Antiproliferative Activity of Mycelium vs. Fruiting Body: Ganoderma subincrustatum and G. weberianum from Sonora, Mexico
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Strains
2.2. Liquid and Solid Culture
2.3. Extracts
2.4. Bioguided Fractionation and Chemical Analysis
2.5. Cell Culture
2.6. Antiproliferative Activity
2.7. Flow Cytometry
2.8. Statistical Analysis
3. Results and Discussions
3.1. Antiproliferative Activity of G. subincrustatum and G. weberianum
3.2. Bioactive Fractions of G. subincrustatum
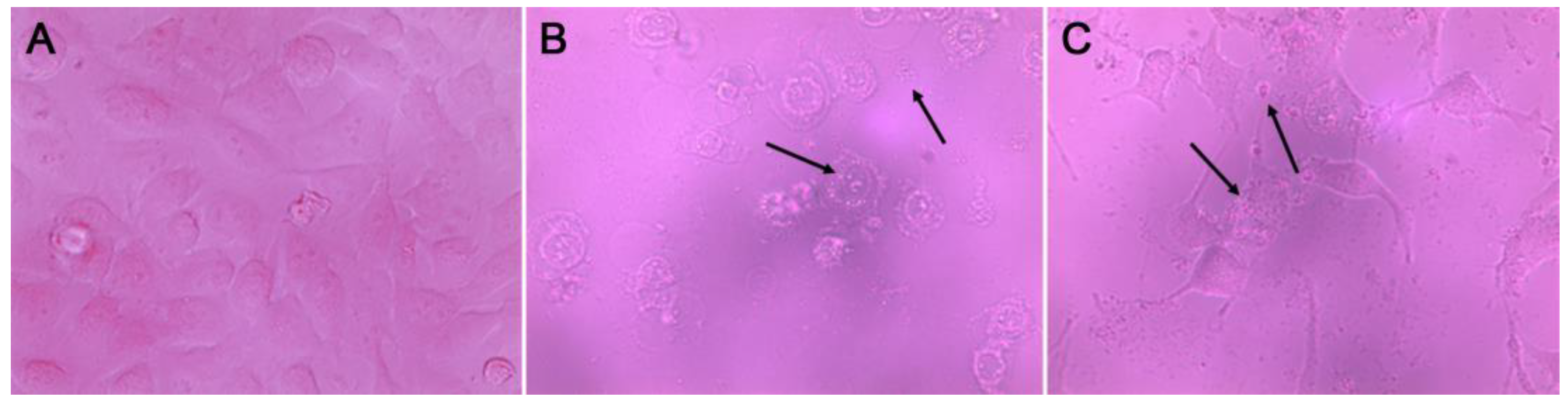
3.3. Apoptosis Induction of F7
3.4. NMR Analysis of F7 Chromatographic Fraction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, M.F.; Wahab, S.; Ahmad, F.A.; Ashraf, S.A.; Abullais, S.S.; Saad, H.H. Ganoderma lucidum: A potential pleiotropic approach of ganoderic acid in health reinforcement and factors influencing their production. Fungal Biol. Rev. 2022, 39, 100–125. [Google Scholar] [CrossRef]
- Angulo-Sanchez, L.T.; López-Peña, D.; Torres-Moreno, H.; Gutiérrez, A.; Gaitán-Hernández, R.; Esqueda, M. Biosynthesis, gene expression, and pharmacological properties of triterpenoids of Ganoderma species (Agaricomycetes): A review. Int. J. Med. Mushrooms 2022, 24, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, J.Q.; Zhang, J.; Li, Z.M.; Liu, H.G.; Wang, Y.Z. Traditional uses, chemical components and pharmacological activities of the genus Ganoderma P. Karst.: A review. RSC Adv. 2020, 10, 42084–42097. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ng, J.M.; Wong, C.C.; Ng, E.K.W.; Yu, J. Molecular alterations of cancer cell and tumour microenvironment in metastatic gastric cancer. Oncogene 2018, 37, 4903–4920. [Google Scholar] [CrossRef]
- Lichota, A.; Gwozdzinski, K. Anticancer activity of natural compounds from plant and marine environment. Int. J. Mol. Sci. 2018, 19, 3533. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A target for anticancer therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef]
- Galappaththi, M.C.A.; Patabendige, N.M.; Premarathne, B.M.; Hapuarachchi, K.K.; Tibpromma, S.; Dai, D.Q.; Suwannarach, N.; Rapior, S.; Karunarathna, S.C. A review of Ganoderma triterpenoids and their bioactivities. Biomolecules 2022, 13, 24. [Google Scholar] [CrossRef]
- Paterson, R.R. Ganoderma—A therapeutic fungal biofactory. Phytochemistry 2006, 67, 1985–2001. [Google Scholar] [CrossRef]
- Yang, L.; Kong, D.X.; Xiao, N.; Ma, Q.Y.; Xie, Q.Y.; Guo, J.C.; Ying, D.C.; Ma, H.X.; Hua, Y.; Dai, H.F.; et al. Antidiabetic lanostane triterpenoids from the fruiting bodies of Ganoderma weberianum. Bioorg. Chem. 2022, 127, 106025. [Google Scholar] [CrossRef]
- Cao, L.; Jin, H.; Liang, Q.; Yang, H.; Li, S.; Liu, Z.; Yuan, Z. A new anti-tumor cytotoxic triterpene from Ganoderma lucidum. Nat. Prod. Res. 2021, 36, 4125–4131. [Google Scholar] [CrossRef]
- Li, X.C.; Liu, F.; Su, H.G.; Peng, C.; Zhou, Q.M.; Liu, J.; Huang, Y.J.; Guo, L.; Xiong, L. Twelve undescribed derivatives of ganoderic acid isolated from Ganoderma luteomarginatum and their cytotoxicity against three human cancer cell lines. Phytochemistry 2021, 183, 112617. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Zhang, H.; Sun, X.; Zhao, H.; Wu, L.; Zhu, D.; Yang, G.; Shao, Y.; Zhang, X.; Mao, X.; et al. A comprehensive review of the structure elucidation and biological activity of triterpenoids from Ganoderma spp. Molecules 2014, 19, 17478–17535. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.L.; Yen, G.C. Ganoderic acid and lucidenic acid (triterpenoid). Enzymes 2014, 36, 33–56. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.S.; Ji, S.L.; Ren, M.F.; He, Y.L.; Jing, X.R.; Xu, J.W. Enhanced accumulation of individual ganoderic acids in a submerged culture of Ganoderma lucidum by the overexpression of squalene synthase gene. Biochem. Eng. J. 2014, 90, 178–183. [Google Scholar] [CrossRef]
- Chen, S.; Xu, J.; Liu, C.; Zhu, Y.; Nelson, D.R.; Zhou, S.; Li, C.; Wang, L.; Guo, X.; Sun, Y.; et al. Genome sequence of the model medicinal mushroom Ganoderma lucidum. Nat. Commun. 2012, 3, 913. [Google Scholar] [CrossRef]
- Xu, J.W.; Zhao, W.; Zhong, J.J. Biotechnological production and application of ganoderic acids. Appl. Microbiol. Biotechnol. 2010, 87, 457–466. [Google Scholar] [CrossRef]
- Zhou, X.W.; Su, K.Q.; Zhang, Y.M. Applied modern biotechnology for cultivation of Ganoderma and development of their products. Appl. Microbiol. Biotechnol. 2012, 93, 941–963. [Google Scholar] [CrossRef]
- Hsu, K.D.; Cheng, K.C. From nutraceutical to clinical trial: Frontiers in Ganoderma development. Appl. Microbiol. Biotechnol. 2018, 102, 9037–9051. [Google Scholar] [CrossRef]
- Swallah, M.S.; Bondzie-Quaye, P.; Wu, Y.; Acheampong, A.; Sossah, F.L.; Elsherbiny, S.M.; Huang, Q. Therapeutic potential and nutritional significance of Ganoderma lucidum—A comprehensive review from 2010 to 2022. Food Funct. 2023, 14, 1812–1838. [Google Scholar] [CrossRef]
- López-Peña, D.; Gutiérrez, A.; Hernández-Navarro, E.; Valenzuela, R.; Esqueda, M. Diversidad y distribución de Ganoderma (Polyporales: Ganodermataceae) en Sonora, México. Bot. Sci. 2016, 94, 431–439. [Google Scholar] [CrossRef]
- López-Peña, D.; Samaniego-Rubiano, C.; Estrada-Morales, I.; Gutiérrez, A.; Gaitán-Hernández, R.; Esqueda, M. Morphological characteristics of wild and cultivated Ganoderma subincrustatum Murrill (Polyporales: Ganodermataceae) from Sonora, Mexico. Sci. Fungorum 2019, 49, e1213. [Google Scholar] [CrossRef]
- Xu, P.; Ding, Z.Y.; Qian, Z.; Zhao, C.X.; Zhang, K.C. Improved production of mycelial biomass and ganoderic acid by submerged culture of Ganoderma lucidum SB97 using complex media. Enzyme Microb. Technol. 2008, 42, 325–331. [Google Scholar] [CrossRef]
- Morales-Estrada, R.I.; Gaitán-Hernández, R.; Gutiérrez, A.; Vargas, G.; Jiménez, A.; Rascón, A.; Esqueda, M. Vineyard pruning waste improves bioconversion and chemical composition of native Ganoderma spp. (Agaricomycetes) strain from Mexico. Int. J. Med. Mushrooms 2018, 20, 775–789. [Google Scholar] [CrossRef] [PubMed]
- López-Romero, J.C.; González-Ríos, H.; Peña-Ramos, A.; Velazquez, C.; Navarro, M.; Robles-Zepeda, R.; Martínez-Benavidez, E.; Higuera-Ciapara, I.; Virués, C.; Olivares, J.L.; et al. Seasonal effect on the biological activities of Litsea glaucescens Kunth extracts. Evid. Based Complement. Altern. Med. 2018, 2018, 2738489. [Google Scholar] [CrossRef] [PubMed]
- Torres-Moreno, H.; Marcotullio, M.C.; Velázquez, C.; Ianni, F.; Garibay-Escobar, A.; Robles-Zepeda, R.E. Cucurbitacin IIb, a steroidal triterpene from Ibervillea sonorae induces antiproliferative and apoptotic effects on cervical and lung cancer cells. Steroids 2020, 157, 108597. [Google Scholar] [CrossRef]
- Müller, C.I.; Kumagai, T.; O’Kelly, J.; Seeram, N.P.; Heber, D.; Koeffler, H.P. Ganoderma lucidum causes apoptosis in leukemia, lymphoma, and multiple myeloma cells. Leuk. Res. 2006, 30, 841–848. [Google Scholar] [CrossRef]
- Mfotie, E.; Munvera, A.M.; Mkounga, P.; Nkengfack, A.E.; McGaw, L.J. Phytochemical analysis with free radical scavenging, nitric oxide inhibition and antiproliferative activity of Sarcocephalus pobeguinii extracts. BMC Complement. Altern. Med. 2017, 17, 199. [Google Scholar] [CrossRef]
- Zolj, S.; Smith, M.P.; Goines, J.C.; Ali, T.S.; Huff, M.O.; Robinson, D.L.; Lau, J.M. Antiproliferative effects of a triterpene-enriched extract from lingzhi or reishi medicinal mushroom, Ganoderma lucidum (Agaricomycetes), on human lung cancer cells. Int. J. Med. Mushrooms 2018, 20, 1173–1183. [Google Scholar] [CrossRef]
- Liu, Y.W.; Gao, J.L.; Guan, J.; Qian, Z.M.; Feng, K.; Li, S.P. Evaluation of antiproliferative activities and action mechanisms of extracts from two species of Ganoderma on tumor cell lines. J. Agric. Food Chem. 2009, 57, 3087–3093. [Google Scholar] [CrossRef]
- Saraste, A.; Pulkki, K. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc. Res. 2000, 45, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.J.; Han, M.H.; Lee, B.H.; Kim, B.W.; Kim, C.H.; Yoon, H.M.; Choi, Y.H. Induction of apoptosis by ethanol extracts of Ganoderma lucidum in human gastric carcinoma cells. J. Acupunct. Meridian Stud. 2010, 3, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Elkhateeb, W.A.; Zaghlol, G.M.; El-Garawani, I.M.; Ahmed, E.F.; Rateb, M.E.; Moneim, A.E.A. Ganoderma applanatum secondary metabolites induced apoptosis through different pathways: In vivo and in vitro anticancer studies. Biomed. Pharmacother. 2018, 101, 264–277. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.M.; Zhong, J.J. Ganoderic acid Mf and S induce mitochondria mediated apoptosis in human cervical carcinoma HeLa cells. Phytomedicine 2011, 18, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Kis, B.; Pavel, I.Z.; Haidu, D.; Ștefănuț, M.N.; Diaconeasa, Z.; Moacă, E.A.; Dehelean, C.A.; Șipos, S.; Ivan, A.; Danciu, C. Inorganic element determination of romanian Populus nigra L. buds extract and in vitro antiproliferative and pro-apoptotic evaluation on A549 human lung cancer cell line. Pharmaceutics 2021, 13, 986. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekhar, M.; Nayak, V.L.; Ramakrishna, S.; Mallavadhani, U.V. Novel triazole hybrids of myrrhanone C, a natural polypodane triterpene: Synthesis, cytotoxic activity and cell based studies. Eur. J. Med Chem. 2016, 14, 293–307. [Google Scholar] [CrossRef]
- Madasu, C.; Karri, S.; Sangaraju, R.; Sistla, R.; Uppuluri, M.V. Synthesis and biological evaluation of some novel 1,2,3-triazole hybrids of myrrhanone B isolated from Commiphora mukul gum resin: Identification of potent antiproliferative leads active against prostate cancer cells (PC-3). Eur. J. Med. Chem. 2020, 188, 111974. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, H.; Zhou, J.; Li, F.; Wang, J.; Chen, M.; Liu, Q. Cytotoxicity, DNA damage, and apoptosis induced by titanium dioxide nanoparticles in human non-small cell lung cancer A549 cells. Environ. Sci. Pollut. Res. Int. 2015, 22, 5519–5530. [Google Scholar] [CrossRef]
- Khan, I.; Bahuguna, A.; Bhardwaj, M.; Khaket, T.P.; Kang, S.C. Carvacrol nanoemulsion evokes cell cycle arrest, apoptosis induction and autophagy inhibition in doxorubicin resistant-A549 cell line. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. S1), 664–675. [Google Scholar] [CrossRef]
- Rieger, A.M.; Nelson, K.L.; Konowalchuk, J.D.; Barreda, D.R. Modified annexin V/propidium iodide apoptosis assay for accurate assessment of cell death. J. Vis. Exp. 2011, 50, 2597. [Google Scholar] [CrossRef]
- Tang, W.; Liu, J.W.; Zhao, W.M.; Wei, D.Z.; Zhong, J.J. Ganoderic acid T from Ganoderma lucidum mycelia induces mitochondria mediated apoptosis in lung cancer cells. Life Sci. 2006, 80, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Li, S.; Zhuo, Y.; Chen, J.; Qin, X.; Guo, G. Anticancer effect of triterpenes from Ganoderma lucidum in human prostate cancer cells. Oncol. Lett. 2017, 14, 7467–7472. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Dai, L.; Wang, L.; Zhu, J. Ganoderic acid DM induces autophagic apoptosis in non-small cell lung cancer cells by inhibiting the PI3K/Akt/mTOR activity. Chem. Biol. Interact. 2020, 316, 108932. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shimizu, K.; Tanaka, A.; Shinobu, W.; Ohnuki, K.; Nakamura, T.; Kondo, R. Target proteins of ganoderic acid DM provides clues to various pharmacological mechanisms. Sci. Rep. 2012, 2, 905. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, L. In vitro and in vivo cardioprotective effects of curcumin against doxorubicin-induced cardiotoxicity: A systematic review. J. Oncol. 2022, 2022, 7277562. [Google Scholar] [CrossRef]
- Supasena, W.; Muangnoi, C.; Praengam, K.; Wui Wong, T.; Qiu, G.; Ye, S.; Wu, J.; Tanasupawat, S.; Rojsitthisak, P. Enhanced selective cytotoxicity of doxorubicin to breast cancer cells by methoxypolyethylene glycol conjugation via a novel beta-thiopropanamide linker. Eur. Polym. J. 2020, 141, 110056. [Google Scholar] [CrossRef]
- Osman, A.M.M.; Bayoumi, H.M.; Al-Harthi, S.E.; Damanhouri, Z.A.; ElShal, M.F. Modulation of doxorubicin cytotoxicity by resveratrol in a human breast cancer cell line. Cancer Cell. Int. 2012, 12, 47. [Google Scholar] [CrossRef]
- Torres-Moreno, H.; Marcotullio, M.C.; Velazquez, C.; Arenas-Luna, V.M.; Hernández-Gutiérrez, S.; Robles-Zepeda, R.E. Cucurbitacin IIb from Ibervillea sonorae induces apoptosis and cell cycle arrest via STAT3 inhibition. Anticancer Agents Med. Chem. 2020, 20, 1188–1196. [Google Scholar] [CrossRef]
- Ouyang, J.J.; Wang, Y.Q.; Tang, W. Ganoderic acid restores the sensitivity of multidrug resistance cancer cells to doxorubicin. Adv. Mater. Res. 2014, 834, 573–576. [Google Scholar] [CrossRef]
- Liang, C.; Tian, D.; Liu, Y.; Li, H.; Zhu, J.; Li, M.; Xin, M.; Xia, J. Review of the molecular mechanisms of Ganoderma lucidum triterpenoids: Ganoderic acids A, C2, D, F, DM, X and Y. Eur. J. Med. Chem. 2019, 174, 130–141. [Google Scholar] [CrossRef]



| Extracts | Cell Lines IC50 (µg/mL) | |||
|---|---|---|---|---|
| HeLa | RAW 264.7 | A549 | L929 | |
| Gsm | ND | 81.9 ± 4.3 | ND | ND |
| Gsfb | <25 | <25 | <25 | <25 |
| Gwm | ND | ND | ND | ND |
| Gwfb | 57.7 ± 6.8 | <25 | 42.8 ± 5.5 | <25 |
| 13C a | Functional Group | C Position |
|---|---|---|
| 164.0 | C=C b | C-9 |
| 171.8 | COOH | C-26 |
| 199.3 | C=O | C-11 |
| 206.6 | C=O | C-23 |
| 219.1 | C=O | C-3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Peña, D.; Torres-Moreno, H.; Vidal-Gutiérrez, M.; Robles-Zepeda, R.E.; Gutiérrez, A.; Esqueda, M. Antiproliferative Activity of Mycelium vs. Fruiting Body: Ganoderma subincrustatum and G. weberianum from Sonora, Mexico. Microbiol. Res. 2023, 14, 1534-1544. https://doi.org/10.3390/microbiolres14040105
López-Peña D, Torres-Moreno H, Vidal-Gutiérrez M, Robles-Zepeda RE, Gutiérrez A, Esqueda M. Antiproliferative Activity of Mycelium vs. Fruiting Body: Ganoderma subincrustatum and G. weberianum from Sonora, Mexico. Microbiology Research. 2023; 14(4):1534-1544. https://doi.org/10.3390/microbiolres14040105
Chicago/Turabian StyleLópez-Peña, Damian, Heriberto Torres-Moreno, Max Vidal-Gutiérrez, Ramón Enrique Robles-Zepeda, Aldo Gutiérrez, and Martín Esqueda. 2023. "Antiproliferative Activity of Mycelium vs. Fruiting Body: Ganoderma subincrustatum and G. weberianum from Sonora, Mexico" Microbiology Research 14, no. 4: 1534-1544. https://doi.org/10.3390/microbiolres14040105
APA StyleLópez-Peña, D., Torres-Moreno, H., Vidal-Gutiérrez, M., Robles-Zepeda, R. E., Gutiérrez, A., & Esqueda, M. (2023). Antiproliferative Activity of Mycelium vs. Fruiting Body: Ganoderma subincrustatum and G. weberianum from Sonora, Mexico. Microbiology Research, 14(4), 1534-1544. https://doi.org/10.3390/microbiolres14040105







