Evaluation of Lacc134 Oxidoreductase of Ganoderma multistipitatum in Detoxification of Dye Wastewater under Different Nutritional Conditions
Abstract
1. Introduction
2. Materials and Methods
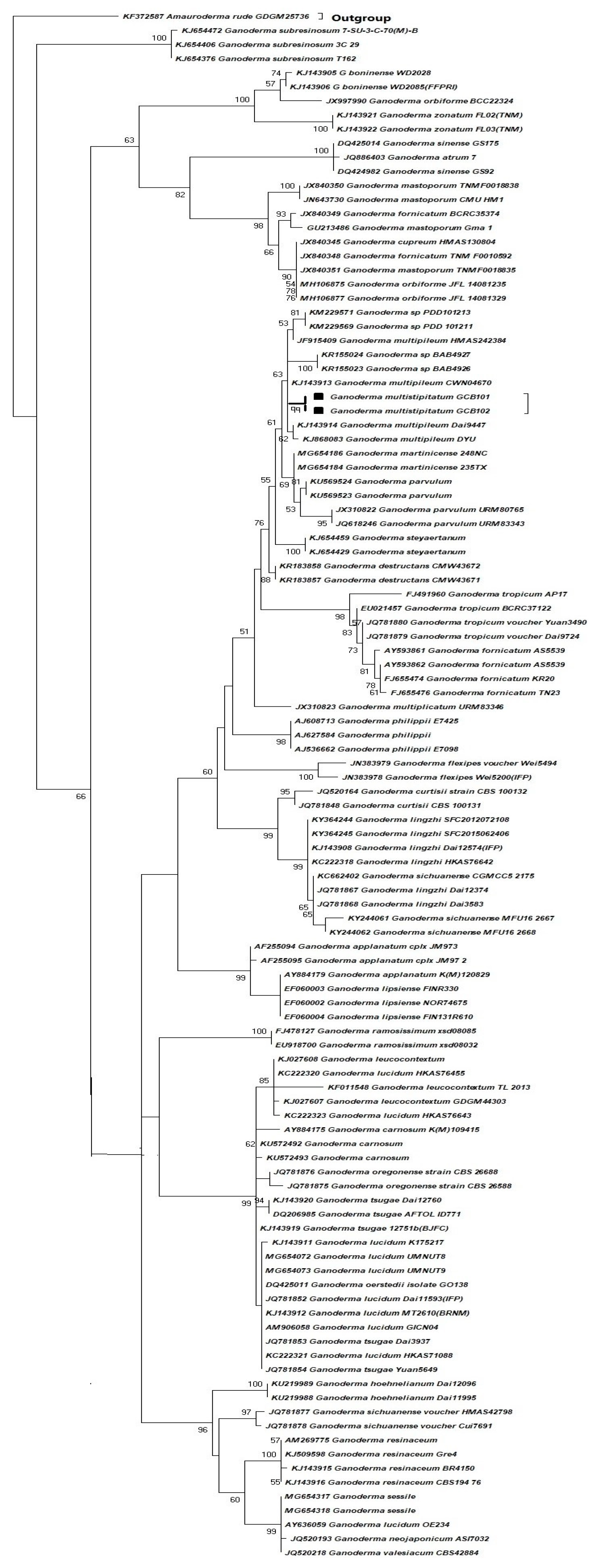
2.1. Species Collection, DNA Extraction, Sequence Alignment, and Molecular Phylogeny
2.2. Qualitative Laccase Analysis
2.3. Quantitative Laccase Analysis
- € = 6740 M−1 cm−1 extinction coefficient of guaiacol
- Vt = Total vol. of the reaction mixture (mL)
- Vs = Vol. of the sample (mL)
- l = Length of the cuvette (1 cm)
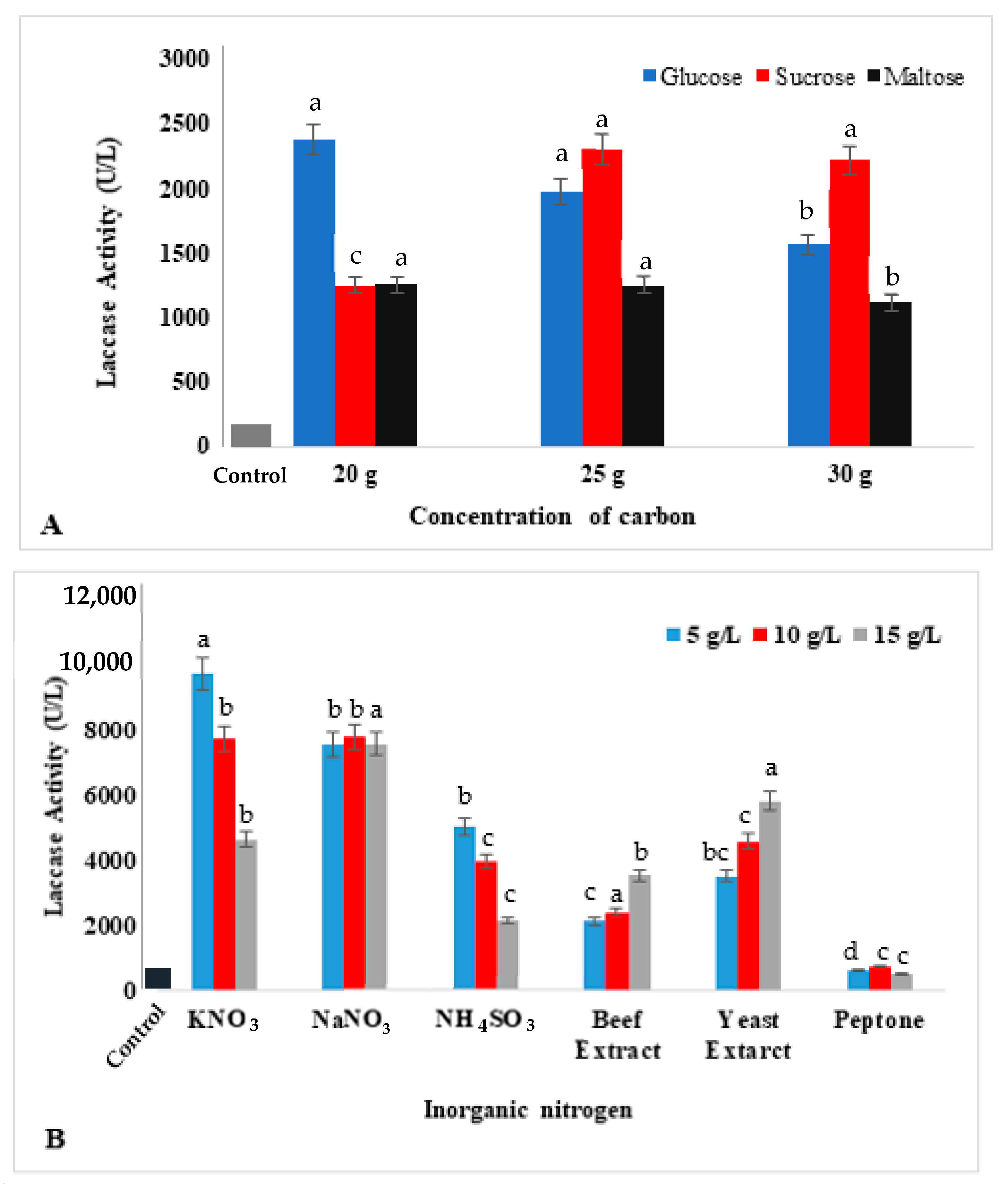
2.4. Effects of Nutritional Sources on Laccase Production
2.5. Partial Purification of Laccase
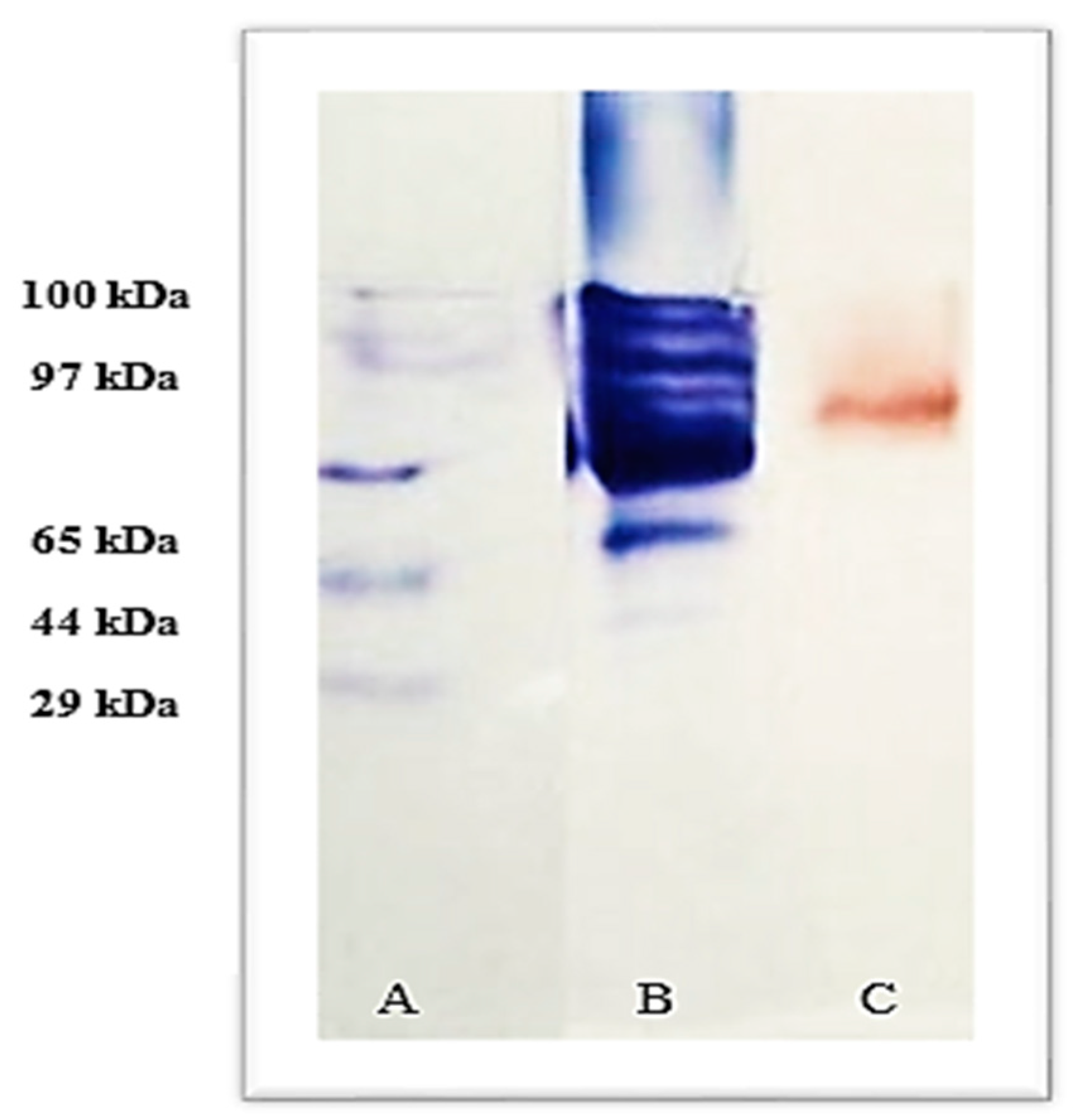
2.6. Laccase Molecular Weight
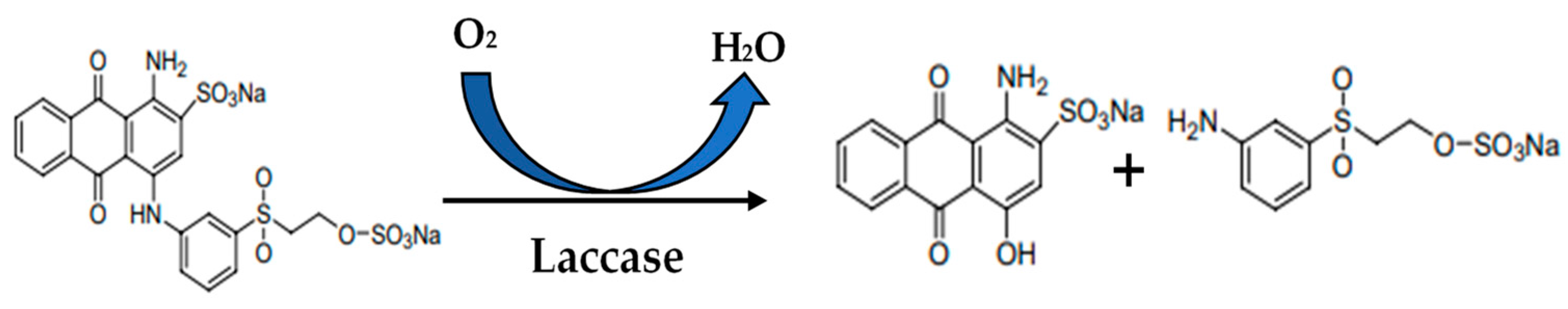
2.7. Dye Decolorization
2.8. Estimation of TOC (Total Organic Carbon)
2.9. Km & Vmax Value
2.10. Statistical Analysis
3. Results
3.1. Species Identification by Phylogenetic Analysis
3.2. Qualitative Estimation of Laccase
3.3. Effects of Nutritional Sources on Laccase Production
3.4. Purification and Molecular Weight Determination of Lacc134
3.5. Dye Decolorization by Lacc134
3.6. Concentration of TOC after Dye Removal Efficiency
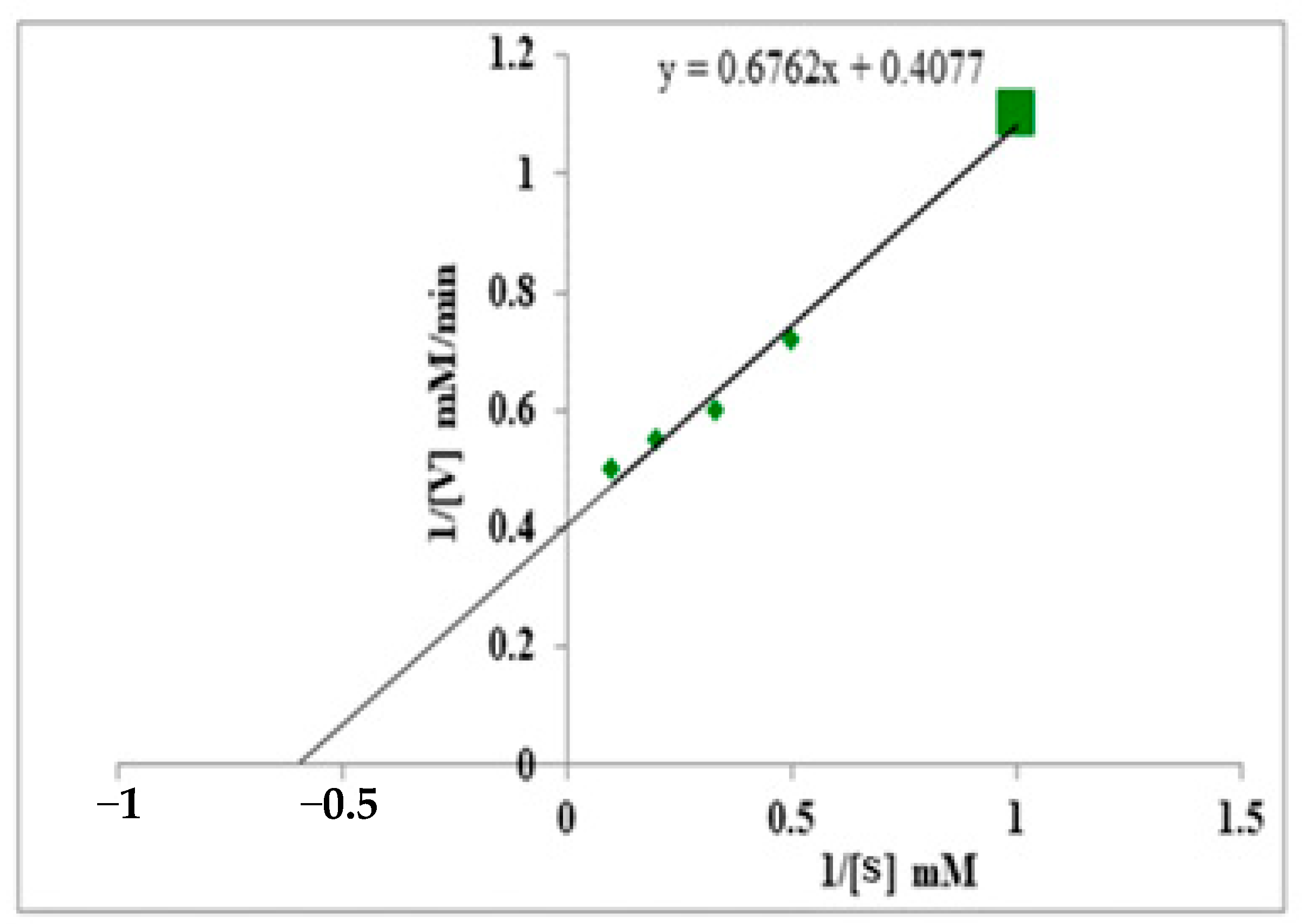
3.7. Km & Vmax Value
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El Sheikha, A.F. Nutritional profile and health benefits of Ganoderma lucidum “Lingzhi, Reishi, or Mannentake” as functional foods: Current scenario and future perspectives. Foods 2022, 11, 1030. [Google Scholar] [CrossRef] [PubMed]
- Han, M.L.; Lin, L.; Guo, X.X.; An, M.; Geng, Y.J.; Xin, C.; Ma, L.-C.; Mi, Q.; Ping, A.-Q.; Yang, Q.-Y.; et al. Comparative Analysis of the Laccase Secretion Ability of Five White-rot Fungi in Submerged Fermentation with Lignocellulosic Biomass. BioResources 2023, 18, 584–598. [Google Scholar] [CrossRef]
- Selvam, K.; Ameen, F.; Amirul Islam, M.; Sudhakar, C.; Selvankumar, T. Laccase production from Bacillus aestuarii KSK using Borassus flabellifer empty fruit bunch waste as a substrate and assessing their malachite green dye degradation. J. Appl. Microbiol. 2022, 133, 3288–3295. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, X.; Lin, Y.; Ng, T.B.; Lin, J.; Ye, X. Laccase-catalyzed decolorization of Malachite Green: Performance optimization and degradation mechanism. PLoS ONE 2015, 10, e0127714. [Google Scholar] [CrossRef] [PubMed]
- Shah, H.; Yusof, F.; Alam, M.Z. A new technique to estimate percentage decolorization of synthetic dyes on solid media by extracellular laccase from white-rot fungus. Bioremed. J. 2023, 27, 66–74. [Google Scholar] [CrossRef]
- Sun, S.; Liu, P.; Ullah, M. Efficient Azo Dye Biodecolorization System Using Lignin-Co-Cultured White-Rot Fungus. J. Fungi 2023, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Alam, R.; Mahmood, R.A.; Islam, S.; Ardiati, F.C.; Solihat, N.N.; Alam, M.B.; Lee, S.H.; Yanto, D.H.Y.; Kim, S. Understanding the biodegradation pathways of azo dyes by immobilized white-rot fungus, Trametes hirsuta D7, using UPLC-PDA-FTICR MS supported by in silico simulations and toxicity assessment. Chemosphere 2023, 313, 137505. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.D.; Tiwari, A.; Anisha, G.S.; Chen, C.W.; Singh, A.; Haldar, D.; Patel, A.K.; Singhania, R.R. Laccase: A potential biocatalyst for pollutant degradation. Environ. Pollut. 2023, 319, 120999. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Hoyos, C.M.; Morales-Álvarez, E.D.; Abelló-Esparza, J.; Buitrago-Pérez, D.F.; Martínez-Aldana, N.; Salcedo-Reyes, J.C.; Poutou-Piñales, R.A.; Pedroza-Rodríguez, A.M. Detoxification of pulping black liquor with Pleurotus ostreatus or recombinant Pichia pastoris followed by CuO/TiO2/visible photocatalysis. Sci. Rep. 2018, 8, 3503. [Google Scholar] [CrossRef]
- Choi, K.Y. Discoloration of indigo dyes by eco-friendly biocatalysts. Dye. Pigment. 2021, 184, 198749. [Google Scholar] [CrossRef]
- Ardila-Leal, L.D.; Hernández-Rojas, V.; Céspedes-Bernal, D.N.; Mateus-Maldonado, J.F.; Rivera-Hoyos, C.M.; Pedroza-Camacho, L.D.; Poutou-Piñales, R.A.; Pedroza-Rodríguez, A.M.; Pérez-Florez, A.; Quevedo-Hidalgo, B.E. Tertiary treatment (Chlorella sp.) of a mixed effluent from two secondary treatments (immobilized recombinant P. pastori and rPOXA 1B concentrate) of coloured laboratory wastewater (CLWW). 3Biotech 2020, 10, 233. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, S.; Chaudhuri, S. Laccase activity and azo dye decolorization potential of Podoscypha elegans. Mycobiology 2018, 46, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Deska, M.; Kończak, B. Immobilized fungal laccase as” green catalyst” for the decolourization process–State of the art. Process Biochem. 2019, 84, 112–123. [Google Scholar] [CrossRef]
- Backes, E.; Kato, C.G.; da Silva, T.B.; Uber, T.M.; Pasquarelli, D.L.; Bracht, A.; Peralta, R.M. Production of fungal laccase on pineapple waste and application in detoxification of malachite green. J. Environ. Sci. Health Part B. 2022, 57, 90–101. [Google Scholar] [CrossRef]
- Velásquez-Quintero, C.; Merino-Restrepo, A.; Hormaza-Anaguano, A. Production, extraction, and quantification of laccase obtained from an optimized solid-state fermentation of corncob with white-rot fungi. J. Clean. Prod. 2022, 370, 133598. [Google Scholar] [CrossRef]
- Kirk, T.K.; Schultz, E.; Connors, W.J.; Lorenz, L.F.; Zeikus, J.G. Influence of culture parameters on lignin metabolism by Phanerochaete chrysosporium. Arch. Microbiol. 1978, 117, 277–285. [Google Scholar] [CrossRef]
- Jhadav, A.; Vamsi, K.K.; Khairnar, Y.; Boraste, A.; Gupta, N.; Trivedi, S.; Patil, P.; Gupta, G.; Gupta, M.; Mujapara, A.K.; et al. Optimization of production and partial purification of laccase by Phanerochaete chrysosporium using submerged fermentation. Int. J. Microbiol. Res. 2009, 1, 9–12. [Google Scholar]
- Das, N.; Chakraborty, T.K.; Mukherjee, M. Purification and characterization of a growth regulating laccase from Pleurotus forida. J. Basic Microbiol. 2001, 41, 261–267. [Google Scholar] [CrossRef]
- Rice, E.W. Standard Methods for the Examination of Water and Wastewater 2540 A, 23rd ed.; Water Environment Federation: Alexandria, Egypt, 2017. [Google Scholar]
- Teerapatsakul, C.; Parra, R.; Bucke, C.; Chitradon, L. Improvement of laccase production from Ganoderma sp. KU-Alk4 by medium engineering. World J. Microbiol. Biotechnol. 2007, 23, 1519–1527. [Google Scholar] [CrossRef]
- Zilly, A.; da Silva Coelho-Moreira, J.; Bracht, A.; De Souza, C.G.M.; Carvajal, A.E.; Koehnlein, E.A.; Peralta, R.M. Influence of NaCl and Na2SO4 on the kinetics and dye decolorization ability of crude laccase from Ganoderma lucidum. Int. Biodeter. Biodegrad. 2011, 65, 340–344. [Google Scholar] [CrossRef]
- Li, S.; Tang, B.; Liu, Y.; Chen, A.; Tang, W.; Wei, S. High-level production and characterization of laccase from a newly isolated fungus Trametes sp. LS-10C. Biocatal. Agric. Biotech. 2016, 8, 278–285. [Google Scholar] [CrossRef]
- Songulashvili, G.; Elisashvili, V.; Wasser, S.; Nevo, E.; Hadar, Y. Laccase and manganese peroxidase activities of Phellinus robustus and Ganoderma adspersum grown on food industry wastes in submerged fermentation. Biotechnol. Lett. 2006, 28, 1425–1429. [Google Scholar] [CrossRef]
- Hou, H.; Zhoua, J.; Wanga, J.; Dua, C.; Yana, B. Enhancement of laccase production by Pleurotus ostreatus and its use for the decolorization of anthraquinone dye. Process Biochem. 2004, 39, 1415–1419. [Google Scholar] [CrossRef]
- Mikiashvili, N.; Wasser, S.P.; Nevo, E.; Elisashvili, V. Effects of carbon and nitrogen sources on P. ostreatus ligninolytic enzyme activity. World J. Microbiol. Biotechnol. 2006, 22, 999–1002. [Google Scholar] [CrossRef]
- He, F.; Qin, X.; Zhang, H.; Yang, Y.; Zhang, X.; Yang, Y. Characterization of laccase isoenzymes from the white-rot fungus Ganoderma sp.En3 and synergistic action of isoenzymes for dye decolorization. J. Chem. Technol. Biotechnol. 2015, 90, 2265–2279. [Google Scholar] [CrossRef]
- Claudia, M.R.H.; Edwin David, M.A.; Raul, A.P.N.; Aura Marina, P.R.; Refugio, R.V.; Julio, M.D.B. Fungal laccases. Fungi Biol. Rev. 2013, 27, 67–82. [Google Scholar]
- Ding, Z.; Peng, L.; Chen, Y.; Zhang, L.; Gu, Z.; Shi, G.; Zhang, K. Production and characterization of thermostable laccase from the mushroom, Ganoderma lucidum, using submerged fermentation. Afr. J. Microbiol. Res. 2012, 6, 1147–1157. [Google Scholar]
- Galhaup, C.; Wagner, H.; Hinterstoisser, B.; Haltrich, D. Increased production of laccase by the wood-degrading basidiomycete Trametes pubescens. Enzyme Microbiol. Technol. 2002, 30, 529–536. [Google Scholar] [CrossRef]
- Wang, H.; Tang, C.; Yu, G.; Li, P. A novel membrane-surface liquid co-culture to improve the production of laccase from Ganoderma lucidum. Biochem. Eng. J. 2013, 80, 27–36. [Google Scholar]
- Songulashvili, G.; Elisashvili, V.; Wasser, S.P.; Nevo, E.; Hadar, Y. Basidiomycetes laccase and manganese peroxidase activity in submerged fermentation of food industry wastes. Enzyme Microbiol. Technol. 2007, 41, 57–61. [Google Scholar] [CrossRef]
- Moldes, D.; Lorenzo, M.; Sanroman, M.A. Different proportion of laccase isoenzymes produced by submerged cultures of Trametes versicolor grown on lignocellulosic wastes. Biotechnol. Lett. 2004, 26, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Teerapatsakul, C.; Parra, R.; Keshavarz, T.; Chitradon, L. Repeated batch for dye degradation in an airlift bioreactor by laccase entrapped in copper alginate. Int. Biodeterior. Biodegrad. 2017, 120, 52–57. [Google Scholar] [CrossRef]
- Nyanhongo, G.S.; Gomes, J.; Gübitz, G.M.; Zvauya, R.; Read, J.; Steiner, W. Decolorization of textile dyes by laccases from a newly isolated strain of Trametes modesta. Water Res. 2002, 36, 1449–1456. [Google Scholar] [CrossRef] [PubMed]
- Shakhova, N.V.; Golenkina, S.A.; Stepanova, E.V.; Loginov, D.S.; Psurtseva, N.V.; Fedorova, T.V.; Koroleva, O.V. Effect of Submerged Cultivation Conditions and Inducers on Biosynthesis of Extracellular Laccase by a Trametes versicolor 1666 Strain. Appl. Biochem. Microbiol. 2011, 47, 808–816. [Google Scholar] [CrossRef]
- D’Souza-Ticlo, D.; Verma, A.K.; Mathew, M.; Raghukumar, C. Effect of nutrient nitrogen on laccase production, it’s isozyme pattern and effluent decolourization by the fungus NIOCC# 2a, isolated from mangrove wood. Ind. J. Mar. Sci. 2006, 35, 364–372. [Google Scholar]
- Revankar, M.S.; Lele, S.S. Enhanced production of laccase using a new isolate of white rot fungus WR-1. Process Biochem. 2006, 41, 581–588. [Google Scholar] [CrossRef]
- Periasamy, R.; Palvannan, T. Optimization of laccase production by Pleurotus ostreatus IMI 395545 using the Taguchi DOE methodology. J. Basic Microbiol. 2010, 50, 548–556. [Google Scholar] [CrossRef]
- Poojary, H.; Mugeraya, G. Laccase production by Phellinus noxius hpF17: Optimization of submerged culture conditions by Response Surface Methodology. Res. Biotechnol. 2012, 3, 9–20. [Google Scholar]
- Rodrigues, E.M.; Karp, S.G.; Malucelli, L.C.; Helm, C.V.; Alvarez, T.M. Evaluation of laccase production by Ganoderma lucidum in submerged and solid-state fermentation using different inducers. J. Basic Microbiol. 2019, 59, 784–791. [Google Scholar] [CrossRef]
- Sivakumar, R.; Rajendran, R.; Balakumar, C.; Tamilvendan, M. Isolation, Screening and Optimization of Production Medium for Thermostable Laccase Production from Ganoderma sps. Int. J. Engineer. Sci. Technol. 2010, 2, 7133–7141. [Google Scholar]
- Rodríguez-Couto, S. Fungal laccase: A versatile enzyme for biotechnological applications. In Recent Advancement in White Biotechnology through Fungi; Fungal biology; Yadav, A., Mishra, S., Singh, S., Gupta, A., Eds.; Springer: Cham, Switzerland, 2019; pp. 429–457. [Google Scholar]
- Levin, L.; Forchiassin, F.; Ramos, A.M. Copper induction of lignin modifying enzymes in the white-rot fungus Trametes trogii. Mycologia 2002, 94, 377–383. [Google Scholar] [CrossRef]
- Bu, T.; Yang, R.; Zhang, Y.; Cai, Y.; Tang, Z.; Li, C.; Wu, Q.; Chen, H. Improving decolorization of dyes by ccase from Bacillus licheniformis by random and site-directed mutagenesis. PeerJ 2020, 8, e10267. [Google Scholar] [CrossRef] [PubMed]
- Leatham, G.F.; Kirk, T.K. Regulation of ligninolytic activity by nutrient nitrogen in white-rot basidiomycetes. FEMS Microbiol. Lett. 1983, 16, 65–67. [Google Scholar] [CrossRef][Green Version]
- Madhavi, S.R.; Lele, S.S. Synthetic dye decolorization by white rot fungus, Ganoderma sp. WR-1. Bioresour. Technol. 2007, 98, 775–780. [Google Scholar]
- Piscitelli, A.; Giardina, P.; Lettera, V.; Pezzella, C.; Sannia, G.; Faraco, V. Induction and transcriptional regulation of laccases in fungi. Curr. Genom. 2011, 12, 104–112. [Google Scholar] [CrossRef]
- Shrestha, P.; Joshi, B.; Joshi, J.; Malla, R.; Sreerama, L. Isolation and physicochemical characterization of laccase from Ganoderma lucidum-CDBT1 isolated from its native habitat in nepal. Biomed. Res. Int. 2016, 2016, 3238909. [Google Scholar] [CrossRef]
- Kuhar, F.; Papinutti, L. Optimización de la producción de lacasa por dos cepas de Ganoderma lucidum utilizando inductores fenólicos y metálicos. Rev. Argent. Microbiol. 2014, 46, 144–149. [Google Scholar]
- Tuncay, D.; Yagar, H. Decolorization of Reactive Blue-19 textile dye by Boletus edulis laccase immobilized onto rice husks. Int. J. Environ. Sci. Technol. 2020, 17, 3177–3188. [Google Scholar] [CrossRef]
- Zouari-Mechichi, H.; Mechichi, T.; Dhouib, A.; Sayadi, S.; Martínez, A.T.; Martínez, M.J. Laccase purification and characterization from Trametes trogii isolated in Tunisia: Decolorization of textile dyes by the purified enzyme. Enzyme Microbiol. Technol. 2006, 39, 141–148. [Google Scholar] [CrossRef]
- Ameen, F.; Dawoud, T.; AlNadhari, S. Ecofriendly and low-cost synthesis of ZnO nanoparticles from Acremonium potronii for the photocatalytic degradation of azo dyes. Environ. Res. 2021, 202, 111700. [Google Scholar] [CrossRef]
- Adnan, L.A.; Sathishkumar, P.; Yusoff, A.R.M.; Hadibarata, T.; Ameen, F. Rapid bioremediation of Alizarin Red S and Quinizarine Green SS dyes using Trichoderma lixii F21 mediated by biosorption and enzymatic processes. Bioprocess Biosyst. Eng. 2017, 40, 85–97. [Google Scholar] [CrossRef]
- Ameen, F.; Aygun, A.; Seyrankaya, A.; Tiri, R.N.E.; Gulbagca, F.; Kaynak, İ.; Majrashi, N.; Orfali, R.; Dragoi, E.N.; Sen, F. Photocatalytic investigation of textile dyes and E. coli bacteria from wastewater using Fe3O4@ MnO2 heterojunction and investigation for hydrogen generation on NaBH4 hydrolysis. Environ. Res. 2023, 220, 115231. [Google Scholar] [CrossRef]
- Zeng, X.; Cai, Y.; Liao, X.; Zeng, X.; Luo, S.; Zhang, D. Anthraquinone dye assisted the decolorization of azo dyes by a novel Trametes trogii laccase. Process Biochem. 2012, 47, 160–163. [Google Scholar] [CrossRef]
- Afreen, S.; Anwer, R.; Singh, R.K.; Fatma, T. Extracellular laccase production and its optimization from Arthrospira maxima catalyzed decolorization of synthetic dyes. Saudi J. Biol. Sci. 2018, 25, 1446–1453. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Cai, Y.; Liao, X.; Zeng, X.; Li, W.; Zhang, D. Decolorization of synthetic dyes by crude laccase from a newly isolated Trametes trogii strain cultivated on solid agro-industrial residue. J. Hazard. Mater. 2018, 187, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Malvis, A.; Hodaifa, G.; Halioui, M.; Seyedsalehi, M.; Sa, S. Integrated process for olive oil mill wastewater treatment and its revalorization through the generation of high added value algal biomass. Water Res. 2019, 151, 332–342. [Google Scholar] [CrossRef]
- Bilinska, L.; Gmurek, M.; Ledakowicz, S. Comparison between industrial and simulated textile wastewater treatment by AOPs—Biodegradability, toxicity and cost assessment. Chem. Eng. J. 2016, 306, 550–559. [Google Scholar] [CrossRef]
- Ulu, A. Metal–organic frameworks (MOFs): A novel support platform for ASNase immobilization. J. Mater. Sci. 2020, 55, 6130–6144. [Google Scholar] [CrossRef]

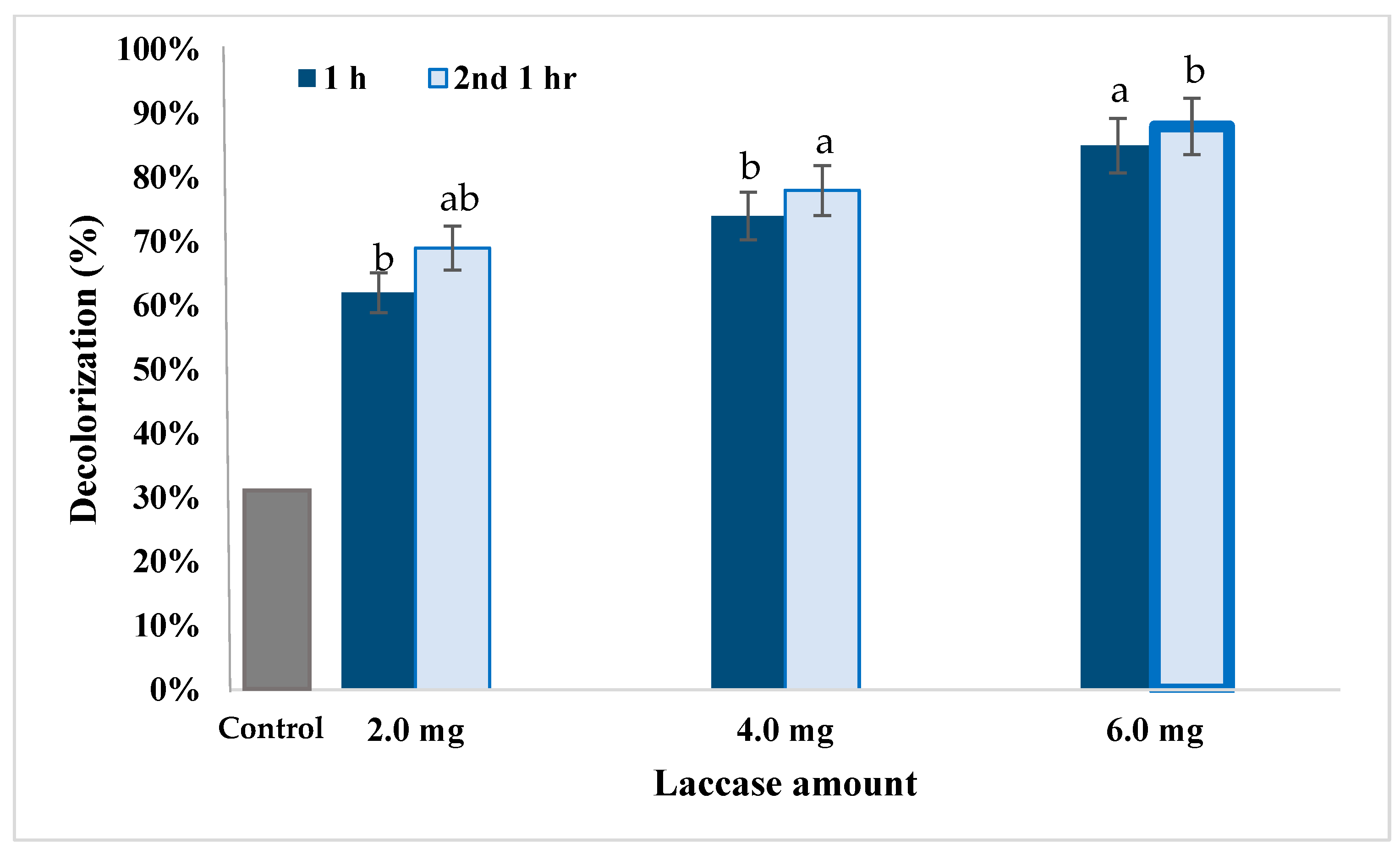
 ), and TOC (total organic carbon) content (%
), and TOC (total organic carbon) content (%  ). (g = gram). The data collected from each treatment was expressed as mean ± SE and statistically analyzed through analytical software. The means were compared by Least Significant Difference. The statistical analysis was done at the significance level α = 0.05 using Co-Stat version 3.01. Similar letters indicated non-significant effects at p < 0.05 between different treatments.
). (g = gram). The data collected from each treatment was expressed as mean ± SE and statistically analyzed through analytical software. The means were compared by Least Significant Difference. The statistical analysis was done at the significance level α = 0.05 using Co-Stat version 3.01. Similar letters indicated non-significant effects at p < 0.05 between different treatments.
 ), and TOC (total organic carbon) content (%
), and TOC (total organic carbon) content (%  ). (g = gram). The data collected from each treatment was expressed as mean ± SE and statistically analyzed through analytical software. The means were compared by Least Significant Difference. The statistical analysis was done at the significance level α = 0.05 using Co-Stat version 3.01. Similar letters indicated non-significant effects at p < 0.05 between different treatments.
). (g = gram). The data collected from each treatment was expressed as mean ± SE and statistically analyzed through analytical software. The means were compared by Least Significant Difference. The statistical analysis was done at the significance level α = 0.05 using Co-Stat version 3.01. Similar letters indicated non-significant effects at p < 0.05 between different treatments.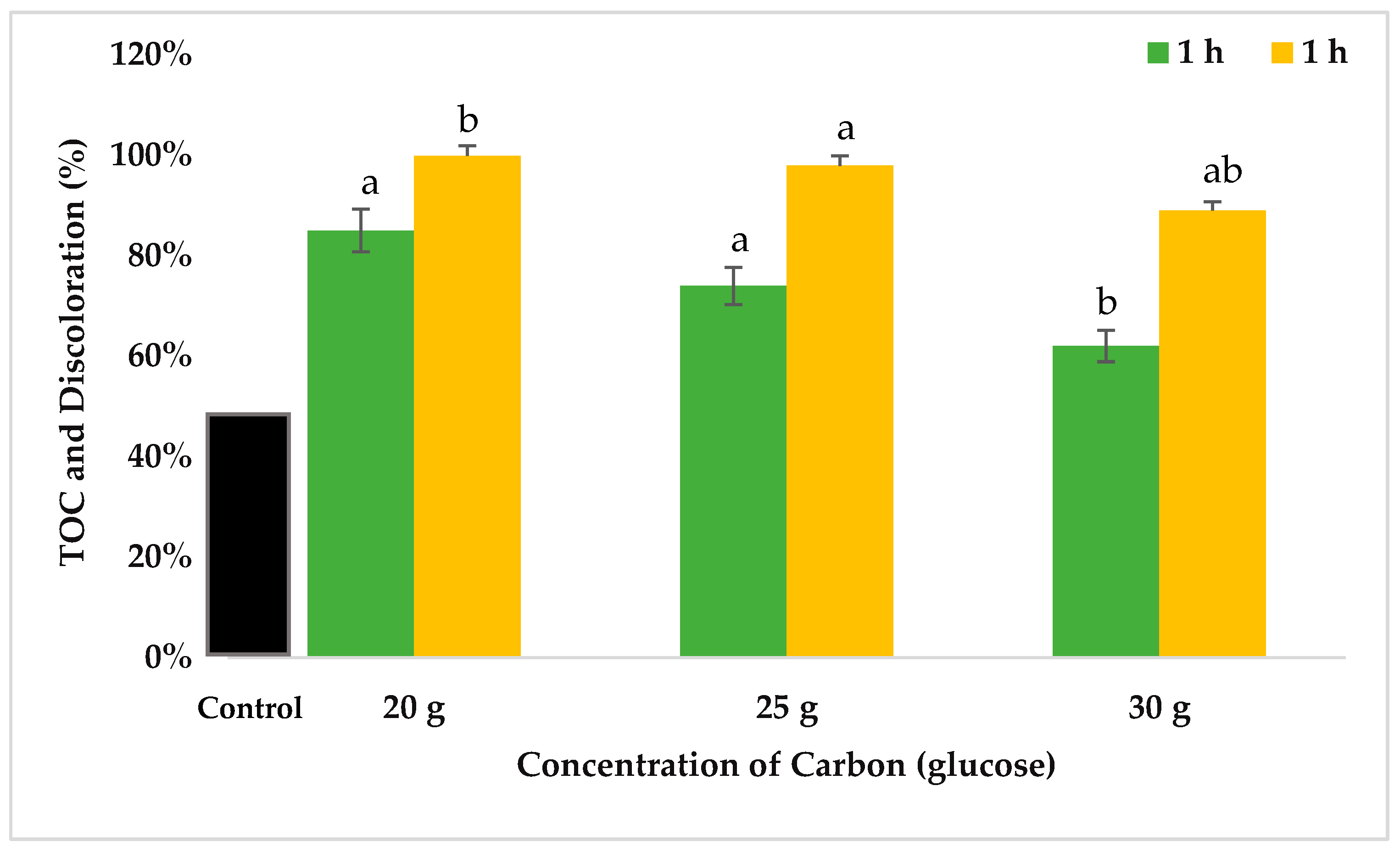
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhomaidi, E.A.; Umar, A.; Alsharari, S.S.; Alyahya, S. Evaluation of Lacc134 Oxidoreductase of Ganoderma multistipitatum in Detoxification of Dye Wastewater under Different Nutritional Conditions. Microbiol. Res. 2023, 14, 1398-1412. https://doi.org/10.3390/microbiolres14030095
Alhomaidi EA, Umar A, Alsharari SS, Alyahya S. Evaluation of Lacc134 Oxidoreductase of Ganoderma multistipitatum in Detoxification of Dye Wastewater under Different Nutritional Conditions. Microbiology Research. 2023; 14(3):1398-1412. https://doi.org/10.3390/microbiolres14030095
Chicago/Turabian StyleAlhomaidi, Eman A., Aisha Umar, Salam S. Alsharari, and Sami Alyahya. 2023. "Evaluation of Lacc134 Oxidoreductase of Ganoderma multistipitatum in Detoxification of Dye Wastewater under Different Nutritional Conditions" Microbiology Research 14, no. 3: 1398-1412. https://doi.org/10.3390/microbiolres14030095
APA StyleAlhomaidi, E. A., Umar, A., Alsharari, S. S., & Alyahya, S. (2023). Evaluation of Lacc134 Oxidoreductase of Ganoderma multistipitatum in Detoxification of Dye Wastewater under Different Nutritional Conditions. Microbiology Research, 14(3), 1398-1412. https://doi.org/10.3390/microbiolres14030095





