Arbuscular Mycorrhizal Fungus Funneliformis mosseae Improves Soybean Growth Even in Soils with Good Nutrition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Cultivation Pots and Substrate
2.3. Microbial Inoculation
2.4. Model Plant, Plant Cultivation, and Harvest
2.5. Analyses and Calculations
2.6. Statistical Analyses
3. Results
3.1. Plant Biomass and Mineral Nutrition
3.2. Stability of Soil Aggregates
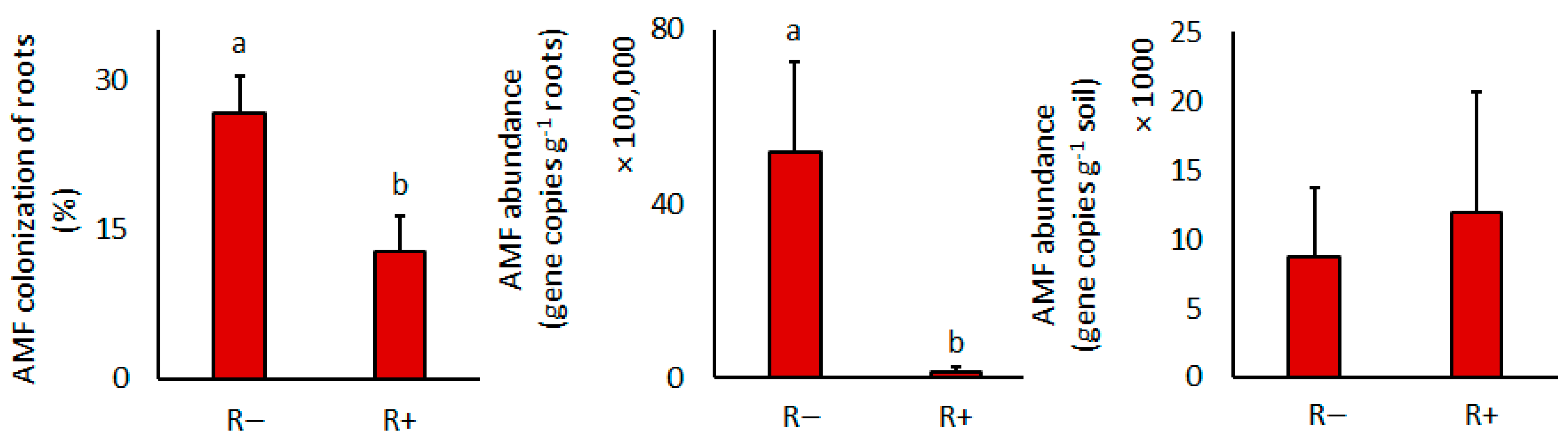
3.3. Arbuscular Mycorrhizal Fungal and Rhizobial Development
3.4. Total Glomalin
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Spatafora, J.W.; Chang, Y.; Benny, G.L.; Lazarus, K.; Smith, M.E.; Berbee, M.L.; Bonito, G.; Corradi, N.; Grigoriev, I.; Gryganskyi, A.; et al. A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia 2016, 108, 1028–1046. [Google Scholar] [CrossRef]
- Jeffries, P.; Barea, J.M. Arbuscular mycorrhiza—A key component of sustainable plant–soil ecosystems. In The Mycota. IX Fungal Associations; Hock, B., Ed.; Springer: Berlin, Germany, 2012. [Google Scholar]
- Schϋßler, A.; Schwarzott, D.; Walker, C. A new fungal phylum, the Glomeromycota: Phylogeny and evolution. Mycol. Res. 2001, 105, 1413–1421. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Jansa, J.; Šmilauer, P.; Borovička, J.; Hršelová, H.; Forczek, S.T.; Slámová, K.; Řezanka, T.; Rozmoš, M.; Bukovská, P.; Gryndler, M.; et al. Dead Rhizophagus irregularis biomass mysteriously stimulates plant growth. Mycorrhiza 2020, 30, 63–77. [Google Scholar] [CrossRef]
- Kiers, E.T.; Duhamel, M.; Beesetty, Y.; Mensah, J.A.; Franken, O.; Verbruggen, E.; Fellbaum, C.R.; Kowalchuk, G.A.; Hart, M.M.; Bago, A.; et al. Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science 2011, 333, 880–882. [Google Scholar] [CrossRef]
- Lekberg, Y.; Hammer, E.C.; Olsson, P.A. Plants as resource islands and storage units—Adopting the mycocentric view of arbuscular mycorrhizal networks. FEMS Microbiol. Ecol. 2010, 74, 336–345. [Google Scholar] [CrossRef]
- Řezáčová, V.; Slavíková, R.; Zemková, L.; Konvalinková, T.; Procházková, V.; Šťovíček, V.; Hršelová, H.; Beskid, O.; Huhslova, M.; Gryndlerova, H.; et al. Mycorrhizal symbiosis induces plant carbon reallocation differently in C3 and C4 Panicum grasses. Plant Soil 2018, 425, 441–456. [Google Scholar] [CrossRef]
- Aroca, R.; Porcel, R.; Ruiz-Lozano, J.M. How does arbuscular mycorrhizal symbiosis regulate root hydraulic properties and plasma membrane aquaporins in Phaseolus vulgaris under drought, cold or salinity stresses? New Phytol. 2007, 173, 808–816. [Google Scholar] [CrossRef]
- Augé, R.M.; Toler, H.D.; Saxton, A.M. Arbuscular mycorrhizal symbiosis and osmotic adjustment in response to NaCl stress: A meta-analysis. Front. Plant Sci. 2014, 5, 562. [Google Scholar]
- Augé, R.M.; Toler, H.D.; Saxton, A.M. Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: A meta-analysis. Mycorrhiza 2015, 25, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.S.; Siddiqui, Z.A.; Wiemken, A. Arbuscular mycorrhizal fungi and Rhizobium to control plant fungal diseases. In Alternative Farming Systems, Biotechnology, Drought Stress and Ecological Fertilisation. Sustainable Agriculture Reviews; Lichtfouse, E., Ed.; Springer: Dordrecht, The Netherlands, 2011; Volume 6. [Google Scholar]
- Matsubara, Y.; Hirano, I.; Sassa, D.; Koshikawa, K. Increased tolerance to Fusarium wilt in mycorrhizal strawberry plants raised by capillary watering methods. Environ. Control. Biol. 2004, 42, 185–191. [Google Scholar] [CrossRef]
- Newsham, K.K.; Fitter, A.H.; Watkinson, A.R. Arbuscular mycorrhiza protect an annual grass from root pathogenic fungi in the field. J. Ecol. 1995, 83, 991–1000. [Google Scholar] [CrossRef]
- Pozo, M.J.; Cordier, C.; Dumas-Gaudot, E.; Gianinazzi, S.; Barea, J.M.; Azcón-Aguilar, C. Localized versus systemic effect of arbuscular mycorrhizal fungi on defense responses to Phytophthora infection in tomato plants. J. Ex. Bot. 2002, 53, 525–534. [Google Scholar] [CrossRef]
- Vigo, C.; Norman, J.R.; Hooker, J.E. Biocontrol of the pathogen Phytophthora parasitica by arbuscular mycorrhizal fungi is a consequence of effects on infection loci. Plant Pathol. 2000, 49, 509–514. [Google Scholar] [CrossRef]
- Joner, E.J.; van Aarle, I.M.; Vosátka, M. Phosphatase activity of extra-radical arbuscular mycorrhizal hyphae: A review. Plant Soil 2000, 226, 199–210. [Google Scholar] [CrossRef]
- Pearson, J.N.; Jakobsen, I. The relative contribution of hyphae and roots to phosphorus uptake by arbuscular mycorrhizal plants measured by dual labelling with 32P and 33P. New Phytol. 1993, 124, 489–494. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A.; Jakobsen, I. Mycorrhizal fungi can dominate phosphate supply to plants irrespective of growth responses. Plant Physiol. 2003, 133, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Mendoza, I.E.; Dewbre, G.R.; Harrison, M.J.; Tsuzuki, S.; Handa, Y.; Takeda, N.; Kawaguchi, M.; Ikeda, Y.; Shimura, H.; Kitahara, R.; et al. A phosphate transporter gene from the extra-radical mycelium of an arbuscular mycorrhizal fungus Glomus intraradices is regulated in response to phosphate in the environment. Mol. Plant Microbe Interact. 2001, 14, 1140–1148. [Google Scholar] [CrossRef]
- Řezáčová, V.; Konvalinková, T.; Jansa, J. Carbon fluxes in mycorrhizal plants. In Mycorrhiza: Eco-Physiology, Secondary Metabolites, Nanomaterials; Varma, A., Prasad, R., Tuteja, N., Eds.; Springer: Berlin, Germany; Cham, Switzerland, 2017. [Google Scholar]
- Bethlenfalvay, G.J.; Cantrell, I.C.; Mihara, K.L.; Schreiner, R.P. Relationships between soil aggregation and mycorrhizae as influenced by soil biota and nitrogen nutrition. Biol. Fertil. Soil. 1999, 28, 356–363. [Google Scholar] [CrossRef]
- Miller, R.M.; Jastrow, J.D. Mycorrhizal fungi influence soil structure. In Arbuscular Mycorrhizas: Physiology and Function; Kapulnik, Y., Douds Jr, D.D., Eds.; Kluwer Academic: Dordrecht, The Netherlands, 2000. [Google Scholar]
- Rashid, M.I.; Mujawar, L.H.; Shahzad, T.; Almeelbi, T.; Ismail, I.M.; Oves, M. Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. Microbiol. Res. 2016, 183, 26–41. [Google Scholar] [CrossRef]
- Velmourougane, K.; Prasanna, R.; Saxena, A.K. Agriculturally important microbial biofilms: Present status and future prospects. J. Basic Microbiol. 2017, 57, 548–573. [Google Scholar] [CrossRef]
- González-Chávez, M.C.; Carrillo-González, R.; Wright, S.F.; Nichols, K.A. The role of glomalin, a protein produced by arbuscular mycorrhizal fungi, in sequestering potentially toxic elements. Environ. Pollut. 2004, 130, 317–323. [Google Scholar] [CrossRef]
- Wright, S.F.; Upadhyaya, A. A survey of soils for aggregate stability and glomalin, a glycoprotein produced by hyphae of arbuscular mycorrhizal fungi. Plant Soil. 1998, 198, 97–107. [Google Scholar] [CrossRef]
- Costa, O.Y.A.; Raaijmakers, J.M.; Kuramae, E.E. Microbial extracellular polymeric substances: Ecological function and impact on soil aggregation. Front. Microbiol. 2018, 9, 1636. [Google Scholar] [CrossRef] [PubMed]
- Dodd, J.C. Approaches to the study of the extraradical mycelium of arbuscular mycorrhizal fungi. In Impact of Arbuscular Mycorrhizas on Sustainable Agriculture and Natural Ecosystems; Gianinazzi, S., Schüepp, H., Eds.; Birkhäuser: Basel, Switzerland, 1994. [Google Scholar]
- Baldock, J.A. Interactions of organic materials and microorganisms with minerals in the stabilization of soil structure. In Interactions between Soil Particles and Microorganisms; Huang, P.M., Bollag, J.-M., Senesi, N., Eds.; John Wiley & Sons: Chichester, UK, 2001. [Google Scholar]
- Barea, J.M.; Azcón, R.; Azcón-Aguilar, C. Interactions between mycorrhizal fungi and bacteria to improve plant nutrient cycling and soil structure. In Microorganisms in Soils: Roles in Genesis and Functions. Soil Biology; Varma, A., Buscot, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 3. [Google Scholar]
- Antunes, P.M.; Goss, M.J. Communication in the tripartite symbiosis formed by arbuscular mycorrhizal fungi, rhizobia and legume plants: A review. In Roots and Soil Management: Interactions between Roots and the Soil; Zobel, R.W., Wright, S.F., Eds.; Am. Soc. Agronom., Inc.: Madison, WI, USA, 2005. [Google Scholar]
- Püschel, D.; Janoušková, M.; Voříšková, A.; Gryndlerová, H.; Vosátka, M.; Jansa, J. Arbuscular mycorrhiza stimulates biological nitrogen fixation in two Medicago spp. through improved phosphorus acquisition. Front. Plant Sci. 2017, 8, 390. [Google Scholar] [CrossRef]
- Wagner, S.C. Biological nitrogen fixation. Nat. Ed. Know. 2011, 3, 15. [Google Scholar]
- Kassaw, T.; Bridges, W., Jr.; Frugoli, J. Multiple autoregulation of nodulation (AON) signals identified through split root analysis of Medicago truncatula sunn and rdn1 mutants. Plants 2015, 4, 209–224. [Google Scholar] [CrossRef]
- Mortimer, P.E.; Pérez-Fernández, M.A.; Valentine, A.J. Arbuscular mycorrhizae affect the N and C economy of nodulated Phaseolus vulgaris (L.) during NH4+ nutrition. Soil Biol. Biochem. 2009, 41, 2115–2121. [Google Scholar] [CrossRef]
- Zhang, X.; Qiu, Y.; Gilliam, F.S.; Gillespie, C.J.; Tu, C.; Reberg-Horton, S.C.; Hu, S. Arbuscular mycorrhizae shift community composition of N-cycling microbes and suppress soil N2O Emission. Environ. Sci. Tech. 2022, 56, 13461–13472. [Google Scholar] [CrossRef] [PubMed]
- Barea, J.M.; Toro, M.; Orozco, M.O.; Campos, E.; Azcón, R. The application of isotopic 32P and 15N-dilution techniques to evaluate the interactive effect of phosphate-solubilizing rhizobacteria, mycorrhizal fungi and Rhizobium to improve the agronomic efficiency of rock phosphate for legume crops. Nutr. Cycl. Agroecosyst. 2002, 63, 35–42. [Google Scholar] [CrossRef]
- Harris, D.; Pacovsky, R.S.; Paul, E.A. Carbon economy of soybean-Rhizobium-Glomus associations. New Phytol. 1985, 101, 427–440. [Google Scholar] [CrossRef]
- Larimer, A.L.; Clay, K.; Bever, J.D. Synergism and context dependency of interactions between arbuscular mycorrhizal fungi and rhizobia with a prairie legume. Ecology 2014, 95, 1045–1054. [Google Scholar] [CrossRef]
- Toro, M.; Azcón, R.; Barea, J.M. The use of isotopic dilution techniques to evaluate the interactive effects of Rhizobium genotype, mycorrhizal fungi, phosphate-solubilizing rhizobacteria and rock phosphate on nitrogen and phosphorus acquisition by Medicago sativa. New Phytol. 1998, 138, 265–273. [Google Scholar] [CrossRef]
- Johnson, N.C.; Wilson, G.W.T.; Wilson, J.A.; Miller, R.M.; Bowker, M.A. Mycorrhizal phenotypes and the law of the minimum. New Phytol. 2015, 205, 1473–1484. [Google Scholar] [CrossRef] [PubMed]
- Konvalinková, T.; Püschel, D.; Řezáčová, V.; Gryndlerová, H.; Jansa, J. Carbon flow from plant to arbuscular mycorrhizal fungi is reduced under phosphorus fertilization. Plant Soil 2017, 419, 319–333. [Google Scholar] [CrossRef]
- Takács, T.; Cseresnyés, I.; Kovács, R.; Parádi, I.; Kelemen, B.; Szili-Kovács, T.; Füzy, A. Symbiotic effectivity of dual and tripartite associations on soybean (Glycine max L. Merr.) cultivars inoculated with Bradyrhizobium japonicum and AM Fungi. Front. Plant Sci. 2018, 9, 1631. [Google Scholar] [CrossRef]
- Antunes, P.M.; Deaville, D.; Goss, M.J. Effect of two AMF life strategies on the tripartite symbiosis with Bradyrhizobium japonicum and soybean. Mycorrhiza 2006, 16, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.; Cuevas, F. The effect of inoculating with arbuscular mycorrhiza and Brydyrhizobium strains on soybean (Glycine max (L) Merrill) crop development. Cultivos Tropic. 2003, 24, 19–21. [Google Scholar]
- Sheteiwy, M.S.; Ali, D.F.I.; Xiong, Y.-C.; Brestic, M.; Skalicky, M.; Hamoud, Y.A.; Ulhassan, Z.; Shaghaleh, H.; AbdElgawad, H.; Farooq, M.; et al. Physiological and biochemical responses of soybean plants inoculated with arbuscular mycorrhizal fungi and Bradyrhizobium under drought stress. BMC Plant Biol. 2021, 21, 195. [Google Scholar] [CrossRef]
- Oliveira, T.C.; Cabral JS, R.; Santana, L.R.; Tavares, G.G.; Santos LD, S.; Paim, T.P.; Müller, C.; Silva, F.G.; Costa, A.C.; Souchie, E.L.; et al. The arbuscular mycorrhizal fungus Rhizophagus clarus improves phasiological tolerance to drought stress in soybean plants. Sci. Rep. 2022, 12, 9044. [Google Scholar] [CrossRef]
- Mehlich, A. Mehlich 3 soil test extractant: A modification of mehlich-2 extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Kandeler, E. Aggregate stability. In Methods in Soil Biology; Schiner, F., Öhlinger, R., Kandeler, E., Eds.; Springer: Berlin, Germany, 2016. [Google Scholar]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A new method which gives an objective-measure of colonization of roots by vesicular arbuscular mycorrhizal fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Koske, R.E.; Gemma, J.N. A modified procedure for staining roots to detect VA-mycorrhizas. Mycol. Res. 1989, 92, 486–505. [Google Scholar] [CrossRef]
- Řezáčová, V.; Czakó, A.; Stehlík, M.; Mayerová, M.; Šimon, T.; Smatanová, M.; Madaras, M. Organic fertilization improves soil aggregation through increases in abundance of eubacteria and products of arbuscular mycorrhizal fungi. Sci. Rep. 2021, 11, 12548. [Google Scholar] [CrossRef]
- Řezáčová, V.; Řezáč, M.; Líblová, Z.; Michalová, T.; Heneberg, P. Stable colonization of native plants and early invaders by arbuscular mycorrhizal fungi after exposure to recent invaders from the Asteraceae family. IPSM 2021, 14, 147–155. [Google Scholar] [CrossRef]
- Simon, L.M.; Lalonde, T.D.; Bruns, T.D. Specific amplification of 18S fungal ribosomal genes from vesicular arbuscular endomycorrhizal fungi colonizing roots. Appl. Environ. Microbiol. 1992, 58, 291–295. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Young, J.P.W. Improved PCR primers for the detection and identification of arbuscular mycorrhizal fungi. FEMS Microbiol. Ecol. 2008, 65, 339–349. [Google Scholar] [CrossRef]
- Wright, S.; Upadhyaya, A. Extraction of an abundant and unusual protein from soil and comparison with hyphal protein of arbuscular mycorrhizal fungi. Soil Sci. 1996, 161, 575–586. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and senstive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 9 September 2022).
- Buil, P.A.; Jansa, J.; Blažková, A.; Holubík, O.; Duffková, R.; Rozmoš, M.; Püschel, D.; Kotianová, M.; Janoušková, M. Plant soil Infectivity and symbiotic efficiency of native arbuscular mycorrhizal fungi from high-input arable soils. Plant Soil. 2022, 482, 627–645. [Google Scholar] [CrossRef]
- Gitonga, N.M.; Koskey, G.; Njeru, E.M.; Maingi, J.M.; Cheruiyot, R. Dual inoculation of soybean with Rhizophagus irregularis and commercial Bradyrhizobium japonicum increases nitrogen fixation and growth in organic and conventional soils. AIMS Agri. Food 2021, 6, 478–495. [Google Scholar]
- Kohler, J.; Roldán, A.; Campoy, M.; Caravaca, F. Unraveling the role of hyphal networks from arbuscular mycorrhizal fungi in aggregate stabilization of semiarid soils with different textures and carbonate contents. Plant Soil 2017, 410, 273–281. [Google Scholar] [CrossRef]
- Lehmann, A.; Zheng, W.S.; Rillig, M.C. Soil biota contributions to soil aggregation. Nat. Ecol. Evol. 2017, 1, 1828–1835. [Google Scholar] [CrossRef] [PubMed]
- Malekian, B.; Parsa, M.; Vessal, S.; Khorassani, R. Split Application of Nitrogen Fertilizer and Inoculation with Arbuscular Mycorrhiza and Rhizobium ciceri Improve Grain Quality of Chickpea. Crop Forage Turfgrass Manag. 2019, 5, 190048. [Google Scholar] [CrossRef]
- Řezáčová, V.; Konvalinková, T.; Hujslová, M.; Gryndlerová, H.; Gryndler, M.; Püschel, D.; Jansa, J. Imbalanced carbon-for-phosphorus exchange between European arbuscular mycorrhizal fungi and non-native Panicum grasses—A case of dysfunctional symbiosis. Pedobiologia 2017, 62, 48–55. [Google Scholar] [CrossRef]
- Glyan’ko, A.K.; Vasil’eva, G.G.; Mitanova, N.B.; Ishchenko, A.A. The influence of mineral nitrogen on legume-rhizobium symbiosis. Biol. Bull. 2009, 36, 250–258. [Google Scholar] [CrossRef]
- Lucinski, R.; Polcyn, W.; Ratajczak, L. Nitrate reduction and nitrogen fixation in symbiotic association Rhizobium—legumes. Acta Biochim. Pol. 2002, 49, 537–546. [Google Scholar] [CrossRef]
- Morgan, J.A.W.; Bending, G.D.; White, P.J. Biological costs and benefits to plant-microbe interactions in the rhizosphere. J. Exp. Bot. 2005, 56, 1729–1739. [Google Scholar] [CrossRef]
- Mortimer, P.E.; Pérez-Fernández, M.A.; Valentine, A.J. The role of arbuscular mycorrhizal colonization in the carbon and nutrient economy of the tripartite symbiosis with nodulated Phaseolus vulgaris. Soil Biol. Biochem. 2008, 40, 1019–1027. [Google Scholar] [CrossRef]
- Alami, Y.; Achouak, W.; Marol, C.; Heulin, T. Rhizosphere soil aggregation and plant growth promotion of sunflowers by an exopolysaccharide-producing Rhizobium sp. strain isolated from sunflower roots. Appl. Environ. Microbiol. 2000, 66, 3393–3398. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, D.; Luo, C.B.; Zhang, F.; Zhang, C.M. Arbuscular mycorrhizal fungal species identity governs plant water content and soil aggregation improvements under wet-dry climate conditions. Environ. Sci. Pollut. Res. 2020, 27, 37377–37383. [Google Scholar] [CrossRef]
- Zhang, J.; Su, L.; Yan, K.; Li, M.; He, Y.; Zu, Y.; Zhan, F.; Li, T. An arbuscular mycorrhizal fungus increased the macroaggregate proportion and reduced cadmium leaching from polluted soil. Int. J. Phytoremed. 2020, 23, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Heydari, L.; Bayat, H.; Gregory, A.S. Investigating the effect of inoculation of chickpea with rhizobium and mycorrhizal fungi (Funneliformis mosseae) on soil mechanical and physical behavior. Geoderma 2021, 385, 114860. [Google Scholar] [CrossRef]
- Rillig, M.C.; Maestre, F.T.; Lamit, L.J. Microsite differences in fungal hyphal length, glomalin, and soil aggregate stability in semiarid Mediterranean steppes. Soil Biol. Biochem. 2003, 35, 1257–1260. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, A.; Wang, F.; Han, X.; Wang, D.; Li, S. Arbuscular mycorrhizal fungi and rhizobium facilitate nitrogen uptake and transfer in soybean/maize intercropping system. Front. Plant Sci. 2015, 6, 339. [Google Scholar] [CrossRef]
- Salloum, M.S.; Guzzo, M.C.; Valazquez, M.B.; Sagadin, M.B.; Luna, C.M. Variability in colonization of arbuscular mycorrhizal fungi and its effect on mycorrhizal dependency of improved and unimproved soybean cultivars. Can. J. Microbiol. 2016, 62, 1034–1040. [Google Scholar] [CrossRef]
- Rao, A.V. Root Exudation in Relation to Inoculation with Rhizobia. Zentralbl Bakteriol Parasitenkd Infekt. Hyg. 1976, 131, 79–82. [Google Scholar] [CrossRef]



| AMF Inoculation | Rhizobial Inoculation | AMF × Rhizobial Inoculation | ||||
|---|---|---|---|---|---|---|
| Parameter | F | p | F | p | F | p |
| Shoot biomass | 14.4 | 3 × 10−3 | 2.9 | 0.11 | 0.0 | 0.96 |
| N concentration | 13.2 | 0.3 × 10−2 | 0.6 | 0.56 | 0.6 | 0.59 |
| P concentration | 14.9 | 0.2 × 10−2 | 1.6 | 0.23 | 0.6 | 0.44 |
| SAS | 0.8 | 0.38 | 5.5 | 0.03 | 3.0 | 0.10 |
| Total glomalin | 1.4 | 0.26 | 1.3 | 0.28 | 1.6 | 0.23 |
| AMF colonization | - | - | 7.6 | 0.03 | - | - |
| AMF gene copies in roots | - | - | 13.2 | 0.01 | - | - |
| AMF gene copies in soil | - | - | 0.0 | 0.94 | - | - |
| Number of nodules | 6.2 | 0.04 | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Řezáčová, V.; Némethová, E.; Stehlíková, I.; Czakó, A.; Gryndler, M. Arbuscular Mycorrhizal Fungus Funneliformis mosseae Improves Soybean Growth Even in Soils with Good Nutrition. Microbiol. Res. 2023, 14, 1252-1263. https://doi.org/10.3390/microbiolres14030084
Řezáčová V, Némethová E, Stehlíková I, Czakó A, Gryndler M. Arbuscular Mycorrhizal Fungus Funneliformis mosseae Improves Soybean Growth Even in Soils with Good Nutrition. Microbiology Research. 2023; 14(3):1252-1263. https://doi.org/10.3390/microbiolres14030084
Chicago/Turabian StyleŘezáčová, Veronika, Ema Némethová, Iva Stehlíková, Alena Czakó, and Milan Gryndler. 2023. "Arbuscular Mycorrhizal Fungus Funneliformis mosseae Improves Soybean Growth Even in Soils with Good Nutrition" Microbiology Research 14, no. 3: 1252-1263. https://doi.org/10.3390/microbiolres14030084
APA StyleŘezáčová, V., Némethová, E., Stehlíková, I., Czakó, A., & Gryndler, M. (2023). Arbuscular Mycorrhizal Fungus Funneliformis mosseae Improves Soybean Growth Even in Soils with Good Nutrition. Microbiology Research, 14(3), 1252-1263. https://doi.org/10.3390/microbiolres14030084





