Evaluation of Compounds from Balanites aegyptiaca against Squalene Epoxidase of Micropsorum gypseum—In Vitro and In Silico Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Species Collection and Identification
2.2. Purchasing Pathogen
2.3. Anti-Dermatophytic Assay
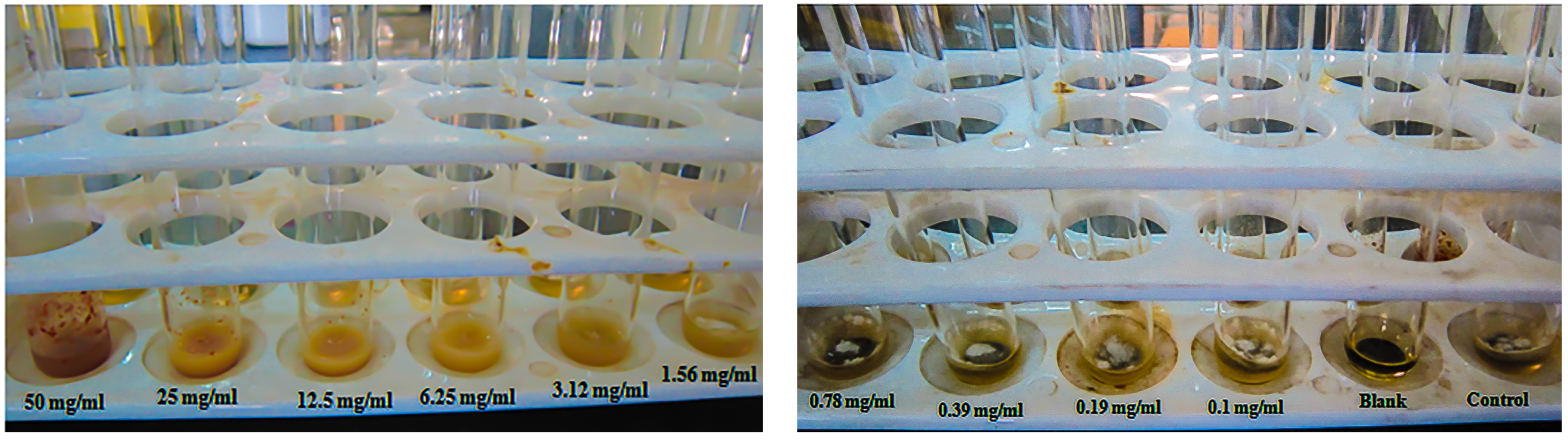
2.4. Minimum Inhibitory Concentration (MIC)
2.5. Phytochemical Screening
2.6. Compound Elucidation
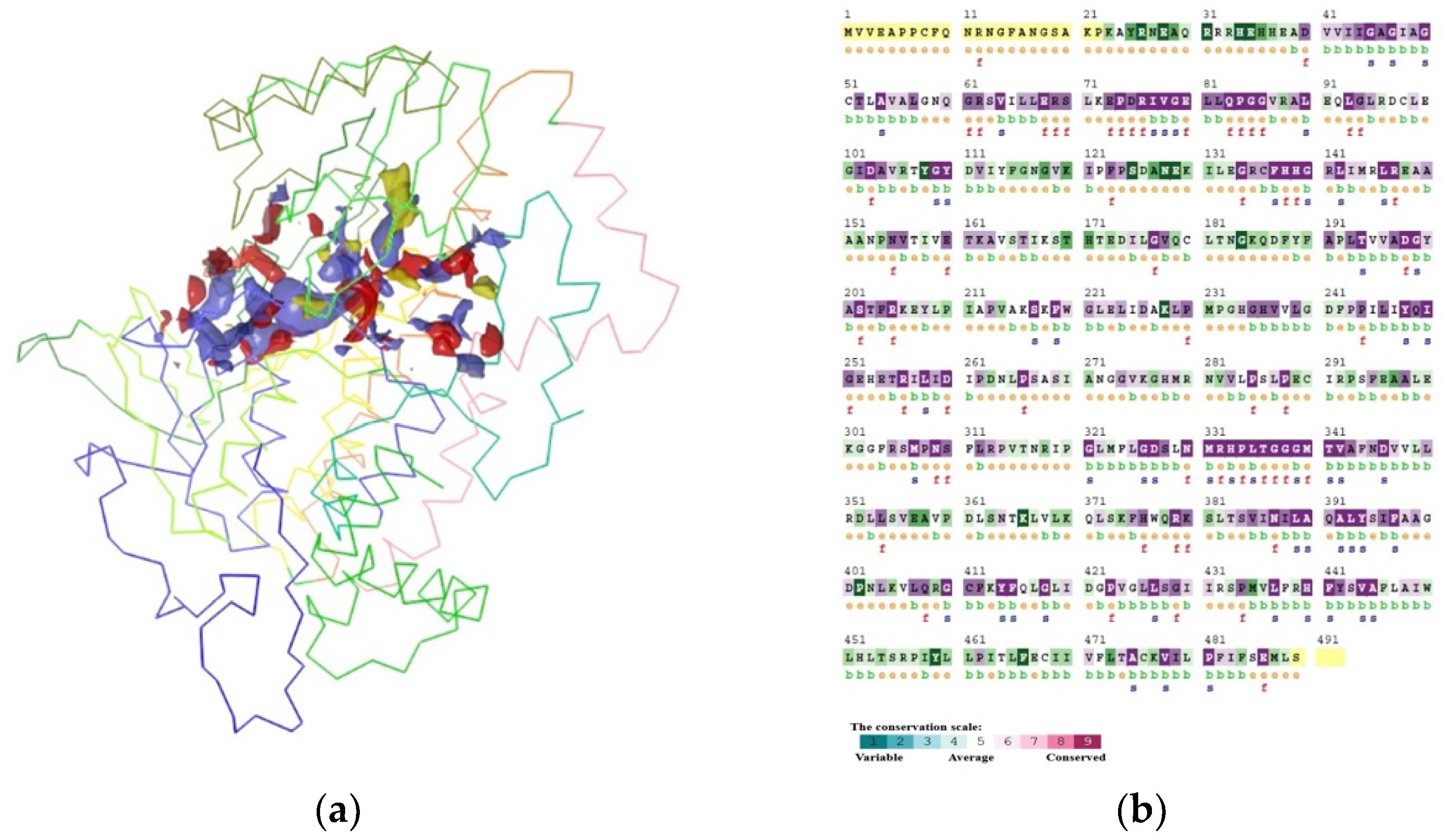
2.7. Modeling and Validation of Protein
2.8. Binding Site Prediction
2.9. Ligand Preparation
2.10. Protein–Ligand Docking
2.11. MM-GBSA Analysis
2.12. Molecular Dynamics Simulation
3. Results
3.1. Plant Verification
3.2. Anti-Dermatophytic Assay
3.3. Phytochemical, LC–MS, and ADMETox Analysis
3.4. Modeling and Confirmation
3.5. Binding Site Prediction
3.6. Molecular Docking
3.7. MMGB-SA Analysis
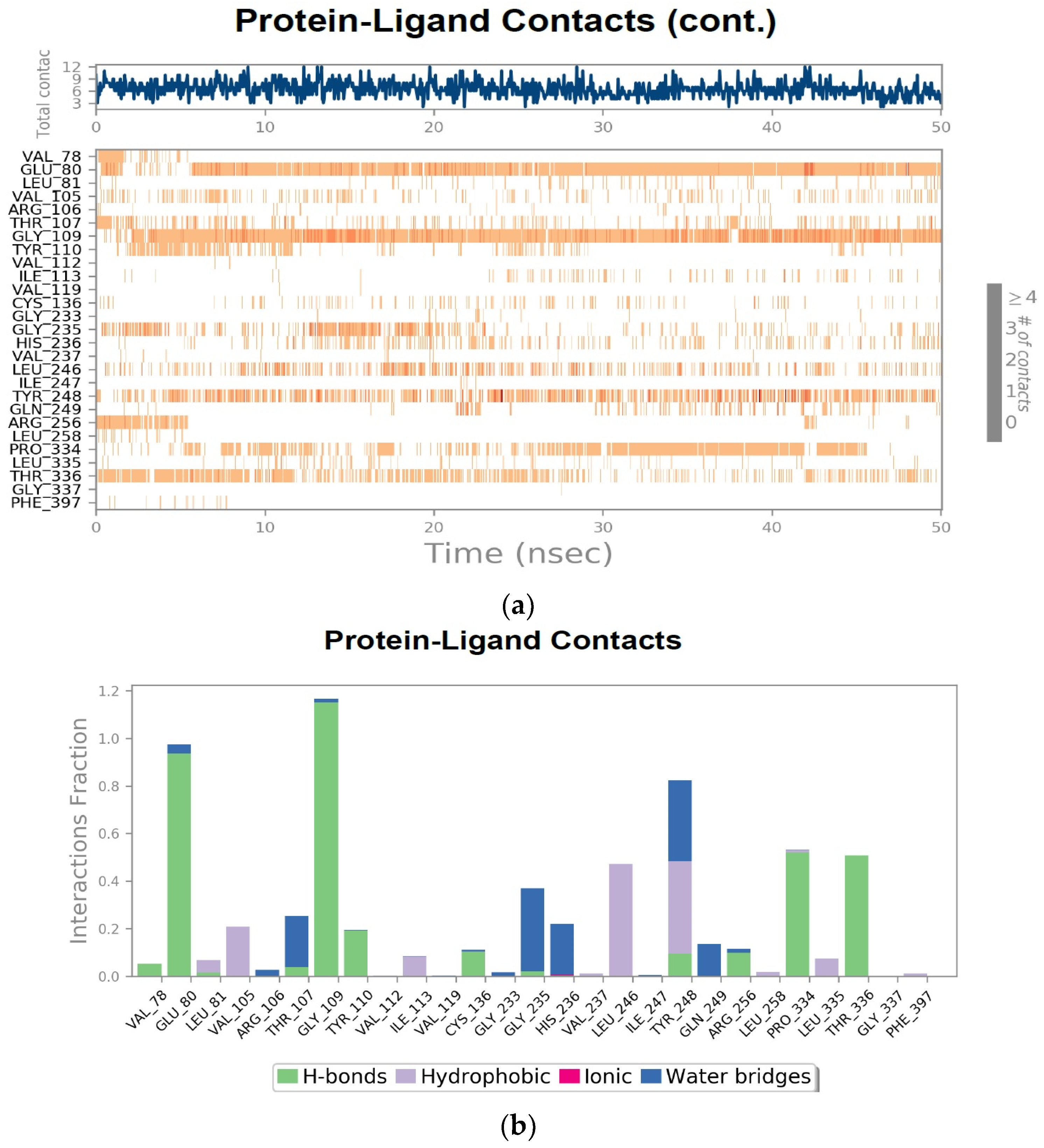
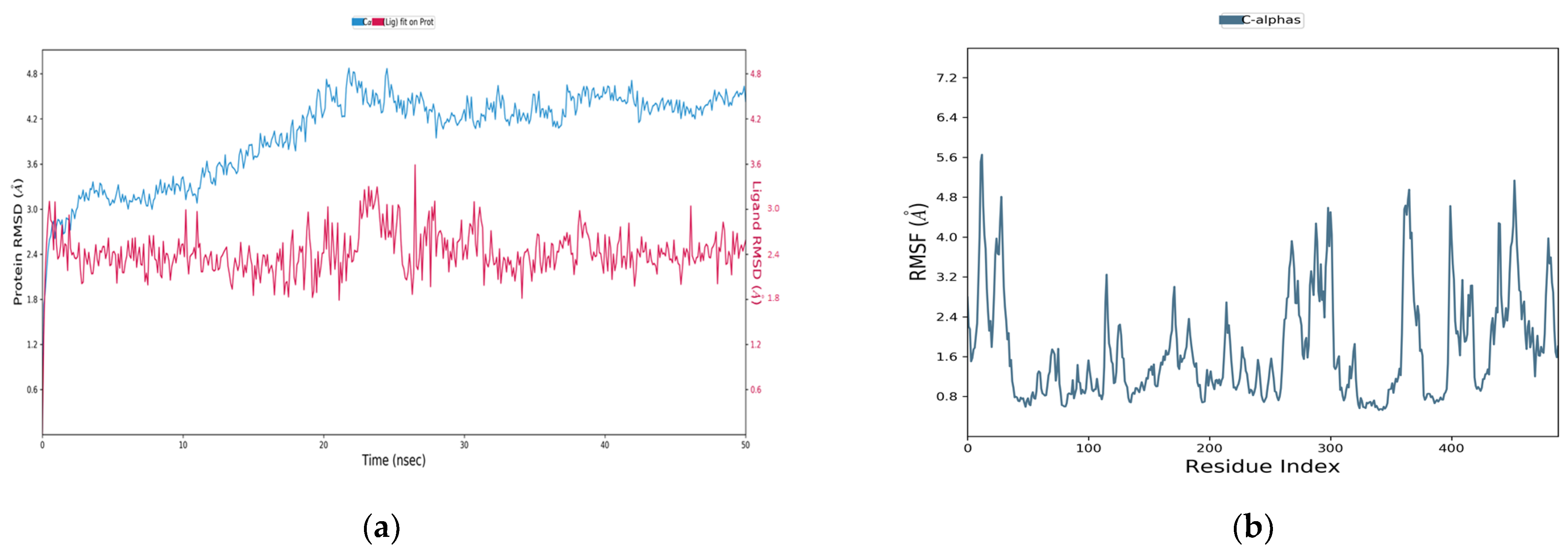
3.8. Molecular Dynamics Simulation
3.9. Squalene Epoxidase (SE) with Compound 56776227
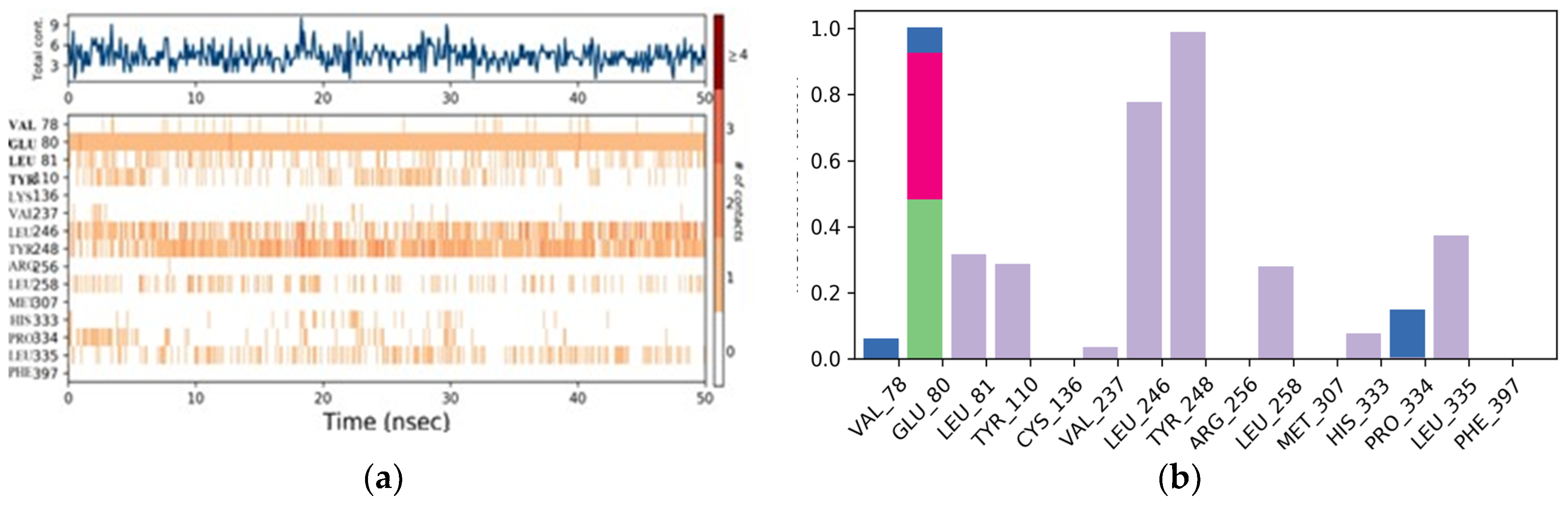
3.10. Naftifine with Squalene Epoxidase
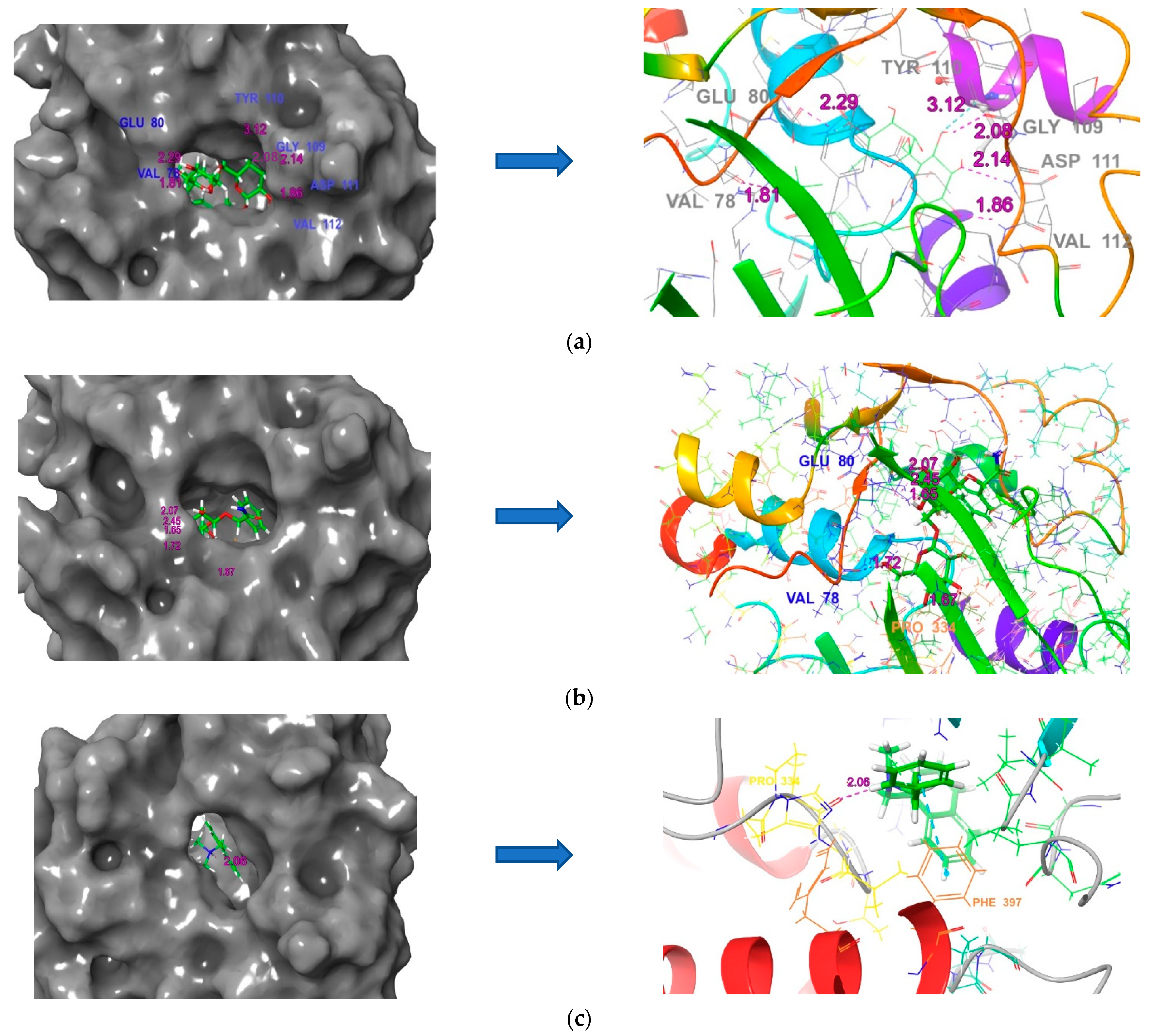
3.11. Protein–Ligand Interactions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moskaluk, A.E.; VandeWoude, S. Current Topics in Dermatophyte Classification and Clinical Diagnosis. Pathogens 2022, 11, 957. [Google Scholar] [CrossRef] [PubMed]
- Shaoo, A.K.; Mahajan, R. Management of tinea corporis, tine cruris and tinea pedis: A comprehensive review. Indian Dermatol. Online J. 2016, 7, 77–86. [Google Scholar]
- Ginter, G. Ecology, epidemiology and clinical symptomatology of infections due to Microsporum gypseum. Mycoses 2009, 32, 531–535. [Google Scholar] [CrossRef]
- Souza, L.K.H.; Oliveira, C.M.A.; Ferri, P.H.; Santos, S.C.; Oliveira Júnior, J.G.; Miranda, A.T.B.; Liao, L.M.; Silva, M.R.R. Antifungal properties of Brazilian cerrado plants. Braz. J. Microbiol. 2022, 33, 247–249. [Google Scholar] [CrossRef]
- Lee, W.J.; Park, J.H.; Kim, J.Y.; Jang, Y.H.; Lee, S.; Bang, Y.J.; Jun, J.B. Low but continuous occurrence of Microsporum gypseum infection in the study on 198 cases in South Korea from 1979 to 2016. Ann. Dermatol. 2018, 30, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Dolenc-Voljc, M.; Gasparic, J. Human Infections with Microsporum gypseum Complex (Nannizzia gypsea) in Slovenia. Mycopathologia 2017, 181, 1069–1075. [Google Scholar] [CrossRef]
- Chugh, A.; Ray, A.; Gupta, J.B. Squalene epoxidase as hypocholesterolmic drug target revisited. Prog. Lipid Res. 2003, 42, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Sahni, K.; Singh, S.; Dogra, S. Newer topical treatments in skin and nail dermatophytes infections. India Dermatol. Online J. 2018, 9, 149–158. [Google Scholar]
- Ghorbani, A.; Naghibi, F.; Mosaddegh, M. Ethnobotany, ethnopharmacology and drug discovery. Iran. J. Pharm. Sci. 2006, 2, 109–118. [Google Scholar]
- Al-Thobaiti, S.A.; Zeid, I.A. Medicinal properties of desert plants (Balanites aegyptiaca)—An overview. Glob. J. Pharmacol. 2018, 12, 1–12. [Google Scholar]
- Grover, R.K.; Moore, J.D. Toxicometric Studies of Fungicides against Brown Rot Organisms Sclerotinia fructicola and S. laxa. Phytopathology 1962, 52, 876–880. [Google Scholar]
- Maurya, V.K.; Kachhwaha, D.; Bora, A.; Khatri, P.K.; Rathore, L. Determination of antifungal minimum inhibitory concentration and its clinical correlation among treatment failure cases of dermatophytosis. J. Family Med. Prim. Care 2019, 8, 2577–2581. [Google Scholar] [PubMed]
- Rao, U.S.M.; Abdurrazak, M.; Mohd, K.S. Phytochemical screening, total flavonoid and phenolic content assays of various solvent extracts of tepal of Musa paradisiaca. Malays. J. Anal. Sci. 2016, 20, 1181–1190. [Google Scholar] [CrossRef]
- Oh, M.; Park, S.; Kim, H.; Choi, G.J.; Kim, S.H. Application of UPLC-QTOF-MS Based Untargeted Metabolomics in Identification of Metabolites Induced inPathogen-Infected Rice. Plants 2021, 10, 213. [Google Scholar] [CrossRef]
- Demarque, D.P.; Dusi, R.G.; Sousa, F.D.M.; Grossi, S.M.; Silverio, M.R.S.; Lopes, N.P.; Espindola, L.S. Mass spectrometry-based metabolomics approach in the isolation of bioactive natural products. Sci. Rep. 2020, 10, 1051. [Google Scholar] [CrossRef] [PubMed]
- Sheik, S.; Sundararajan, P.; Hussain, A.S.Z.; Sekar, K. Ramachandran plot on the web. Bioinformatics 2002, 18, 1548–1549. [Google Scholar] [CrossRef] [PubMed]
- Halgren, T. New method for fast and accurate binding-site identification and analysis. Chem. Biol. Drug Des. 2007, 69, 146–148. [Google Scholar] [CrossRef]
- Ashkenazy, H.; Abadi, S.; Martz, E.; Chay, O.; Mayrose, I.; Pupko, T.; Ben-Tal, N. ConSurf 2016: An improved methodology to estimate and visualize evolutionary conservation in macromolecules. Nucleic Acids Res. 2016, 44, W344–W350. [Google Scholar] [CrossRef]
- Zhang, Q.; Khetan, A.; Er, S. A quantitative evaluation of computational methods to accelerate the study of alloxazine-derived electroactive compounds for energy storage. Sci. Rep. 2021, 11, 4089. [Google Scholar] [CrossRef]
- Hage-Melim, L.I.S.; Federico, L.B.; Oliveria, N.K.S.; Francisco, V.C.C.; Correia, L.C.; Lima, H.B.; Gomes, S.Q.; Barcelos, M.P.; Francischini, I.A.G.; de Paula da Silva, C.H.T. Virtual screening, ADME/Tox predictions and the drug repurposing concept for future use of old drugs against the COVID-19. Life Sci. 2020, 256, 117963. [Google Scholar] [CrossRef]
- Bathula, R.; Muddagoni, N.; Lanka, G.; Dasari, M.; Potlapally, R. Glide Docking, Autodock, Binding Free Energy and Drug-Likeness Studies for Prediction of Potential Inhibitors of Cyclin-Dependent Kinase 14 Protein in Wnt Signaling Pathway. Biointerface Res. Appl. Chem. 2022, 12, 2473–2488. [Google Scholar]
- Ramirez, D.; Caballero, J. Is It Reliable to Use Common Molecular Docking Methods for Comparing the Binding Affinities of Enantiomer Pairs for Their Protein Target? Int. J. Mol. Sci. 2016, 17, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Arokia, A.M.V.; Anantha, K.D.; Hemalatha, M.; Krishnasamy, G.; Ernest, D. In silico studies towards enhancing the anticancer activity of phytochemical Phloretin against cancer drug targets. Curr. Drug Ther. 2018, 13, 174–188. [Google Scholar]
- Liang, D.; Chen, Q.; Guo, Y.; Zhang, T.; Guo, W. Insight into resistance mechanisms of AZD4547 and E3810 to FGFR1 gatekeeper mutation via theoretical study. Drug Des. Dev. Ther. 2017, 11, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Alturki, N.A.; Mashraqi, M.M.; Alzamami, A.; Alghamdi, Y.S.; Alharthi, A.A.; Asiri, S.A.; Ahmad, S.; Alshamrani, S. In-Silico Screening and Molecular Dynamics Simulation of Drug Bank Experimental Compounds against SARS-CoV-2. Molecules 2022, 27, 4391. [Google Scholar] [CrossRef]
- Aier, I.; Varadwaj, P.K.; Raj, U. Structural insights into conformational stability of both wild-type and mutant EZH2 receptor. Sci. Rep. 2016, 6, 34984. [Google Scholar] [CrossRef]
- Abuthakir, M.H.S.; Al-Dosary, M.A.; Hatamleh, A.A.; Alodaini, H.A.; Perumal, P.; Jeyam, M. Platyphylloside, a potential inhibitor from epicarp of B. aegyptiaca against CYP450 protein in T. rubrum—In Vitro and in silico approaches. Saudi J. Biol. Sci. 2022, 29, 3899–3910. [Google Scholar] [CrossRef]
- Al-Janabi, A.A.H.S. Dermatophytosis: Causes, clinical features, signs and treatment. J. Symptoms Signs 2014, 3, 200–203. [Google Scholar]
- Khatoon, R.; Jahan, N.; Ahamed, S.; Shahzad, A. In vitro evaluation of antifungal activity of aerial parts of medicinal plants Balanites aegyptiaca Del. and Spilanthes acmella Murr. J. Appl. Pharm. Sci. 2014, 4, 123–127. [Google Scholar]
- Hussain, S.A.M.; Velusamy, S.; Muthusamy, J. Balanites aegyptiaca (L.) Del. for dermatophytoses: Ascertaining the efficacy and mode of action through experimental and computational approaches. Inform. Med. Unlocked 2019, 15, 100177. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; Silva-Espinoza, B.A.; Cruz-Valenzuela, M.R.; Villegas-Ochoa, M.A.; Esqueda, M.; González-Aguilar, G.A.; Calderón-López, Y. Antioxidant and antifungal potential of methanol extracts of Phellinus spp. from Sonora, Mexico. Rev. Iberoam. De Micol. 2012, 29, 132–138. [Google Scholar] [CrossRef]
- Dos Santos, E.C.F.; da Silva, W.A.V.; Machado, J.C.B.; Ferreira, M.R.A.; Soares, L.A.L. Evaluation of the Antioxidant and Antifungal Actions of the Optimized Crude Extract and Fractions from the Aerial Parts of Acanthospermum hispidum. Biopharm. Drug Dispos. 2022, 20, e202200905. [Google Scholar]
- Halat, D.H.; Younes, S.; Mourad, N.; Rahal, M. Allylamines, Benzylamines, and Fungal cell permeability: A review of mechanistic effects and usefulness against fungal pathogens. Membranes 2022, 12, 1171. [Google Scholar] [CrossRef] [PubMed]
- Nowosielski, M.; Hoffmann, M.; Wyrwicz, L.S.; Stepniak, P.; Plewczynski, D.M.; Lazniewski, M.; Ginalski, K.; Rychlewski, L. Detailed mechanism of squalene epoxidase inhibition by terbinafine. J. Chem. Infect. Model 2011, 51, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Maeda, M.; Alshahni, M.M.; Tanaka, R.; Yaguchi, T.; Bontems, O.; Salamin, K.; Fratti, M.; Monod, M. Terbinafine Resistance of Trichophyton Clinical Isolates Caused by Specific Point Mutations in the Squalene Epoxidase Gene. Antimicrob. Agents Chemother. 2017, 61, e00115-17. [Google Scholar] [CrossRef]
- Bournonville, C.G.; Filippone, M.P.; Peto, P.d.L.D.; Trejo, M.F.; Couto, A.S.; de Marchese, A.M.; Ricci, J.C.D.; Welin, B.; Castagnaro, A.P. Strawberry fatty acyl glycosides enhance disease protection, have antibiotic activity and stimulate plant growth. Sci. Rep. 2020, 10, 8196. [Google Scholar] [CrossRef] [PubMed]
- Kawsar, S.M.A.; Almalki, F.A.; Ben Hadd, T.; Laaroussi, H.; Khan, M.A.R.; Hosen, M.A.; Mahmud, S.; Aounti, A.; Maideen, N.M.P.; Heidarizadeh, F.; et al. Potential antifungal activity of novel carbohydrate derivatives validated by POM, molecular docking and molecular dynamic simulations analyses. Mol. Simul. 2022, 48, 60–75. [Google Scholar] [CrossRef]
- Novakovic, M.M.; Novakovic, I.; Cvetkovic, M.; Tesevic, V. Antimicrobial activity of the diarylheptanoids from the black and green alder. Braz. J. Bot. 2015, 38, 441–446. [Google Scholar] [CrossRef]
- Jorda, T.; Puig, S. Regulation of Ergosterol Biosynthesis in Saccharomyces cerevisiae. Genes 2020, 11, 795. [Google Scholar] [CrossRef]
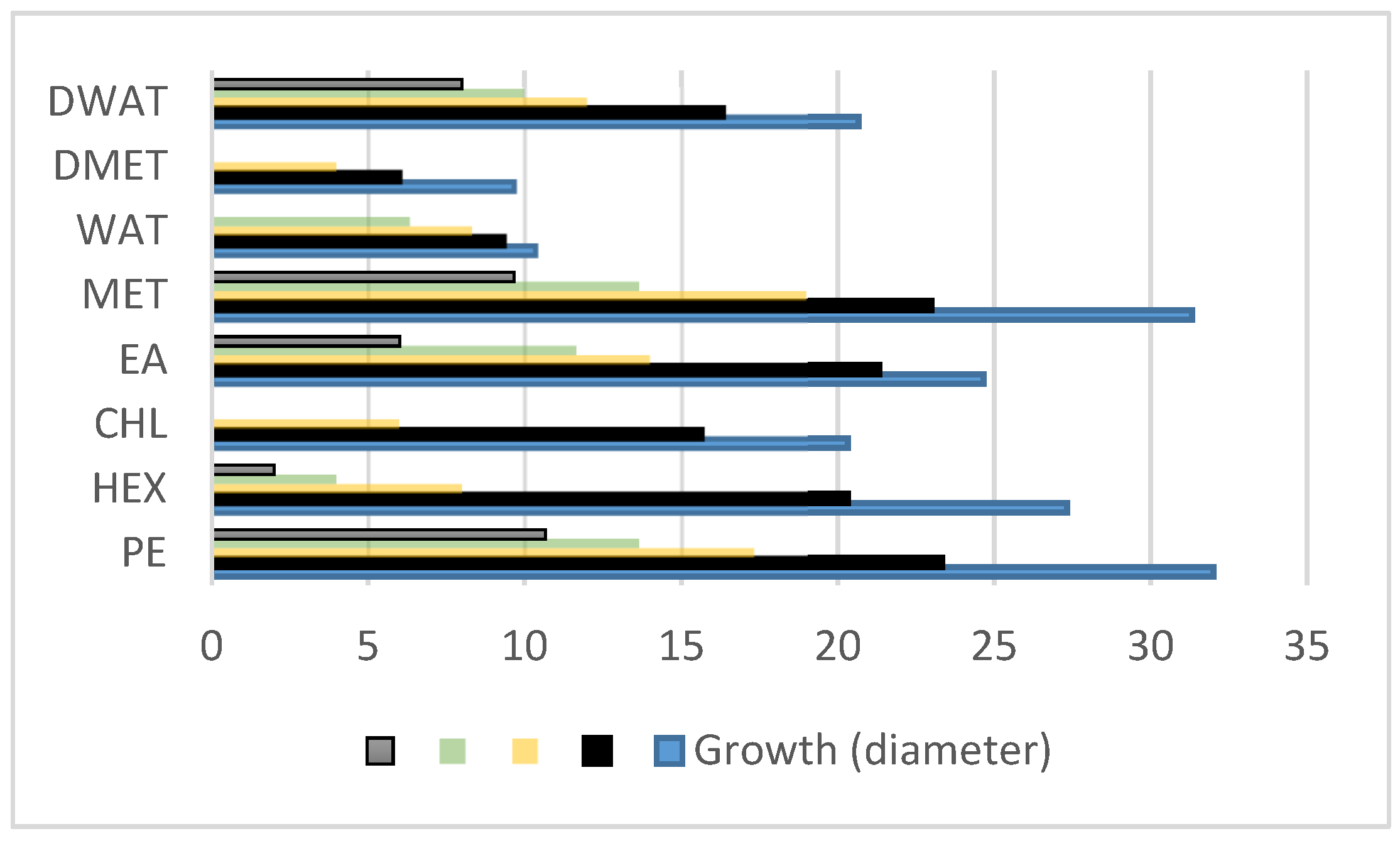
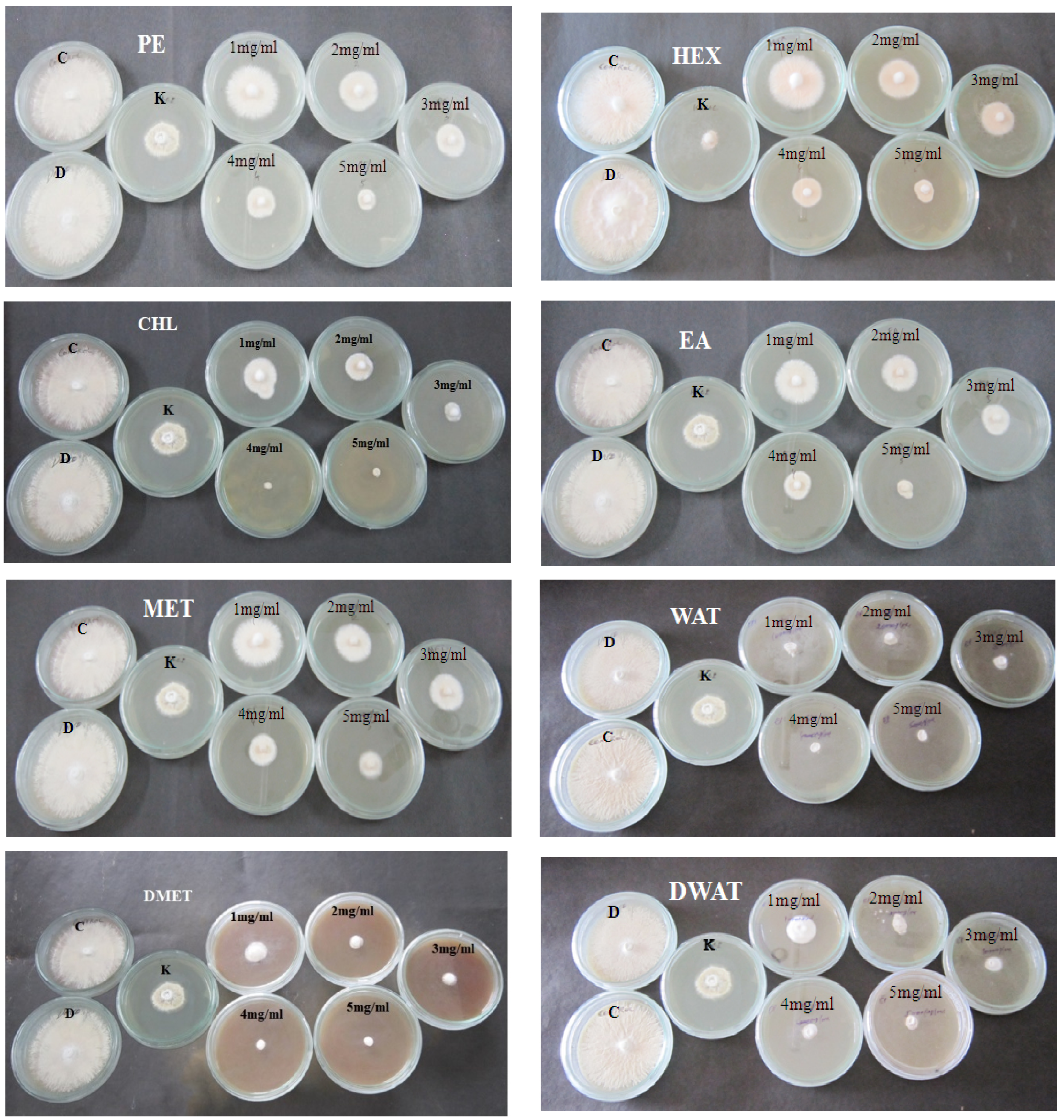



| Extracts | PE | HEX | CHL | EA | MET | WAT | DMET | DWAT | |
|---|---|---|---|---|---|---|---|---|---|
| Growth (diameter) | 1 mg/mL | 32 | 27.33 | 20.33 | 24.66 | 31.33 | 10.33 | 9.66 | 20.66 |
| 2 mg/mL | 23.33 | 20.33 | 15.66 | 21.33 | 23 | 9.33 | 6 | 16.33 | |
| 3 mg/mL | 17.33 | 8 | 6 | 14 | 19 | 8.33 | 4 | 12 | |
| 4 mg/mL | 13.66 | 4 | - | 11.66 | 13.66 | 6.33 | - | 10 | |
| 5 mg/mL | 10.66 | 2 | - | 6 | 9.66 | - | - | 8 | |
| Compound No. | Compound Name | Docking Score (kcal/mol) | Interacting Residues | Bond Length (Å) |
|---|---|---|---|---|
| 1. | (3E)-7-Hydroxy-3,7-dimethyl-3-octen-1-yl 6-O-(6-deoxy-alpha-L-mannopyranosyl)-beta-D-glucopyranoside | −10.1 | Val 78, Glu 80, Gly 109, Tyr 110, Asp 111, Val 112 | 1.81, 2.29, 2.08, 3.12, 2.14, 1.86 |
| 2. | 2-phenyl-2-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-[[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxymethyl]oxan-2-yl]oxyacetamide | −9.8 | Val 78, Glu 80(3), Pro 334 | 1.72, 1.65, 2.07, 2.45, 1.67 |
| 3. | Platyphylloside | −9.2 | Glu 80, Pro 224, Thr 336, Met 340, Phe 397 | 2.09, 2.56, 2.61, 2.05, 4.98 |
| 5. | Forchlorfenuron | −8.8 | Glu 80, Arg 256, Pro 340(2), Phe 397 | 2.57, 5.35, 1.89, 2.12, 5.01 |
| 6. | 1,3,6-trihydroxy-5-methoxy-2-[(2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]xanthen-9-one | −8.5 | Val 78, Glu 80, Leu 81, Thr 336 | 2.04, 2.07, 2.53, 2.17 |
| 7. | Thelephoric acid | −7.9 | Val 78(2), Arg 256 | 1.83, 2.23, 1.92 |
| 8. | 4-[(2-{[(2-Ethyl-2,3-dihydroxybutanoyl)oxy]methyl}phenyl)amino]-4-oxobutanoic acid | −7.7 | Val 78, Val 112, Gly 235, Pro 334, Thr 336 | 1.85, 1.91, 1.95, 1.82, 2.71 |
| 9. | N-Acetyl-D-Galactosamine | −7.6 | Glu 80, Pro 334, Thr 336, Gly 337 | 2.60, 2.08, 2.18, 2.57 |
| 10. | N~5~-Carbamoyl-N~2~-(phenylacetyl)ornithine | −7.4 | Gly 109, Gly 235, Pro 334, Gly 337, Phe 397 | 2.04, 2.35, 2.11, 1.97, 4.90 |
| 11. | N-[2-(4-sec-Butyl-phenoxy)-4,5-dihydroxy-6-hydroxymethyl-tetrahydro-pyran-3-yl]-acetamide | −7.3 | Glu 80(2), Arg 256 | 2.15, 2.24, 2.12 |
| 12. | 2-[5-[2-[2-[5-(2-oxopropyl)oxolan-2-yl]propanoyloxy]butyl]oxolan-2-yl]propanoic acid | −7.2 | Thr 336, Gly 337, Gly 339, Met 340 | 2.04, 2.76, 1.70, 2.11 |
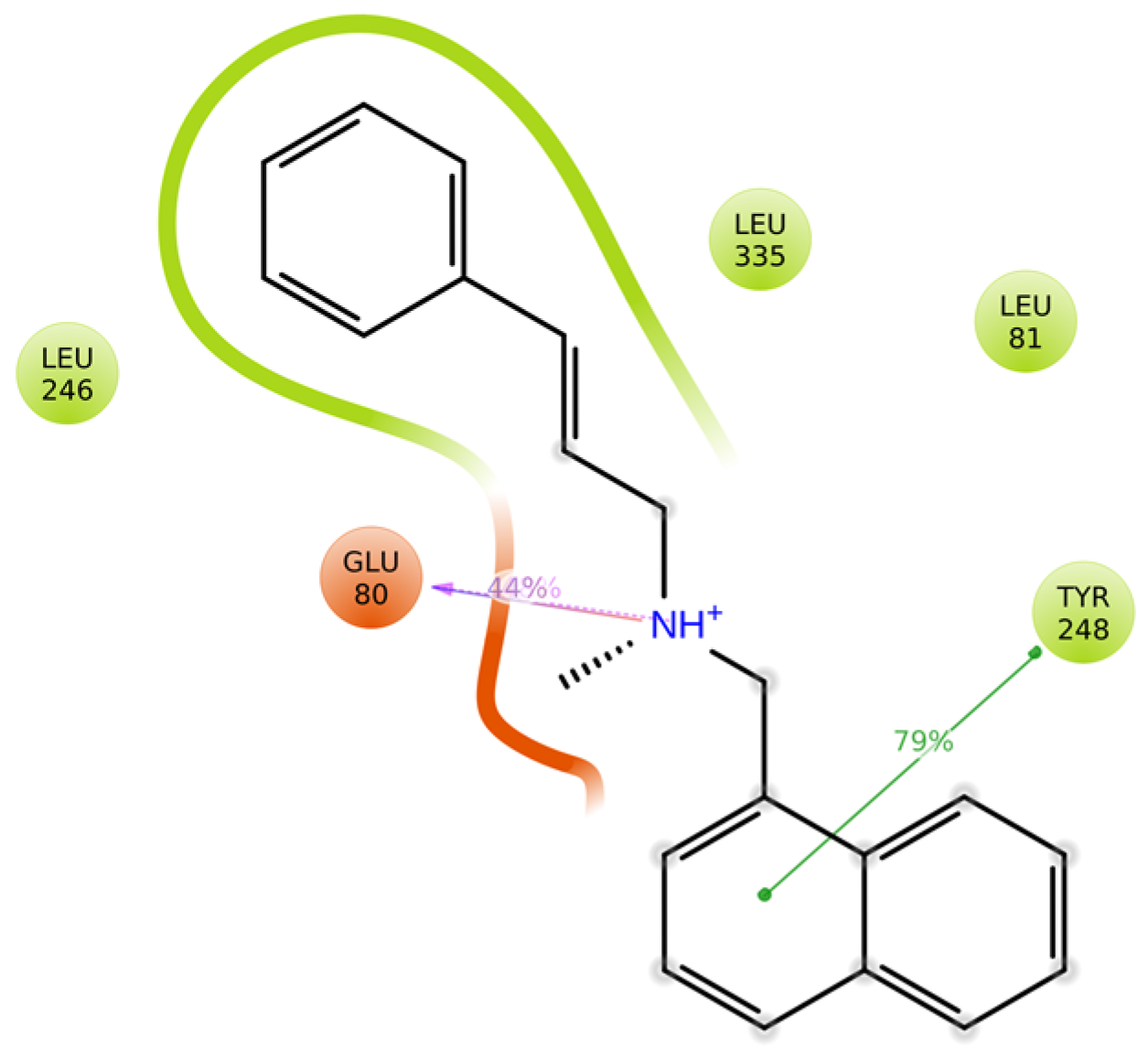
| 13. | Naftifine | −7.1 | Pro 334, Phe 397 | 2.06, 4.88 |
| 14. | 3-[(E)-2-(3-Hydroxyphenyl)ethenyl]-5-methoxyphenol | −7.1 | Leu 258, Phe 397 | 1.64, 5.03 |
| 15. | N-Acetylneuraminate | −6.9 | Val 78, Arg 305, Pro 334(2), Thr 336 | 1.95, 4.05, 1.81, 2.04, 2.18 |
| 16. | Deoxycytidine | −6.4 | Glu 80, Leu 81, Arg 256, Met 340 | 1.92, 2.28, 4.72, 2.64 |
| 17. | 5-hydroxy-7-[4-hydroxy-2-methoxy-3-(3-methylbut-2-enyl)phenyl]-2,2-dimethyl-7,8-dihydropyrano[3,2-g]chromen-6-one | −6.4 | Gly 337 | 2.09 |
| 18. | Adenosine | −6.3 | Glu 80, Arg 256, Pro 334 | 2.52, 6.44, 1.98 |
| 19. | N-Acetyl Phenylalanine | −6.2 | Pro 334, Thr 336, Phe 397 | 2.04, 2.63, 5.00 |
| 20. | Citreorosein | −6.0 | Glu 80, Gly 109, His 236 | 2.09, 2.29, 2.30 |
| 21. | 2-hydroxy-4-methoxy-3-(3-methylbut-2-enyl)-6-(2-phenylethyl)benzoic acid | −6.0 | Thr 336, Gly 337, Phe 397 | 2.08, 2.70, 4.84 |
| 22. | Cortisol | −5.7 | Val 78, Glu 223 | 2.14, 1.83 |
| 23. | Caffeate | −5.5 | Pro 244, Thr 336, Phe 397 | 2.75, 2.18, 4.86 |
| 24. | N-methylaurotetanine | −5.1 | Pro 334 | 1.62 |
| 25. | Phenylalanine | −5.0 | Pro 334, Phe 397 | 2.19, 4.92 |
| 26. | Pulvinic acid | −5.0 | Arg 256, Thr 336, Gly 337 | 2.01, 2.28, 2.31 |
| 27. | 5-Chlorodivaricatinic acid | −4.9 | Val 78, Glu 80, Arg 256 | 2.07, 2.12, 3.53 |
| 28. | Glucosaminate | −4.8 | Tyr 200, Asp 327, Gly 339, Met 340 | 2.01, 1.75, 2.49, 2.13 |
| 29. | 7-methoxy-9,10-dihydrophenanthrene-2,5-diol | −4.8 | Pro 334 | 2.05 |
| 30. | p-Coumaric acid | −4.2 | Pro 244, Thr 336, Phe 397 | 2.67, 2.33, 4.89 |
| 31. | Paraxanthine | −4.0 | Glu 80, Arg 256, Pro 334 | 1.90, 6.34, 2.17 |
| 32. | Lycorine | −4.0 | Glu 80, Gly 109, Gly 235, Tyr 248 | 4.58, 2.32, 1.98, 2.25 |
| 33. | Theobromine | −3.9 | Glu 80, Leu 81, Met 340 | 2.21, 2.38, 2.21 |
| 34. | (4S,4aR,7aS,9aR)-4,6,6-trimethyl-3-oxo-1,3,4,5,6,7,7a,9a-octahydropentaleno[1,6a-c]pyran-9-carboxylic acid | −3.4 | Val 112 | 2.09 |
| 35. | Norepanorin | −3.4 | Glu 80, Val 112, Thr 336 | 2.46, 2.16, 2.06 |
| 36. | Allocryptopine | −3.0 | Thr 336 | 1.82 |
| 37. | Hexadecanoic acid | −2.9 | Gly 339, Met 340 | 2.04, 2.06 |
| 38. | Minoxidil | −2.3 | Tyr 248 | 5.33 |
| 39. | Tetradecanoic acid | −2.1 | Gly 339, Met 340 | 2.70, 1.84 |
| 40. | 11-eicosenoic acid | −2.0 | Asp 111, Val 112 | 2.59, 1.78 |
| S.No | Compound | MMGBSA dG Bind |
|---|---|---|
| 1. | 567776227 | −51.92 |
| 2. | 11248520 | −38.08 |
| 3. | 9826264 | −47.03 |
| 4. | Naftifine | −40.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abuthakir, M.H.S.; Hemamalini, V.; Alahmadi, R.M.; Ahamed, A.; Hatamleh, A.A.; Abdullah, R.; Muthusamy, J. Evaluation of Compounds from Balanites aegyptiaca against Squalene Epoxidase of Micropsorum gypseum—In Vitro and In Silico Studies. Microbiol. Res. 2023, 14, 1264-1278. https://doi.org/10.3390/microbiolres14030085
Abuthakir MHS, Hemamalini V, Alahmadi RM, Ahamed A, Hatamleh AA, Abdullah R, Muthusamy J. Evaluation of Compounds from Balanites aegyptiaca against Squalene Epoxidase of Micropsorum gypseum—In Vitro and In Silico Studies. Microbiology Research. 2023; 14(3):1264-1278. https://doi.org/10.3390/microbiolres14030085
Chicago/Turabian StyleAbuthakir, Mohamed Husain Syed, V. Hemamalini, Reham M. Alahmadi, Anis Ahamed, Ashraf Atef Hatamleh, Razack Abdullah, and Jeyam Muthusamy. 2023. "Evaluation of Compounds from Balanites aegyptiaca against Squalene Epoxidase of Micropsorum gypseum—In Vitro and In Silico Studies" Microbiology Research 14, no. 3: 1264-1278. https://doi.org/10.3390/microbiolres14030085
APA StyleAbuthakir, M. H. S., Hemamalini, V., Alahmadi, R. M., Ahamed, A., Hatamleh, A. A., Abdullah, R., & Muthusamy, J. (2023). Evaluation of Compounds from Balanites aegyptiaca against Squalene Epoxidase of Micropsorum gypseum—In Vitro and In Silico Studies. Microbiology Research, 14(3), 1264-1278. https://doi.org/10.3390/microbiolres14030085






