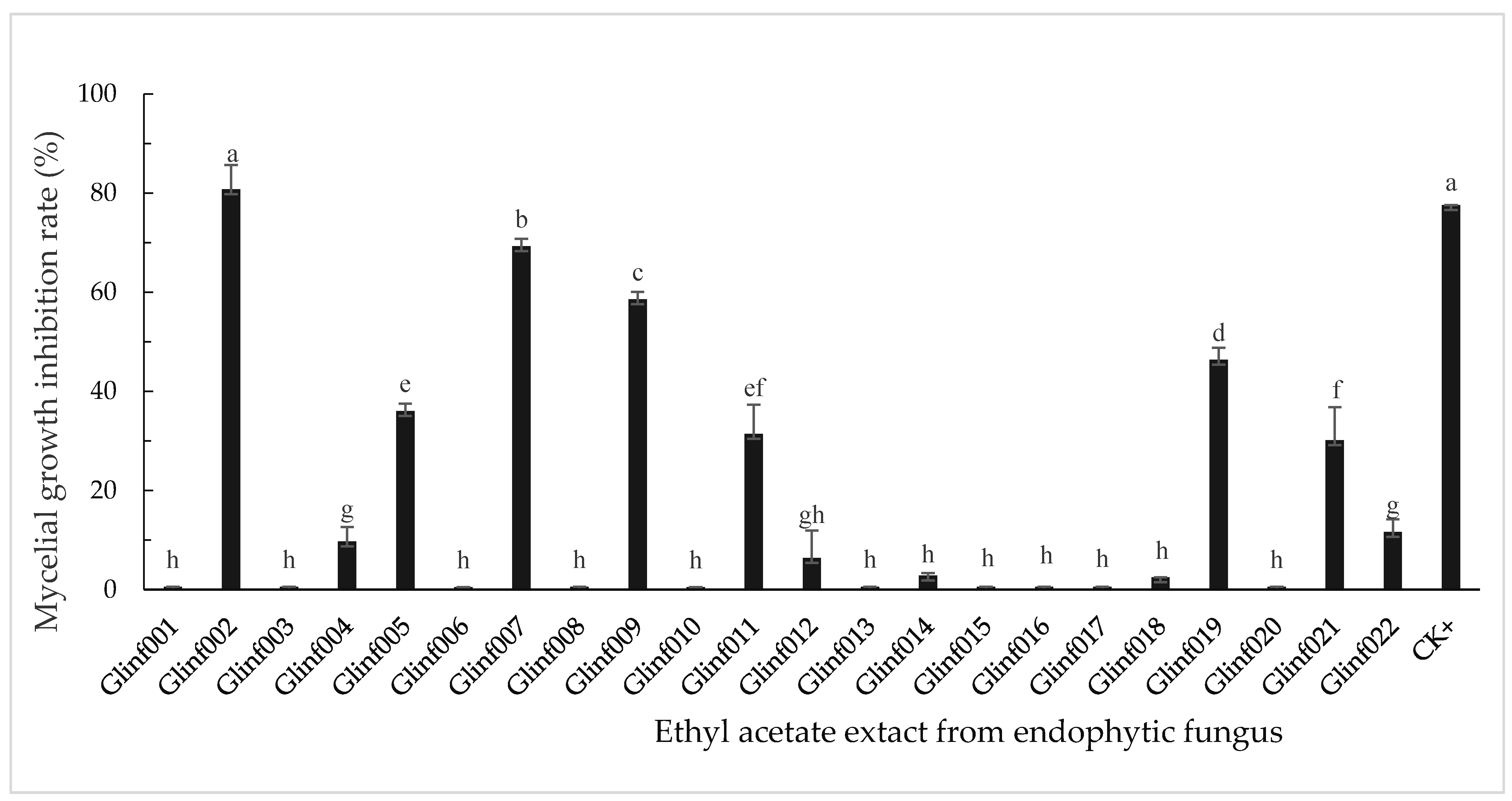
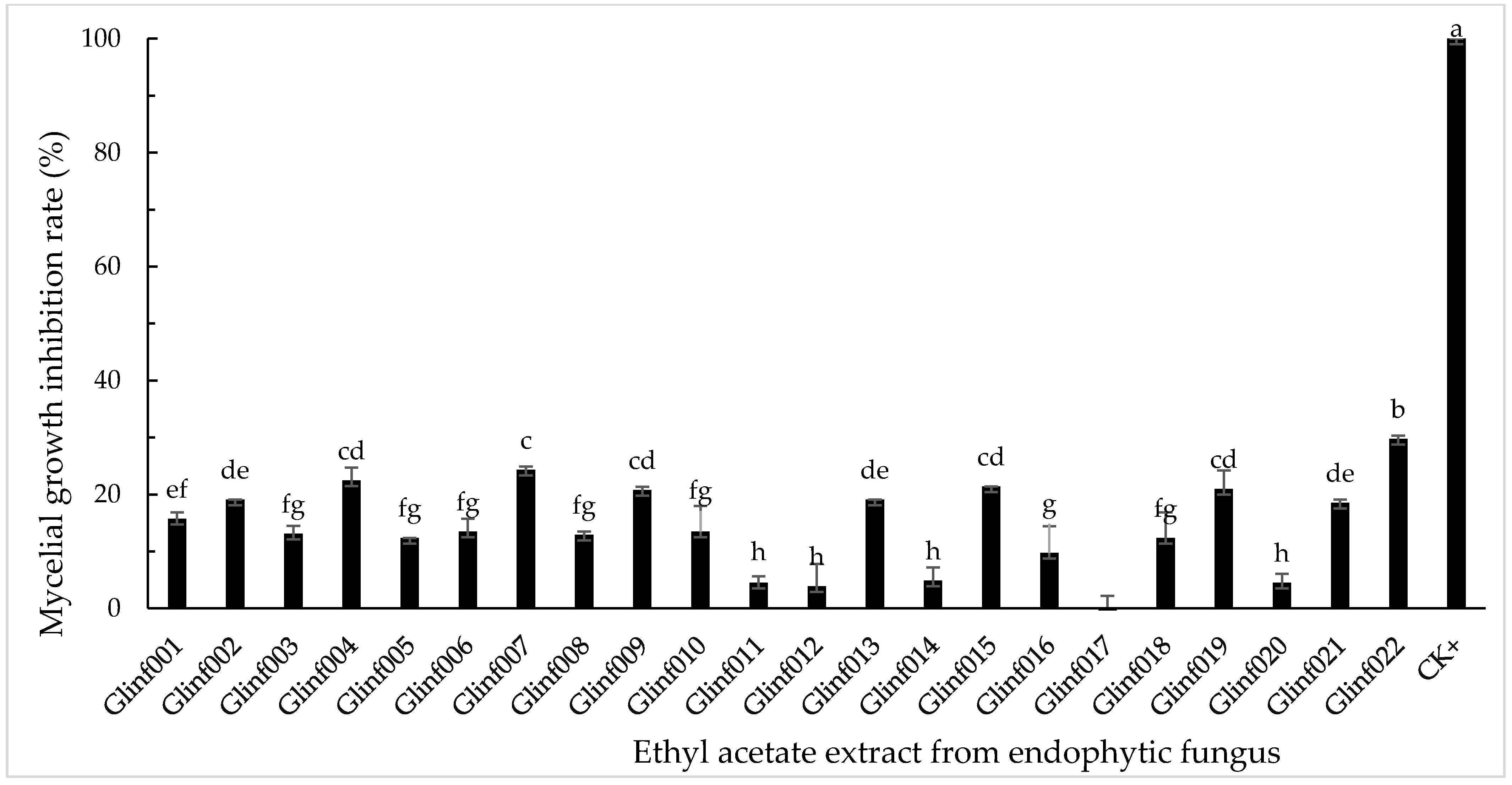
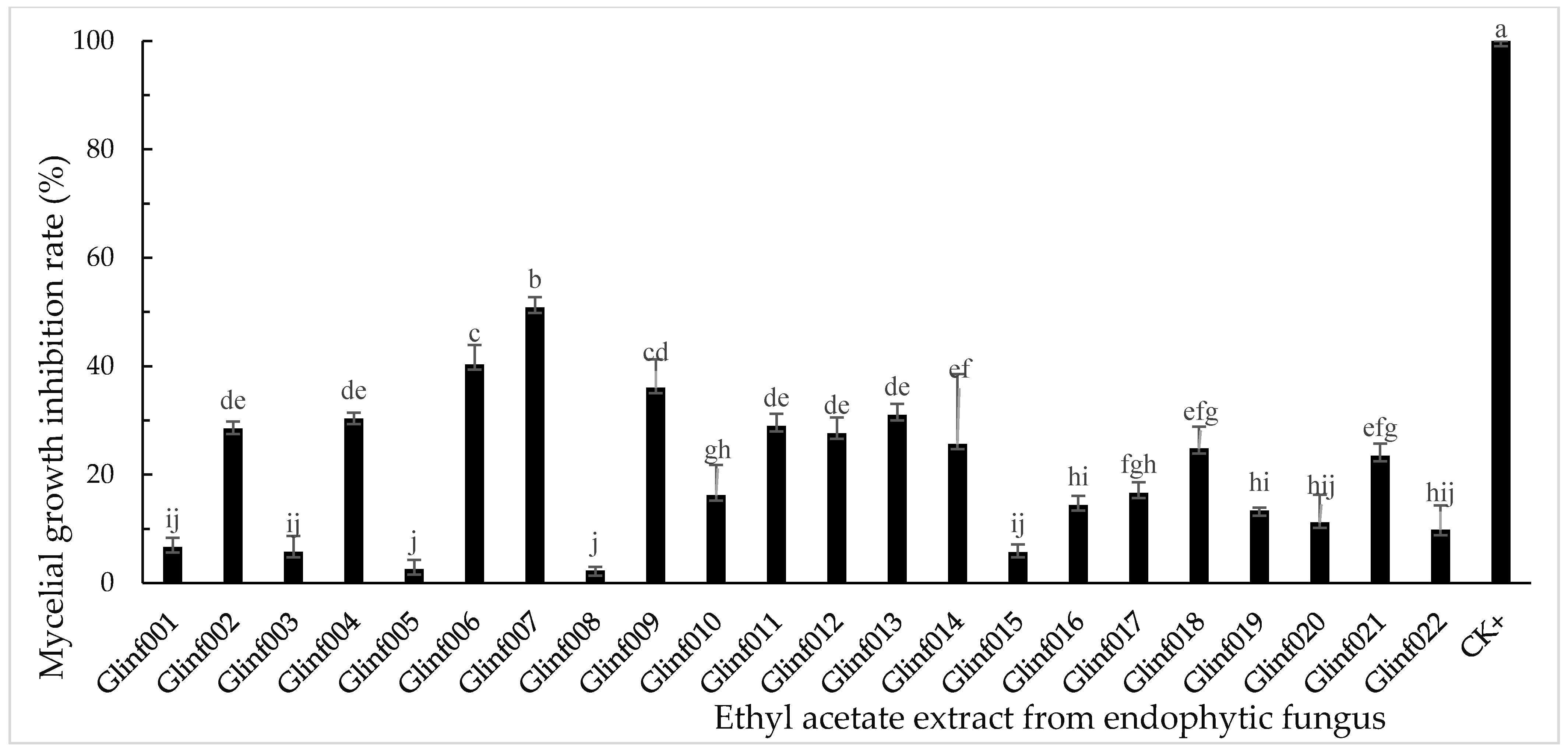
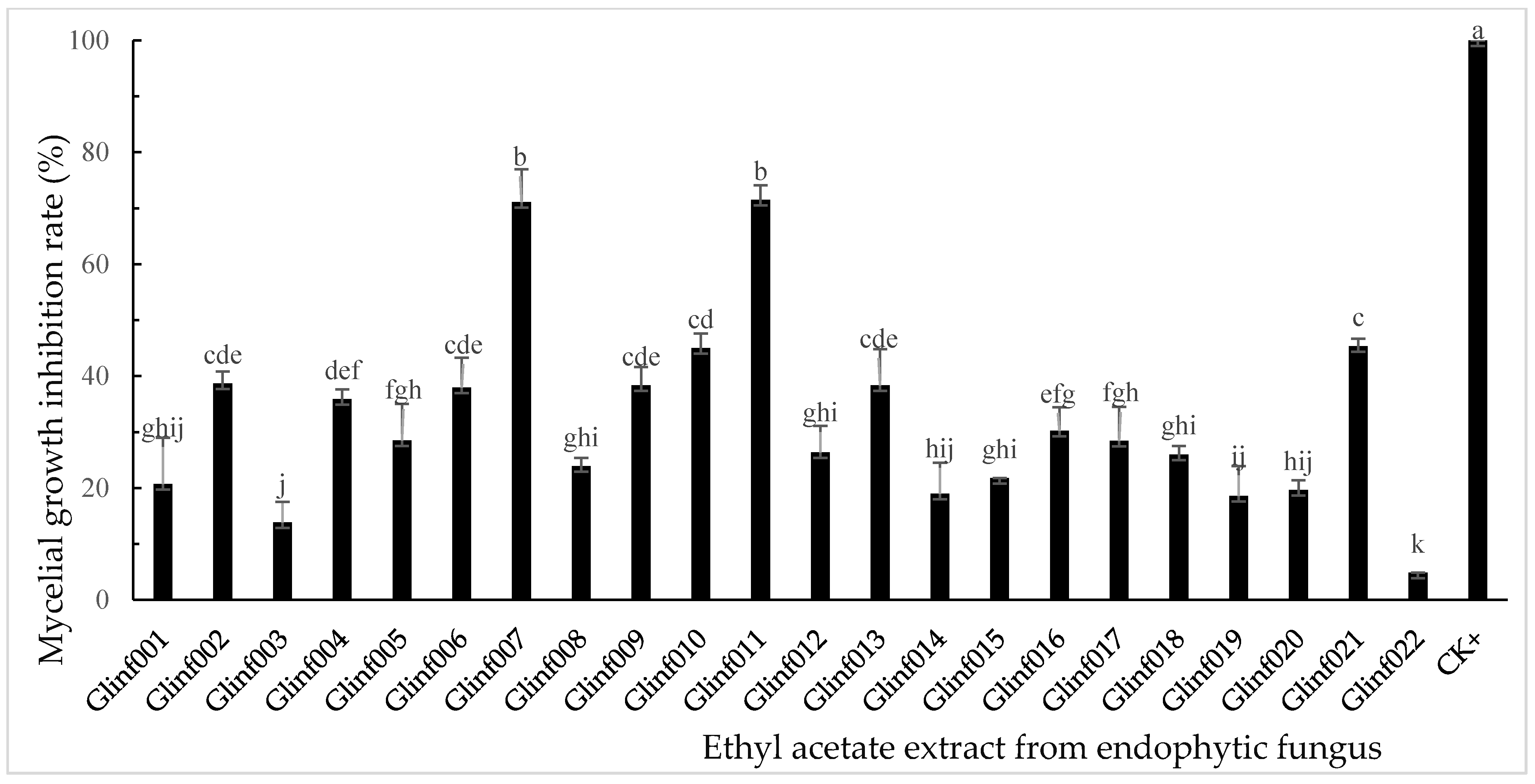
3.2. Inhibitory Activity of Endophytic Fungal Extracts on Mycelial Growth
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saikkonen, K.; Faeth, S.H.; Helander, M.; Sullivan, T.J. Fungal endophytes: A continuum of interactions with host plants. Annu. Rev. Ecol. Syst. 1998, 29, 319–343. [Google Scholar] [CrossRef]
- Hyde, K.D.; Soytong, K. The fungal endophyte dilemma. Fungal Divers. 2008, 33, 163–173. [Google Scholar]
- Bae, H.; Sicher, R.C.; Kim, M.S.; Kim, S.H.; Strem, M.D.; Melnick, R.L.; Bailey, B.A. The beneficial endophyte Trichoderma hamatum isolate DIS 219b promotes growth and delays the onset of the drought response in Theobroma cacao. J. Exp. Bot. 2009, 60, 3279–3295. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, S.K.; Verekar, S.A.; Bhave, S.V. Endophytic fungi: A reservoir of antibacterials. Front. Microbiol. 2015, 5, 715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nisa, H.; Kamili, A.N.; Nawchoo, I.A.; Shafi, S.; Shameem, N.; Bandh, S.A. Fungal endophytes as prolific source of phytochemicals and other bioactive natural products: A review. Microb. Pathog. 2015, 82, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Venugopalan, A.; Srivastava, S. Endophytes as in vitro production platforms of high value plant secondary metabolites. Biotechnol. Adv. 2015, 33, 873–887. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Shan, T.; Mou, Y.; Zhou, L. Plant-derived bioactive compounds produced by endophytic fungi. Mini-Rev. Med. Chem. 2011, 11, 159–168. [Google Scholar] [CrossRef]
- Stierle, A.; Strobel, G.; Stierle, D. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science 1993, 260, 214–216. [Google Scholar] [CrossRef]
- Tan, R.X.; Zou, W.X. Endophytes: A rich source of functional metabolites (1987 to 2000). Nat. Prod. Rep. 2001, 18, 448–459. [Google Scholar] [CrossRef]
- Li, J.; Zhao, J.; Xu, L.; Zhou, L.; Li, X.; Wang, J. Endophytic fungi from rhizomes of Paris polyphylla var. yunnanensis. World J. Microbiol. Biotechnol. 2008, 24, 733–737. [Google Scholar] [CrossRef]
- Xu, L.; Zhou, L.; Zhao, J.; Li, J.; Li, X.; Wang, J. Fungal endophytes from Dioscorea zingiberensis rhizomes and their antibacterial activity. Lett. Appl. Microbiol. 2008, 46, 68–72. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, J.; Zhou, L.; Wang, M.; Wang, J.; Li, X.; Chen, Q. Antimicrobial compounds from the endophytic fungus Fusarium sp. Ppf4 isolated from the medicinal plant Paris polyphylla var. yunnanensis. Nat. Prod. Commun. 2009, 4, 1455–1458. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Mou, Y.; Shan, T.; Li, Y.; Zhou, L.; Wang, M.; Wang, J. Antimicrobial metabolites from the endophytic fungus Pichia guilliermondii isolated from Paris polyphylla var. yunnanensis. Molecules 2010, 15, 7961–7970. [Google Scholar] [CrossRef] [Green Version]
- Lou, J.; Fu, L.; Luo, R.; Wang, X.; Luo, H.; Zhou, L. Endophytic fungi from medicinal herb Salvia miltiorrhiza Bunge and their antimicrobial activity. Afr. J. Microbiol. Res. 2013, 7, 5343–5349. [Google Scholar]
- Cui, J.-L.; Zhang, Y.-Y.; Vijayakumar, V.; Zhang, G.; Wang, M.-L.; Wang, J.-H. Secondary metabolite accumulation associates with ecological succession of endophytic fungi in Cynomorium songaricum Rupr. J. Agric. Food Chem. 2018, 66, 5499–5509. [Google Scholar] [CrossRef]
- Lai, D.; Mao, Z.; Zhou, Z.; Zhao, S.; Xue, M.; Dai, J.; Zhou, L.; Li, D. New chlamydosporol derivatives from the endophytic fungus Pleosporales sp. Sigrf05 and their cytotoxic and antimicrobial activities. Sci. Rep. 2020, 10, 8193. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Xue, M.; Gu, G.; Wang, W.; Li, D.; Lai, D.; Zhou, L. Lophiostomins A–D, new 3,4-dihydroisocoumarin derivatives from the endophytic fungus Lophiostoma sp. Sigrf10. RSC Adv. 2020, 10, 6985–6991. [Google Scholar] [CrossRef] [Green Version]
- Kao, T.-C.; Wu, C.-H.; Yen, G.-C. Bioactivity and potential health benefits of licorice. J. Agric. Food Chem. 2014, 62, 542–553. [Google Scholar] [CrossRef] [PubMed]
- Baltina, L.; Kndratenko, R. Glycyrrhizic acid derivatives as new antiviral and immune modulating agents. Curr. Bioact. Compd. 2021, 17, 41–58. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, L.; Hou, J.; Tian, S.; Liu, Y. Molecular mechanisms underlying the anticancer activities of licorice flafonoids. J. Ethnopharmacol. 2021, 267, 113635. [Google Scholar] [CrossRef] [PubMed]
- Simayi, Z.; Rozi, P.; Yang, X.; Ababaikeri, G.; Maimaitituoheti, W.; Bao, X.; Ma, S.; Askar, G.; Yadikar, N. Isolation, structural characterization, biological activity, and application of Glycyrrhiza polysaccharides: Systematic review. Int. J. Biol. Macromol. 2021, 183, 387–398. [Google Scholar] [CrossRef]
- Arora, P.; Wani, Z.A.; Ahmad, T.; Sultan, P.; Gupta, S.; Riyaz-Ul-Hssan, S. Community structure, spatial distribution, diversity and functional characterization of culturable endophytic fungi associated with Glycyrrhiza glabra L. Fungal Biol. 2019, 123, 373–383. [Google Scholar] [CrossRef]
- Nalli, Y.; Arora, P.; Khan, S.; Malik, F.; Riyaz-Ul-Hassan, S.; Gupta, V.; Ali, A. Isolation, structural modification of macrophin from endophytic fungus Phoma macrostoma and their cytotoxic potential. Med. Chem. Res. 2019, 28, 260–266. [Google Scholar] [CrossRef]
- Dang, H.; Zhang, T.; Wang, Z.; Li, G.; Zhao, W.; Lv, X.; Zhuang, L. Differences in the endophytic fungal community and effective ingredients in root of three Glycyrrhiza species in Xinjiang, China. PeerJ 2021, 9, e11047. [Google Scholar] [CrossRef] [PubMed]
- White, J.R.; Nagarajan, N.; Pop, M. Statistical methods for detecting differentially abundant features in clinical metagenomic samples. PLoS Comput. Biol. 2009, 5, e1000352. [Google Scholar] [CrossRef]
- Ainworth, G.C.; Sparrow, F.K.; Sussman, A.S. The Fungi, An Advanced Treatise. A Taxonomic Review with Keys Ascomycetes and Fungi Imperfecti; Academic Press: New York, NY, USA, 1973; Volume IV(A). [Google Scholar]
- Sun, S.; Lui, Q.; Han, L.; Ma, Q.; He, S.; Li, X.; Zhang, H.; Zhang, J.; Liu, X.; Wang, L. Identification and characterization of Fusarium proliferatum, a new species of fungi that cause fungal keratitis. Sci. Rep. 2018, 8, 4859. [Google Scholar] [CrossRef] [PubMed]
- Raeder, U.; Broda, P. Rapid preparation of DNA from filamentous fungi. Lett. Appl. Microbiol. 1985, 1, 17–20. [Google Scholar] [CrossRef]
- Quiroga, E.N.; Sampietro, A.R.; Vattuone, M.A. Screening antifungal activities of selected medicinal plants. J. Ethnopharmcol. 2001, 74, 89–96. [Google Scholar] [CrossRef]
- Raja, H.A.; Oberlier, N.H.; Stadler, M. Occasional comment: Fungal identification to species-level can be challenging. Phytochemistry 2021, 190, 112855. [Google Scholar] [CrossRef]
- Jiang, Z.-D.; An, Z. Bioactive fungal natural products through classic and biocombinatorial approaches. Stud. Nat. Prod. Chem. 2000, 22, 245–272. [Google Scholar]
- Singh, D.K.; Sharma, V.K.; Kumar, J.; Mishra, A.; Verma, S.K.; Sieber, T.N.; Kharwar, R.N. Diversity of endophytic mycobiota of tropical tree Tectona grandis Linn. f.: Spatiotemporal and tissue type effects. Sci. Rep. 2017, 7, 14. [Google Scholar]
- Hoffman, M.T.; Arnold, A.E. Geographic locality and host identity shape fungal endophyte communities in cupressaceous trees. Mycol. Res. 2008, 112, 331–344. [Google Scholar] [CrossRef]
- Persoh, D. Plant-associated fungal communities in the light of meta’omics. Fungal Divers. 2015, 75, 1–25. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, L.; Huang, Y.; Zhou, L. Detection of antimicrobial components from extracts of the endophytic fungi associated with Paris polyphylla var. yunnanensis using TLC-bioautography-MTT assay. Nat. Prod. Res. Dev. 2008, 20, 28–32. [Google Scholar]
- Okusa, P.N.; Stevigny, C.; Devleeschouwer, M.; Duez, P. Optimization of the culture medium used for direct TLC–bioautography. Application to the detection of antimicrobial compounds from Cordia gilletii De Wild (Boraginaceae). J. Planar Chromat. 2010, 4, 245–249. [Google Scholar] [CrossRef]
- Shan, T.; Tian, J.; Wang, X.; Mou, Y.; Mao, Z.; Lai, D.; Dai, J.; Peng, Y.; Zhou, L.; Wang, M. Bioactive spiro-bisnaphthalenes from the endophytic fungus Berkleasmium sp. J. Nat. Prod. 2013, 77, 2151–2160. [Google Scholar] [CrossRef] [PubMed]

| Fungal Isolate | CF (%) | GenBank Accession Number | Closest Related Species (Accession Number) | Identity (%) | Macro- and Microscopic Identification |
|---|---|---|---|---|---|
| Glinf001 | 17 | MW563907 | Aspergillus ustus (AY373874.1) | 99 | Aspergillus ustus |
| Glinf002 | 3 | MW563908 | Aspergillus keveii (MN542353.1) | 100 | Aspergillus keveii |
| Glinf003 | 1 | MW563909 | Aspergillus germanicus (MN650837.1) | 100 | Aspergillus germanicus |
| Glinf004 | 15 | MW563910 | Alternaria alternata (MN615420.1) | 100 | Alternaria alternata |
| Glinf005 | 1 | MW563911 | Alternaria sp. (MW220839.1) | 99 | Alternaria sp. |
| Glinf006 | 1 | MW563912 | Alternaria tenuissima (MK616250.1) | 100 | Alternaria tenuissima |
| Glinf007 | 1 | MW563913 | Alternaria angustiovoidea (MK910070.1) | 99 | Alternaria angustiovoidea |
| Glinf008 | 1 | MW563914 | Alternaria brassicae (JF439450.1) | 99 | Alternaria brassicae |
| Glinf009 | 29 | MW563915 | Fusarium proliferatum (MT560212.1) | 100 | Fusarium proliferatum |
| Glinf010 | 3 | MW563916 | Fusarium annulatum (MT434005.1) | 100 | Fusarium annulatum |
| Glinf011 | 3 | MW563917 | Fusarium fujikuroi (MT603302.1) | 100 | Fusarium fujikuroi |
| Glinf012 | 2 | MW563918 | Fusarium solani (MN013858.1) | 100 | Fusarium solani |
| Glinf013 | 2 | MW563919 | Fusarium sp. (MT252004.1) | 99 | Fusarium sp. |
| Glinf014 | 3 | MW563920 | Penicillium sizovae (MN858522.1) | 99 | Penicillium sizovae |
| Glinf015 | 2 | MW563921 | Penicillium bilaiae (LN901118.1) | 99 | Penicillium bilaiae |
| Glinf016 | 1 | MW563922 | Acrocalymma sp. (KP170636) | 98 | Acrocalymma sp. |
| Glinf017 | 1 | MW563923 | Athelia bombacina (MH201277.1) | 99 | Athelia bombacina |
| Glinf018 | 2 | MW563924 | Acremonium sclerotigenum (MF077221.1) | 99 | Acremonium sclerotigenum |
| Glinf019 | 2 | MW563925 | Botryotrichum murorum (MG228407.1) | 100 | Botryotrichum murorum |
| Glinf020 | 5 | MW563926 | Earliella scabrosa (MF077243.1) | 99 | Earliella scabrosa |
| Glinf021 | 3 | MW563927 | Rosellinia sp. (KU375680.1) | 100 | Rosellinia sp. |
| Glinf022 | 2 | MW563928 | Trichothecium roseum (MN372207.1) | 99 | Trichothecium roseum |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, G.; Jia, X.; Wang, W.; Li, P.; Zhao, S.; Zhou, Z.; Yin, R.; Lai, D.; Song, S.; Zhou, L. Culturable Endophytic Fungi from Glycyrrhiza inflata Distributed in Xinjiang, China with Antifungal Activity. Microbiol. Res. 2021, 12, 829-839. https://doi.org/10.3390/microbiolres12040060
Gu G, Jia X, Wang W, Li P, Zhao S, Zhou Z, Yin R, Lai D, Song S, Zhou L. Culturable Endophytic Fungi from Glycyrrhiza inflata Distributed in Xinjiang, China with Antifungal Activity. Microbiology Research. 2021; 12(4):829-839. https://doi.org/10.3390/microbiolres12040060
Chicago/Turabian StyleGu, Gan, Xiaowei Jia, Weixuan Wang, Peng Li, Siji Zhao, Zhiyao Zhou, Ruya Yin, Daowan Lai, Suqin Song, and Ligang Zhou. 2021. "Culturable Endophytic Fungi from Glycyrrhiza inflata Distributed in Xinjiang, China with Antifungal Activity" Microbiology Research 12, no. 4: 829-839. https://doi.org/10.3390/microbiolres12040060
APA StyleGu, G., Jia, X., Wang, W., Li, P., Zhao, S., Zhou, Z., Yin, R., Lai, D., Song, S., & Zhou, L. (2021). Culturable Endophytic Fungi from Glycyrrhiza inflata Distributed in Xinjiang, China with Antifungal Activity. Microbiology Research, 12(4), 829-839. https://doi.org/10.3390/microbiolres12040060







