Effect of a Diet Based on Biotransformed Sorghum on Rabbit Intestinal Morphology and Fecal Fiber Composition
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hernández, P.; Dalle Zotte, A. Influence of Diet on Rabbit Meat Quality. In Nutrition of the Rabbit; De Blas, C., Wiseman, J., Eds.; CAB International: Wallingford, UK, 2010; pp. 163–178. [Google Scholar]
- Dalle Zotte, A.; Szendrő, Z. The role of rabbit meat as functional food. Meat Sci. 2011, 88, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Para, P.A.; Ganguly, S.; Wakchaure, R.; Sharma, R.; Mahajan, T.; Praveen, P.K. Rabbit meat has the potential of being a possible alternative to other meats as a protein source: A brief review. Int. J. Phar. Biomedi. Rese. 2015, 2, 17–19. [Google Scholar]
- Dalle Zotte, A.; Cossu, M.E. Dietary inclusion of tannin extract from red quebracho trees (Schinopsis spp.) in the rabbit meat production. Ital. J. Anim. Sci. 2009, 8, 784–786. [Google Scholar] [CrossRef]
- Kowalska, D.; Bielanski, P. Meat quality of rabbit fed a diet supplemented with fish oil and antioxidant. Anim. Sci. Pap. Rep. 2009, 2, 139–148. [Google Scholar]
- De Blas, J.C. Nutritional impact on health and performance in intensively reared rabbits. Animal 2013, 7, 102–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gidenne, T. Dietary fibers in the nutrition of the growing rabbit and recommendations to preserve digestive health: A review. Animal 2015, 9, 227–242. [Google Scholar] [CrossRef] [Green Version]
- Ajila, C.M.; Brar, S.K.; Verma, M.; Tyagi, R.D.; Godbout, S.; Valéro, J.R. Bio-processing of agro-byproducts to animal feed. Crit. Rev. Biotechnol. 2012, 32, 382–400. [Google Scholar] [CrossRef]
- Celia, C.; Cullere, M.; Gerencsér, Z.; Matics, Z.; Giaccone, V.; Kovács, M.; Bónai, A.; Szendrő, Z.; Dalle Zotte, A. Dietary supplementation of Digestarom® herbal formulation: Effect on apparent digestibility, faecal and caecal microbial counts and live performance of growing rabbits. World Rabbit Sci. 2016, 24, 95–105. [Google Scholar] [CrossRef] [Green Version]
- del Toro, M.I.; Aguilar, Y.M.; Navarro, M.V.; Chipres, D.S.; Cortés, M.R. Comportamiento productivo y características de la canal de conejos alimentados con harina de Agave tequilana. Rev. Electron. Vet. 2016, 17, 1–12. [Google Scholar]
- Dalle Zotte, A.; Cullere, M.; Sartori, A.; Szendrő, Z.; Kovàcs, M.; Giaccone, V.; Dal Bosco, A. Dietary Spirulina (Arthrospira platensis) and Thyme (Thymus vulgaris) supplementation to growing rabbits: Effects on raw and cooked meat quality, nutrient true retention and oxidative stability. Meat Sci. 2014, 98, 94–103. [Google Scholar] [CrossRef]
- Phuoc, T.L.; Jamikorn, U. Effects of probiotic supplement (Bacillus subtilis and Lactobacillus acidophilus) on feed efficiency, growth performance, and microbial population of weaning rabbits. Asian-Australas. J. Anim. Sci. 2017, 30, 198–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Martínez, C.A.; Maldonado Herrera, J.A.; Méndez-Zamora, G.; Hernández-Luna, C.E.; Gutiérrez-Soto, G. Enzymatic extract of Trametes maxima CU1 on productive parameters and carcass yield of rabbits. Can. J. Anim. Sci. 2017, 97, 683–688. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, C. Lignocellulosic residues: Biodegradation and bioconversion by fungi. Biotechnol. Adv. 2009, 27, 185–194. [Google Scholar] [CrossRef]
- Akinfemi, A.; Adu, O.A.; Doherty, F. Conversion of sorghum stover into animal feed with white-rot fungi: Pleurotus ostreatus and Pleurotus pulmonarius. Afr. J. Biotechnol. 2010, 9, 1706–1712. [Google Scholar] [CrossRef] [Green Version]
- Medina-González, G.E.; Bernal-Barragán, H.; Hernández-Luna, C.E.; Hernández-Martínez, C.A.; Gutiérrez-Soto, G. Uso de basidiomicetos nativos en la biotransformación del pasto buffel (Cenchrus ciliaris) para mejorar la calidad nutricional. Rev. Mex. Micol. 2016, 43, 31–35. [Google Scholar]
- Shrivastava, B.; Thakur, S.; Khasa, Y.P.; Gupte, A.; Puniya, A.K.; Kuhad, R.C. White-rot fungal conversion of wheat straw to energy rich cattle feed. Biodegradation 2011, 22, 823–831. [Google Scholar] [CrossRef] [PubMed]
- van Kuijk, S.J.A.; Sonnenberg, A.S.; Baars, J.J.; Hendriks, W.H.; Cone, J.W. Fungal treated lignocellulosic biomass as ruminant feed ingredient: A review. Biotechnol. Adv. 2015, 33, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Hatakka, A.; Maijala, P. Can co-culturing of two white-rot fungi increase lignin degradation and the production of lignin-degrading enzymes? Int. Biodeterior. Biodegrad. 2007, 59, 32–39. [Google Scholar] [CrossRef]
- Kuhar, F.; Castiglia, V.; Levin, L. Enhancement of laccase production and malachite green decolorization by co-culturing Ganoderma lucidum and Trametes versicolor in solid-state fermentation. Int. Biodeterior. Biodegrad. 2015, 104, 238–243. [Google Scholar] [CrossRef]
- Gutiérrez-Soto, G.; Medina-Gonzalez, G.E.; Treviño-Ramírez, J.E.; Hernández-Luna, C.E. Native macrofungi that produce lignin-modifying enzymes, cellulases, and xylanases with potential biotechnological applications. Bioresources 2015, 10, 6676–6689. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Soto, G.; Medina-González, G.E.; García-Zambrano, E.A.; Treviño-Ramírez, J.E.; Hernández-Luna, C.E. Selection and characterization of a native Pycnoporus sanguineus strain as a lignocellulolytic extract producer from submerged cultures of various agroindustrial wastes. Bioresources 2015, 10, 3564–3576. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Martínez, C.A.; Treviño-Cabrera, G.F.; Hernández-Luna, C.E.; Silva-Vázquez, R.; Hume, M.E.; Gutiérrez-Soto, G.; Méndez-Zamora, G. The effects of hydrolysed sorghum on growth performance and meat quality of rabbits. World Rabbit Sci. 2018, 26, 155–163. [Google Scholar] [CrossRef]
- Norma Oficial Mexicana. Especificaciones Técnicas para la Producción, Cuidado y Uso de Animales de Laboratorio. NOM-062-ZOO. Available online: https://www.gob.mx/cms/uploads/attachment/file/203498/NOM-062-ZOO-1999_220801.pdf (accessed on 29 November 2022).
- AOAC. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- Van Soest, J.P.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3594. [Google Scholar] [CrossRef] [PubMed]
- Safwat, A.M.; Sarmiento-Franco, L.; Santos-Ricalde, R.H.; Nieves, D.; Sandoval-Castro, C.A. Estimating apparent nutrient digestibility of diets containing Leucaena leucocephala or Moringa oleifera leaf meals for growing rabbits by two methods. Asian Australas. J. Anim. Sci. 2015, 2, 1155–1162. [Google Scholar] [CrossRef]
- Attia, K.A.; Saleh, S.Y.; El-Hamid Safaa, S.A.; ZakiAmal, A.; El-Sawy, M.A. Effects of exogenous multi-enzyme feed additive (kemzyme) on the activities of certain digestive enzymes and intestinal morphology in growing rabbits. J. Agric. Sci. 2012, 4, 35–44. [Google Scholar]
- Koutrotsios, G.; Mountzouris, K.C.; Chatzipavlidis, I.; Zervakis, G.I. Bioconversion of lignocellulosic residues by Agrocybe cylindracea and Pleurotus ostreatus mushroom fungi–Assessment of their effect on the final product and spent substrate properties. Food Chem. 2014, 161, 127–135. [Google Scholar] [CrossRef]
- van Kuijk, S.J.A.; Sonnenberg, A.S.; Baars, J.J.; Hendriks, W.H.; Cone, J.W. Fungal treatment of lignocellulosic biomass: Importance of fungal species, colonization and time on chemical composition and in vitro rumen degradability. Anim. Feed Sci. Technol. 2015, 28, 40–50. [Google Scholar] [CrossRef]
- van Kuijk, S.J.A.; Del Rio, J.C.; Rencoret, J.; Gutiérrez, A.; Sonnenberg, A.S.M.; Baars, J.J.P.; Hendriks, W.H.; Cone, J.W. Selective ligninolysis of wheat straw and wood chips by the white-rot fungus Lentinula edodes and its influence on in vitro rumen degradability. J. Anim. Sci. Biotechnol. 2016, 7, 55–74NRC. [Google Scholar] [CrossRef] [Green Version]
- Oduguwa, O.O.; Edema, M.O.; Ayeni, A.O. Physico-chemical and microbiological analyses of fermented corn cob, rice bran and cowpea husk for use in composite rabbit feed. Bioresour. Technol. 2008, 99, 1816–1820. [Google Scholar] [CrossRef]
- Ribeiro, L.; Pinheiro, V.; Outor-Monteiro, D.; Mourão, J.; Bezerra, R.M.F.; Dias, A.A.; Bennett, R.N.; Marques, G.; Rodrigues, M.A.M. Effects of the dietary incorporation of untreated and white-rot fungi (Ganoderma resinaceum Boud) pre-treated olive leaves on growing rabbits. Anim. Feed Sci. Technol. 2012, 173, 244–251. [Google Scholar] [CrossRef]
- Andrade, E.; Pinheiro, V.; Gonçalves, A.; Cone, J.W.; Marques, G.; Silva, V.; Ferreira, L.; Rodrigues, M. Potential use of cowpea (Vigna unguiculata (L.) Walp.) stover treated with white-rot fungi as rabbit feed. J. Sci. Food Agric. 2017, 97, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, T.; Thomas, P.A.; Sheu, J.R.; Geraldine, P. In-vitro and in-vivo antioxidant effects of the oyster mushroom Pleurotus ostreatus. Int. Food Res. J. 2011, 44, 851–861. [Google Scholar] [CrossRef]
- El-Badawi, A.Y.; El-Wardany, I.; El-Moez, S.A.; Helal, F.I.S.; Ali, N.G.; Shourrap, M.I.; Aboelazab, O.M. Impact of dietary Moringa oleifera leaves on intestinal pathogenic load and histological structure of growing rabbits raised under heat-stress conditions. Anim. Prod. Sci. 2017, 58, 1901–1907. [Google Scholar] [CrossRef]
- Gidenne, T. Dietary fibers: Their analysis in animal feeding, and their role in rabbit nutrition and health. JITV 2013, 23, 195–213. [Google Scholar]
- El-Maaty, H.M.A.; Sherif, S.K.; Foda, L.S. Dietary sugar beet tops and prebiotic effect on nutrient digestibility, caecal activity and organ histology of weaning rabbits. J. Agric. Sci. 2018, 10, 162–177. [Google Scholar] [CrossRef]
| Ingredient | g kg−1 |
|---|---|
| Alfalfa hay | 496.7 |
| Ground sorghum * | 300.0 |
| Soya meal | 146.0 |
| Soya oil | 12.5 |
| Methionine | 1.3 |
| Molasses | 30.0 |
| Phosphate | 4.5 |
| Vitamin and mineral premix | 2.5 |
| Component (%) | Sorghum | SSF-Sorghum | Diet T1 | Diet T2 |
|---|---|---|---|---|
| Dry Matter | 94.25 | 94.42 | 93.93 | 94.99 |
| NDF DM | 17.89 | 15.90 | 75.51 | 43.17 |
| ADF DM | 6.46 | 3.81 | 49.37 | 31.21 |
| Lignin DM | 2.55 | 0.51 | 16.28 | 5.57 |
| Hemicellulose DM | 12.09 | 11.43 | 26.14 | 11.96 |
| Cellulose DM | 5.95 | 1.26 | 33.09 | 25.64 |
| Ash | 9.16 | 8.580 | 1.36 | 1.62 |
| Nitrogen | 1.42 | 1.42 | 2.68 | 2.36 |
| Crude protein | 8.85 | 8.89 | 16.73 | 14.75 |
| PC DM | 9.39 | 9.41 | 17.81 | 15.52 |
| Day | Tx | DM | ASH | OM | NDF DM | ADF DM | LIGNIN | HEMICEL | CEL |
|---|---|---|---|---|---|---|---|---|---|
| (%) | |||||||||
| 7 | T1 | 92.45 a | 7.02 a | 85.42 b | 72.93 a | 52.86 a | 11.03 b | 20.07 a | 41.83 a |
| T2 | 92.62 a | 4.99 b | 87.63 a | 71.10 a | 51.56 a | 21.17 a | 19.55 a | 30.38 b | |
| 21 | T1 | 92.97 a | 7.25 a | 85.72 b | 73.28 a | 52.39 a | 13.70 b | 20.89 a | 38.70 a |
| T2 | 93.85 a | 6.26 b | 87.59 a | 68.27 b | 48.09 b | 19.07 a | 20.18 a | 29.02 b | |
| 35 | T1 | 93.79 a | 7.19 b | 86.60 a | 62.6 a | 42.37 a | 19.70 a | 20.23 a | 22.67 b |
| T2 | 92.78 a | 7.86 a | 84.92 b | 58.17 b | 40.27 a | 15.39 b | 17.91 a | 24.88 a | |
| 49 | T1 | 93.41 a | 6.71 b | 86.70 a | 66.28 a | 47.17 a | 25.72 a | 19.11 a | 21.45 a |
| T2 | 93.25 a | 7.85 a | 85.39 b | 62.91 a | 44.7 a | 19.63 b | 18.21 a | 25.08 a | |
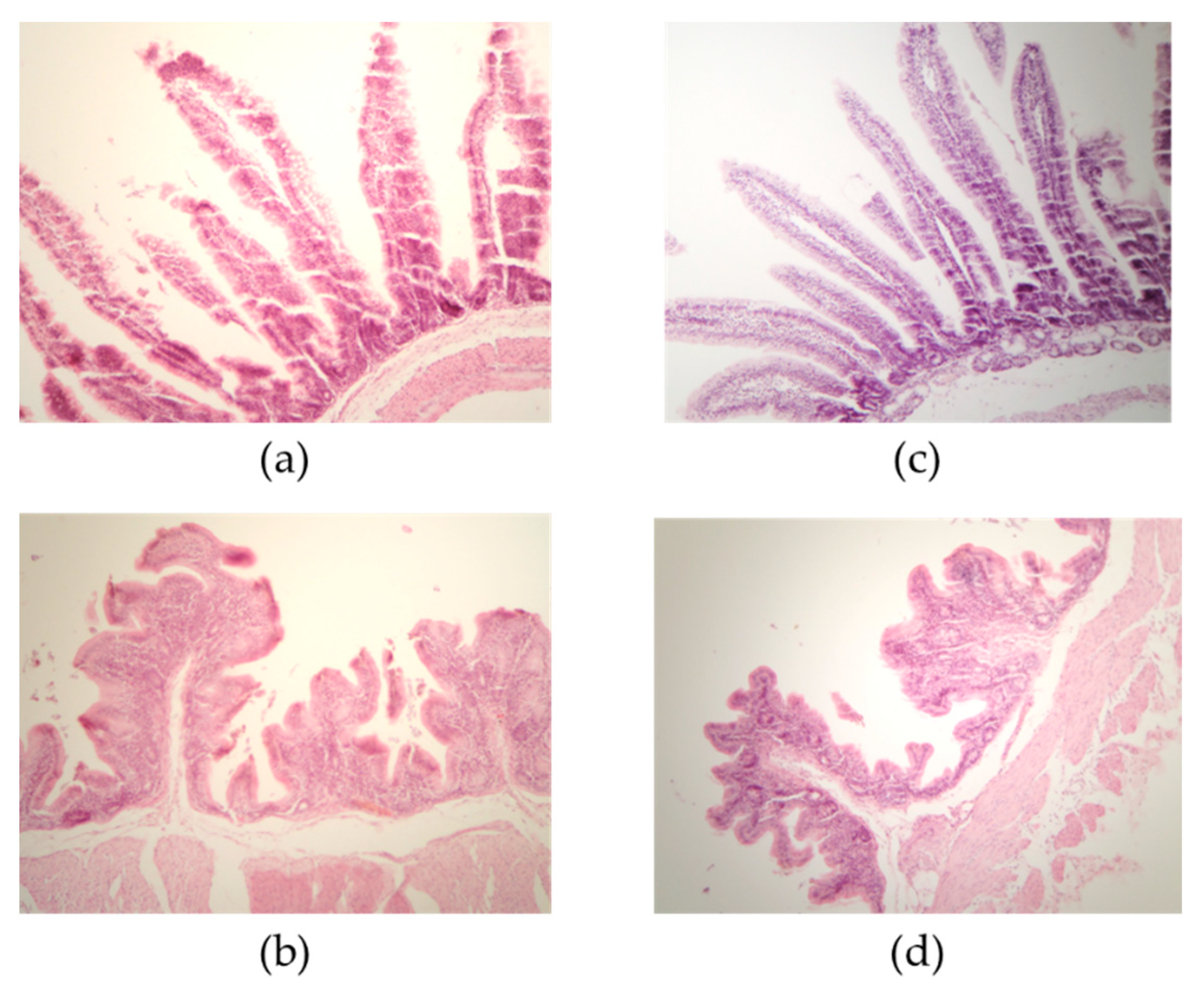
| Segment | T1 * | T2 * | p-Value |
|---|---|---|---|
| Villus Length in Small Intestine and Cecum | |||
| Duodenum | 363.86 ± 93.25 a | 350.85 ± 49.23 a | 0.5019 |
| Jejunum | 424.83 ± 36.43 b | 513.69 ± 70.35 a | <0.0001 |
| Ileum | 872.73 ± 25.47 a | 675.46 ± 88.96 b | <0.0001 |
| Cecum | 157.02 ± 18.44 b | 241.04 ± 53.90 a | <0.0001 |
| Crypt depth in small intestine and cecum | |||
| Duodenum | 58.77 ± 6.59 a | 59.60 ± 6.42 b | 0.6218 |
| Jejunum | 97.43 ± 13.17 a | 84.03 ± 19.95 b | 0.0032 |
| Ileum | 112.53 ± 18.41 a | 93.03 ± 12.83 b | <0.0001 |
| Cecum | 256.87 ± 8.21 b | 356.85 ± 13.37 a | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Martínez, C.A.; Levin, L.; Treviño-Cabrera, G.; Hernández-Luna, C.E.; Bernal-Barragán, H.; Castillo-Velázquez, U.; Rodríguez-Tovar, L.E.; Dávila-Martínez, C.; Trejo-Chávez, A.; Méndez-Zamora, G.; et al. Effect of a Diet Based on Biotransformed Sorghum on Rabbit Intestinal Morphology and Fecal Fiber Composition. Microbiol. Res. 2022, 13, 1018-1026. https://doi.org/10.3390/microbiolres13040074
Hernández-Martínez CA, Levin L, Treviño-Cabrera G, Hernández-Luna CE, Bernal-Barragán H, Castillo-Velázquez U, Rodríguez-Tovar LE, Dávila-Martínez C, Trejo-Chávez A, Méndez-Zamora G, et al. Effect of a Diet Based on Biotransformed Sorghum on Rabbit Intestinal Morphology and Fecal Fiber Composition. Microbiology Research. 2022; 13(4):1018-1026. https://doi.org/10.3390/microbiolres13040074
Chicago/Turabian StyleHernández-Martínez, Carlos A., Laura Levin, Griselda Treviño-Cabrera, Carlos E. Hernández-Luna, Hugo Bernal-Barragán, Uziel Castillo-Velázquez, Luis Edgar Rodríguez-Tovar, Cesar Dávila-Martínez, Armando Trejo-Chávez, Gerardo Méndez-Zamora, and et al. 2022. "Effect of a Diet Based on Biotransformed Sorghum on Rabbit Intestinal Morphology and Fecal Fiber Composition" Microbiology Research 13, no. 4: 1018-1026. https://doi.org/10.3390/microbiolres13040074
APA StyleHernández-Martínez, C. A., Levin, L., Treviño-Cabrera, G., Hernández-Luna, C. E., Bernal-Barragán, H., Castillo-Velázquez, U., Rodríguez-Tovar, L. E., Dávila-Martínez, C., Trejo-Chávez, A., Méndez-Zamora, G., & Gutiérrez-Soto, G. (2022). Effect of a Diet Based on Biotransformed Sorghum on Rabbit Intestinal Morphology and Fecal Fiber Composition. Microbiology Research, 13(4), 1018-1026. https://doi.org/10.3390/microbiolres13040074







