Evaluation of Outer Surface Protein Vaccine Candidates of Borrelia burgdorferi for Lyme Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Production and Purification of Recombinant Proteins
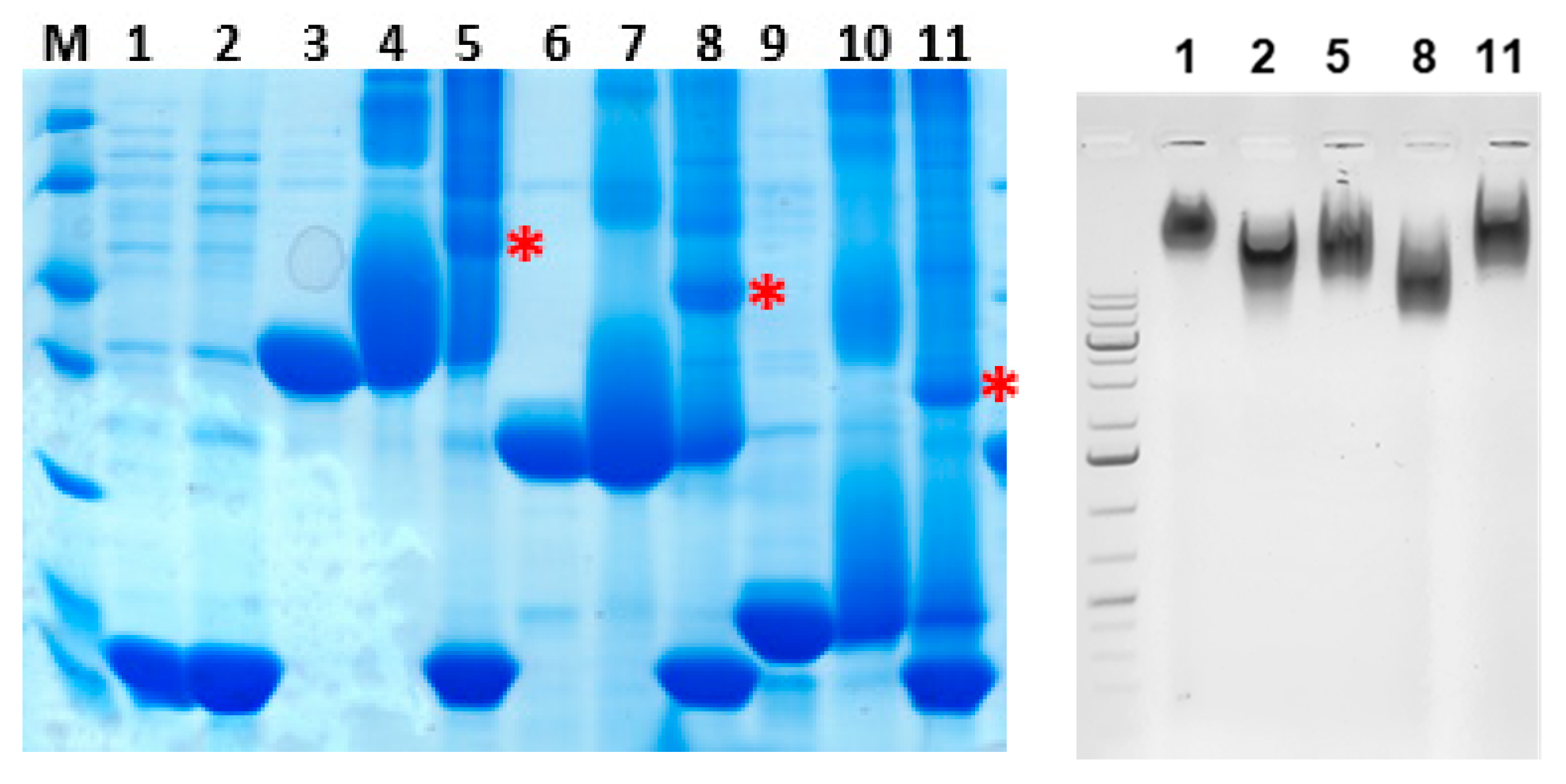
2.2. Generation of Vaccine Candidates and Coupling to Bacteriophage Qβ VLPs
2.3. Animal Studies
2.4. Evaluation of Vaccine-Induced Antibody Responses
2.5. B. burgdorferi Strain and Bactericidal Activity of Serum from Immunized Mice
3. Results
3.1. Choice of Vaccine Candidate Proteins
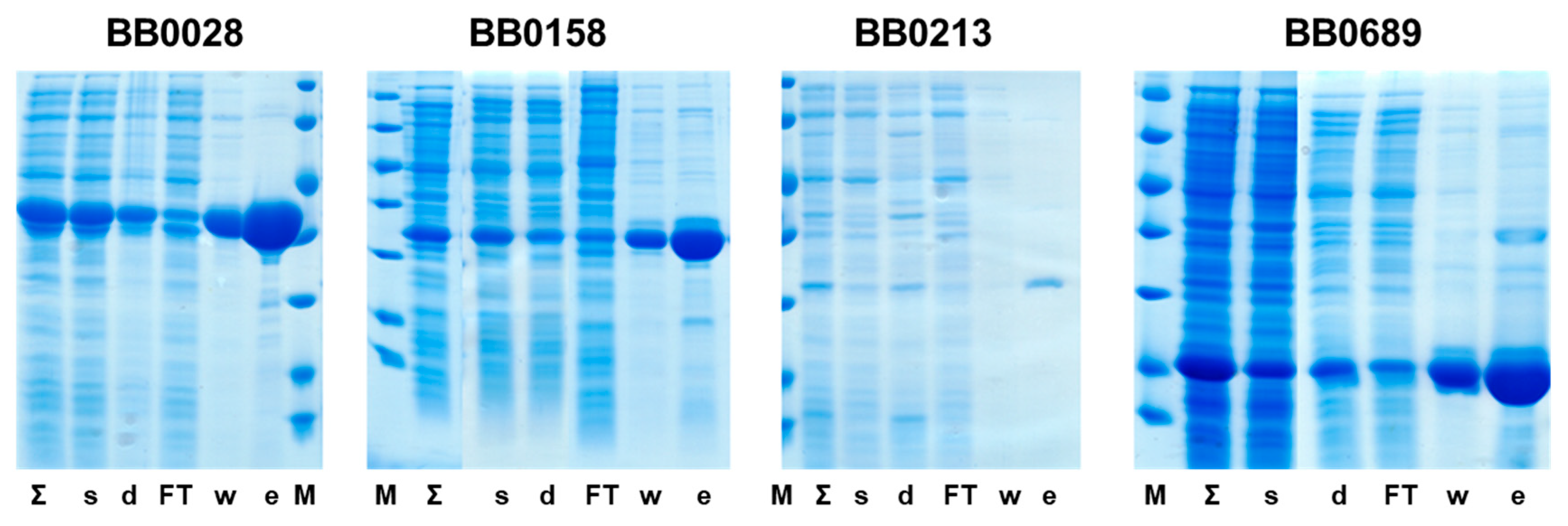
3.2. Production of Proteins for Vaccination
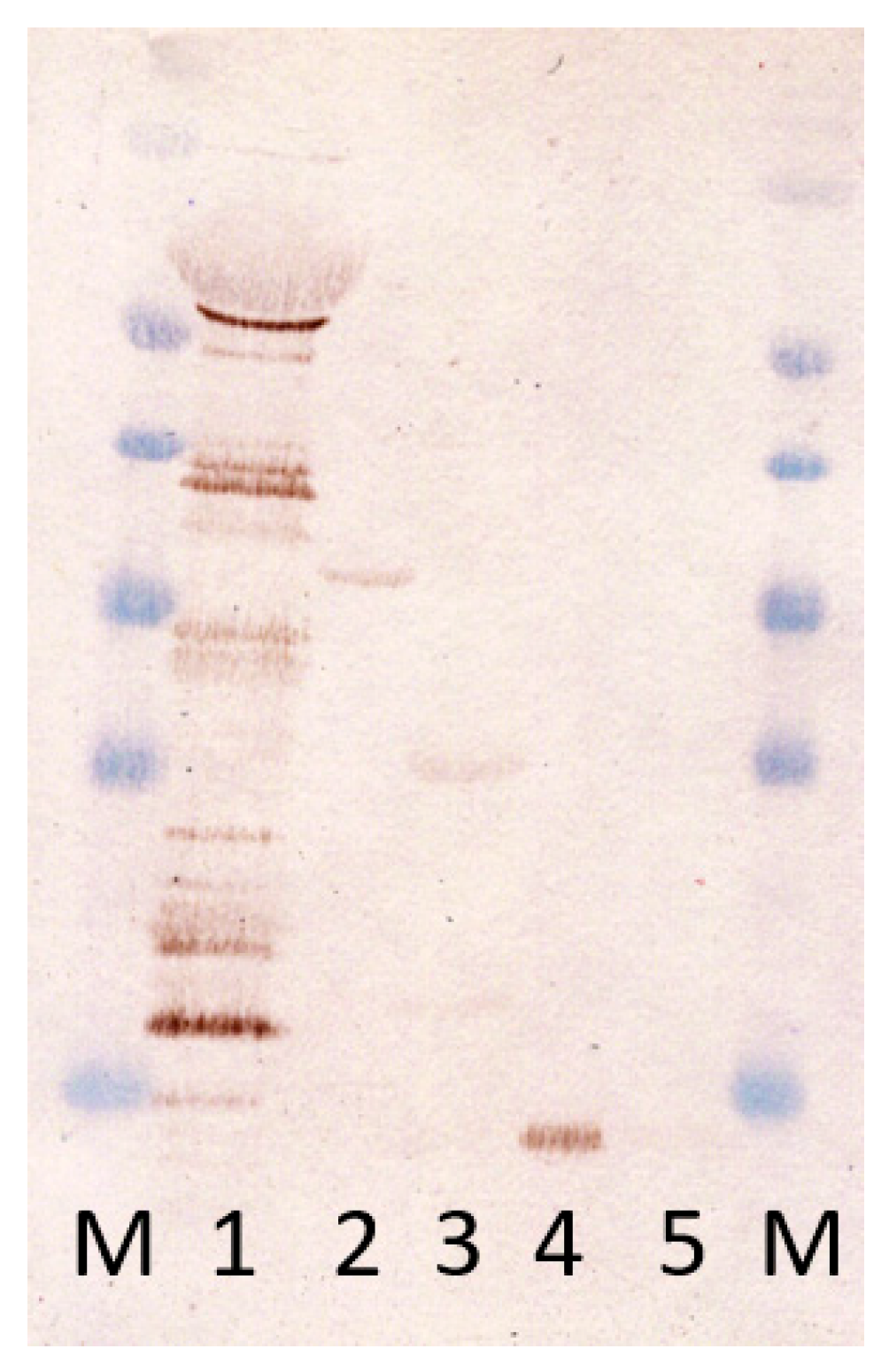
3.3. Presence of Antibodies against Produced Antigens in Infected Mice
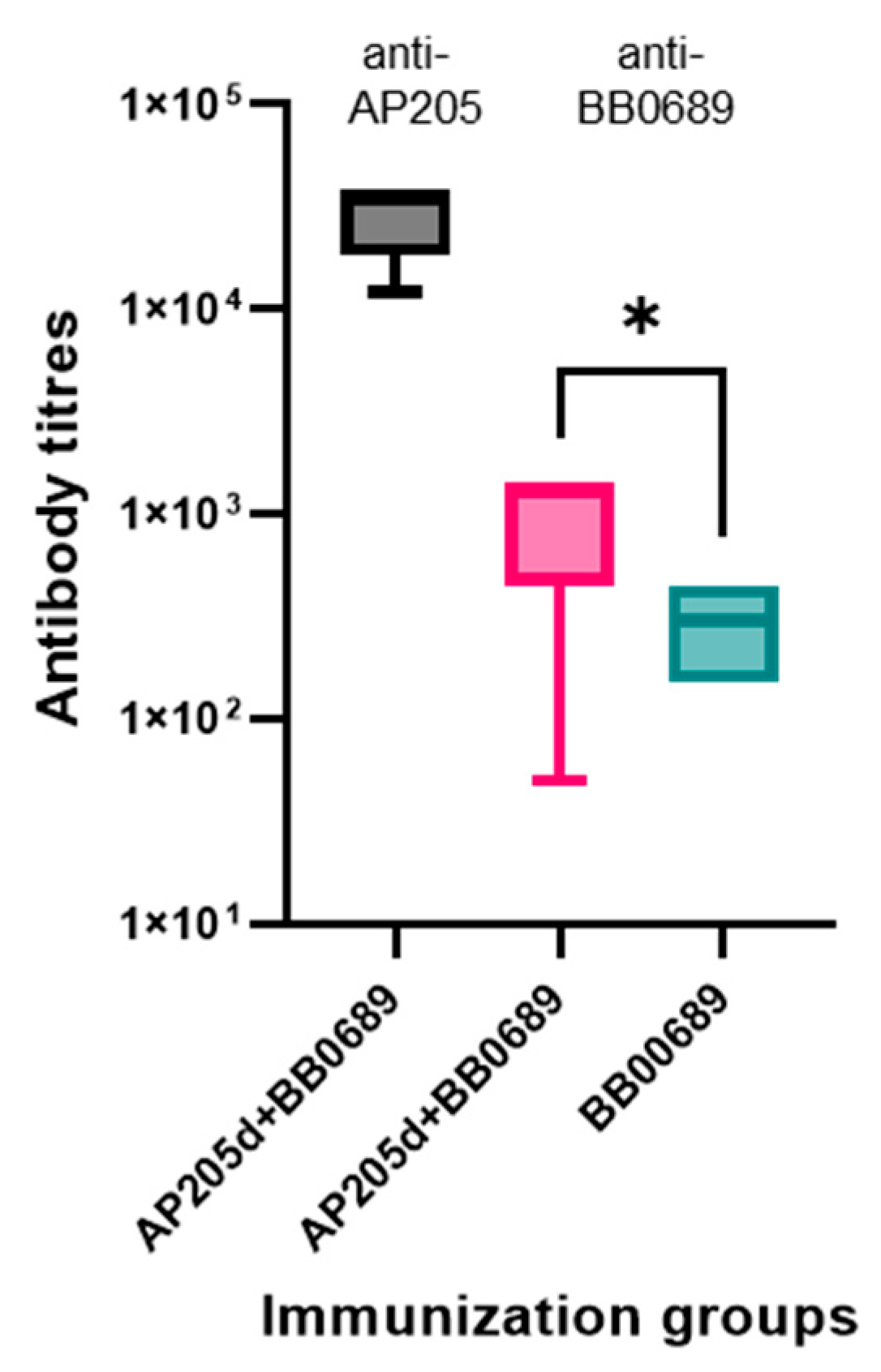
3.4. Immunogenicity Studies in Mice
3.5. Bactericidal Efficacy Testing of Obtained Sera
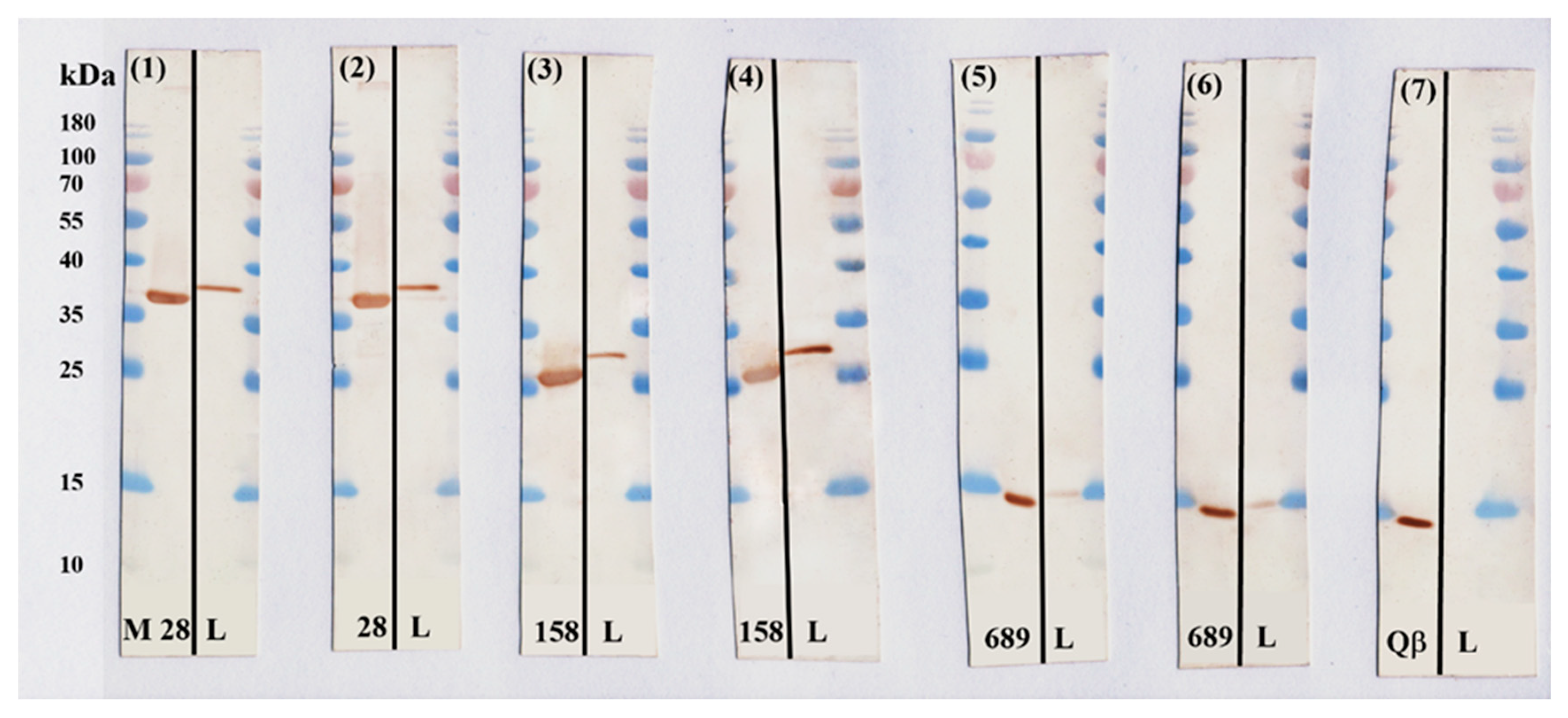
3.6. Confirmation of the Presence of Selected Antigens in B. burgdorferi Bacteria
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burgdorfer, W. Discovery of the Lyme disease spirochete and its relation to tick vectors. Yale J. Biol. Med. 1984, 57, 515–520. [Google Scholar]
- Marques, A.R.; Strle, F.; Wormser, G.P. Comparison of Lyme Disease in the United States and Europe. Emerg. Infect. Dis. 2021, 27, 2017–2024. [Google Scholar] [CrossRef] [PubMed]
- Bobe, J.R.; Jutras, B.L.; Horn, E.J.; Embers, M.E.; Bailey, A.; Moritz, R.L.; Zhang, Y.; Soloski, M.J.; Ostfeld, R.S.; Marconi, R.T.; et al. Recent Progress in Lyme Disease and Remaining Challenges. Front. Med. 2021, 8, 666554. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhou, G.; Cao, W.; Xu, X.; Zhang, Y.; Ji, Z.; Yang, J.; Chen, J.; Liu, M.; Fan, Y.; et al. Global seroprevalence and sociodemographic characteristics of Borrelia burgdorferi sensu lato in human populations: A systematic review and meta-analysis. BMJ Glob. Health 2022, 7, e007744. [Google Scholar] [CrossRef] [PubMed]
- Kugeler, K.J.; Schwartz, A.M.; Delorey, M.J.; Mead, P.S.; Hinckley, A.F. Estimating the Frequency of Lyme Disease Diagnoses, United States, 2010–2018. Emerg. Infect. Dis. 2021, 27, 616–619. [Google Scholar]
- Wright, W.F.; Riedel, D.J.; Talwani, R.; Gilliam, B.L. Diagnosis and management of Lyme disease. Am. Fam. Physician 2012, 85, 1086–1093. [Google Scholar]
- Aronowitz, R.A. The rise and fall of the lyme disease vaccines: A cautionary tale for risk interventions in American medicine and public health. Milbank Q. 2012, 90, 250–277. [Google Scholar] [CrossRef]
- Dattwyler, R.J.; Gomes-Solecki, M. The year that shaped the outcome of the OspA vaccine for human Lyme disease. Npj Vaccines 2022, 7, 10. [Google Scholar] [CrossRef]
- Pfeifle, A.; Raman, S.N.T.; Lansdell, C.; Zhang, W.; Tamming, L.; Cecillon, J.; Laryea, E.; Patel, D.; Wu, J.; Gravel, C.; et al. DNA lipid nanoparticle vaccine targeting outer surface protein C affords protection against homologous Borrelia burgdorferi needle challenge in mice. Front. Immunol. 2023, 14, 1020134. [Google Scholar] [CrossRef]
- Kung, F.; Kaur, S.; Smith, A.A.; Yang, X.; Wilder, C.N.; Sharma, K.; Buyuktanir, O.; Pal, U. A Borrelia burgdorferi Surface-Exposed Transmembrane Protein Lacking Detectable Immune Responses Supports Pathogen Persistence and Constitutes a Vaccine Target. J. Infect. Dis. 2016, 213, 1786–1795. [Google Scholar] [CrossRef]
- Brandt, K.S.; Patton, T.G.; Allard, A.S.; Caimano, M.J.; Radolf, J.D.; Gilmore, R.D. Evaluation of the Borrelia burgdorferi BBA64 protein as a protective immunogen in mice. Clin. Vaccine Immunol. 2014, 21, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Labandeira-Rey, M.; Baker, E.A.; Skare, J.T. VraA (BBI16) protein of Borrelia burgdorferi is a surface-exposed antigen with a repetitive motif that confers partial protection against experimental Lyme borreliosis. Infect. Immun. 2001, 69, 1409–1419. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kumar, M.; Kaur, S.; Kariu, T.; Yang, X.; Bossis, I.; Anderson, J.F.; Pal, U. Borrelia burgdorferi BBA52 is a potential target for transmission blocking Lyme disease vaccine. Vaccine 2011, 29, 9012–9019. [Google Scholar] [CrossRef] [PubMed]
- Fikrig, E.; Feng, W.; Barthold, S.W.; Telford, S.R.; Flavell, R.A. Arthropod- and host-specific Borrelia burgdorferi bbk32 expression and the inhibition of spirochete transmission. J. Immunol. 2000, 164, 5344–5351. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, A.J.; Russell, T.M.; Dolan, M.C.; Williams, M.; Hojgaard, A.; Weiner, Z.P.; Johnson, B.J.B. Evaluation of Borrelia burgdorferi BbHtrA Protease as a Vaccine Candidate for Lyme Borreliosis in Mice. PLoS ONE 2015, 10, e0128868. [Google Scholar] [CrossRef] [PubMed]
- Brissette, C.A.; Bykowski, T.; Cooley, A.E.; Bowman, A.; Stevenson, B. Borrelia burgdorferi RevA antigen binds host fibronectin. Infect. Immun. 2009, 77, 2802–2812. [Google Scholar] [CrossRef]
- Floden, A.M.; Gonzalez, T.; Gaultney, R.A.; Brissette, C.A. Evaluation of RevA, a fibronectin-binding protein of Borrelia burgdorferi, as a potential vaccine candidate for lyme disease. Clin. Vaccine Immunol. 2013, 20, 892–899. [Google Scholar] [CrossRef]
- Rogers, E.A.; Abdunnur, S.V.; McDowell, J.V.; Marconi, R.T. Comparative analysis of the properties and ligand binding characteristics of CspZ, a factor H binding protein, derived from Borrelia burgdorferi isolates of human origin. Infect. Immun. 2009, 77, 4396–4405. [Google Scholar] [CrossRef]
- Marcinkiewicz, A.L.; Lieknina, I.; Yang, X.; Lederman, P.L.; Hart, T.M.; Yates, J.; Chen, W.-H.; Bottazzi, M.E.; Mantis, N.J.; Kraiczy, P.; et al. The Factor H-Binding Site of CspZ as a Protective Target against Multistrain, Tick-Transmitted Lyme Disease. Infect. Immun. 2020, 88, e00956-19. [Google Scholar] [CrossRef]
- Comstedt, P.; Schüler, W.; Meinke, A.; Lundberg, U. The novel Lyme borreliosis vaccine VLA15 shows broad protection against Borrelia species expressing six different OspA serotypes. PLoS ONE 2017, 12, e0184357. [Google Scholar] [CrossRef]
- El-Sayed, S.A.; Rizk, M.A.; Eldoumani, H.; Sorour, S.S.; Terkawi, M.A.; AbouLaila, M.; Igarashi, I.; Sayed-Ahmed, M.Z. Identification and Characterization of P0 Protein as a Vaccine Candidate Against, Blood Parasite of Veterinary and Zoonotic Importance. Front. Vet. Sci. 2022, 8, 795906. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, S.A.E.-S.; Rizk, M.A.; Terkawi, M.A.; Yokoyama, N.; Igarashi, I. Molecular identification and antigenic characterization of Babesia divergens Erythrocyte Binding Protein (BdEBP) as a potential vaccine candidate. Parasitol. Int. 2017, 66, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Grgacic, E.V.; Anderson, D.A. Virus-like particles: Passport to immune recognition. Methods 2006, 40, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Pumpens, P.; Renhofa, R.; Dishlers, A.; Kozlovska, T.; Ose, V.; Pushko, P.; Tars, K.; Grens, E.; Bachmann, M.F. The True Story and Advantages of RNA Phage Capsids as Nanotools. Intervirology 2016, 59, 74–110. [Google Scholar] [CrossRef]
- Nassal, M.; Skamel, C.; Vogel, M.; Kratz, P.A.; Stehle, T.; Wallich, R.; Simon, M.M. Development of hepatitis B virus capsids into a whole-chain protein antigen display platform: New particulate Lyme disease vaccines. Int. J. Med. Microbiol. 2008, 298, 135–142. [Google Scholar] [CrossRef]
- Marcinkiewicz, A.L.; Lieknina, I.; Kotelovica, S.; Yang, X.; Kraiczy, P.; Pal, U.; Lin, Y.-P.; Tars, K. Eliminating Factor H-Binding Activity of Borrelia burgdorferi CspZ Combined with Virus-Like Particle Conjugation Enhances Its Efficacy as a Lyme Disease Vaccine. Front. Immunol. 2018, 9, 181. [Google Scholar] [CrossRef]
- Brangulis, K.; Jaudzems, K.; Petrovskis, I.; Akopjana, I.; Kazaks, A.; Tars, K. Structural and functional analysis of BB0689 from Borrelia burgdorferi, a member of the bacterial CAP superfamily. J. Struct. Biol. 2015, 192, 320–330. [Google Scholar] [CrossRef]
- Brune, K.D.; Liekniņa, I.; Sutov, G.; Morris, A.R.; Jovicevic, D.; Kalniņš, G.; Kazāks, A.; Kluga, R.; Kastaljana, S.; Zajakina, A.; et al. N-Terminal Modification of Gly-His-Tagged Proteins with Azidogluconolactone. Chembiochem 2021, 22, 3199–3207. [Google Scholar] [CrossRef]
- Pine, M.; Arora, G.; Hart, T.M.; Bettini, E.; Gaudette, B.T.; Muramatsu, H.; Tombácz, I.; Kambayashi, T.; Tam, Y.K.; Brisson, T.; et al. Development of an mRNA-lipid nanoparticle vaccine against Lyme disease. Mol. Ther. 2023, 31, 2702–2714. [Google Scholar] [CrossRef]
- Chen, Y.L.; Marcinkiewicz, A.L.; Nowak, T.A.; Kundu, R.T.; Liu, Z.; Strych, U.; Bottazzi, M.E.; Chen, W.-H.; Lin, Y.-P. CspZ FH-Binding Sites as Epitopes Promote Antibody-Mediated Lyme Borreliae Clearance. Infect. Immun. 2022, 90, e0006222. [Google Scholar] [CrossRef]
- Klouwens, M.J.; Salverda, M.; Trentelman, J.; Ersoz, J.; Wagemakers, A.; Gerritzen, M.; van der Ley, P.; Hovius, J. Vaccination with meningococcal outer membrane vesicles carrying OspA protects against experimental Lyme borreliosis. Vaccine 2021, 39, 2561–2567. [Google Scholar] [CrossRef] [PubMed]
- Klouwens, M.J.; Trentelman, J.J.; Ersoz, J.I.; Nieves Marques Porto, F.; Sima, R.; Hajdusek, O.; Thakur, M.; Pal, U.; Hovius, J.W. Investigating BB0405 as a novel vaccination candidate in Lyme borreliosis. Sci. Rep. 2021, 11, 4775. [Google Scholar] [CrossRef] [PubMed]
- Guibinga, G.H.; Sahay, B.; Brown, H.; Cooch, N.; Chen, J.; Yan, J.; Reed, C.; Mishra, M.; Yung, B.; Pugh, H.; et al. Protection against infection mediated by a synthetically engineered DNA vaccine. Hum. Vaccines Immunother. 2020, 16, 2114–2122. [Google Scholar] [CrossRef] [PubMed]
- Dishlers, A.; Petrovskis, I.; Skrastina, D.; Zarina, I.; Lieknina, I.; Jansons, J.; Akopjana, I.; Zakova, J.; Ose, V.; Sominskaya, I. PreS1 Containing HBc VLPs for the Development of a Combined Therapeutic/Prophylactic Hepatitis B Vaccine. Microorganisms 2023, 11, 972. [Google Scholar] [CrossRef] [PubMed]
- Dowdell, A.S.; Murphy, M.D.; Azodi, C.; Swanson, S.K.; Florens, L.; Chen, S.; Zückert, W.R. Comprehensive Spatial Analysis of the Borrelia burgdorferi Lipoproteome Reveals a Compartmentalization Bias toward the Bacterial Surface. J. Bacteriol. 2017, 199, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Lenhart, T.R.; Kenedy, M.R.; Yang, X.; Pal, U.; Akins, D.R. BB0324 and BB0028 are constituents of the Borrelia burgdorferi beta-barrel assembly machine (BAM) complex. BMC Microbiol. 2012, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Seiler, K.P.; Eichwald, E.J.; Weis, J.H.; Teuscher, C.; Weis, J.J. Distinct characteristics of resistance to Borrelia burgdorferi-induced arthritis in C57BL/6N mice. Infect. Immun. 1998, 66, 161–168. [Google Scholar] [CrossRef]
- Klouwens, M.J.; Trentelman, J.J.; Wagemakers, A.; Ersoz, J.I.; Bins, A.D.; Hovius, J.W. Tick-Tattoo: DNA Vaccination Against or Tick Proteins. Front. Immunol. 2021, 12, 615011. [Google Scholar] [CrossRef]
- Gaber, A.M.; Mandric, I.; Nitirahardjo, C.; Piontkivska, H.; Hillhouse, A.E.; Threadgill, D.W.; Zelikovsky, A.; Rogovskyy, A.S. Comparative transcriptome analysis of and C3H mice infected with the Lyme disease pathogen. Front. Cell. Infect. Microbiol. 2023, 13, 33. [Google Scholar] [CrossRef]
- Kamp, H.D.; Swanson, K.A.; Wei, R.R.; Dhal, P.K.; Dharanipragada, R.; Kern, A.; Sharma, B.; Sima, R.; Hajdusek, O.; Hu, L.T.; et al. Design of a broadly reactive Lyme disease vaccine. Npj Vaccines 2020, 5, 1–19. [Google Scholar] [CrossRef]
- Kenedy, M.R.; Lenhart, T.R.; Akins, D.R. The role of Borrelia burgdorferi outer surface proteins. FEMS Immunol. Med. Microbiol. 2012, 66, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Krenger, P.; Krueger, C.C.; Zha, L.; Han, J.; Yermanos, A.; Roongta, S.; Mohsen, M.O.; Oxenius, A.; Vogel, M.; et al. TLR7 Signaling Shapes and Maintains Antibody Diversity Upon Virus-Like Particle Immunization. Front. Immunol. 2021, 12, 827256. [Google Scholar] [CrossRef] [PubMed]
- Doucet, M.; El-Turabi, A.; Zabel, F.; Hunn, B.H.; Bengoa-Vergniory, N.; Cioroch, M.; Ramm, M.; Smith, A.M.; Gomes, A.C.; de Miranda, G.C.; et al. Preclinical development of a vaccine against oligomeric alpha-synuclein based on virus-like particles. PLoS ONE 2017, 12, e0181844. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, N.; Dietmeier, K.; Bauer, M.; Maudrich, M.; Utzinger, S.; Muntwiler, S.; Saudan, P.; Bachmann, M.F. Displaying Fel d1 on virus-like particles prevents reactogenicity despite greatly enhanced immunogenicity: A novel therapy for cat allergy. J. Exp. Med. 2009, 206, 1941–1955. [Google Scholar] [CrossRef]
- Brooks, C.S.; Vuppala, S.R.; Jett, A.M.; Akins, D.R. Identification of Borrelia burgdorferi outer surface proteins. Infect. Immun. 2006, 74, 296–304. [Google Scholar] [CrossRef]
- Golde, W.T.; Piesman, J.; Dolan, M.C.; Kramer, M.; Hauser, P.; Lobet, Y.; Capiau, C.; Desmons, P.; Voet, P.; Dearwester, D.; et al. Reactivity with a specific epitope of outer surface protein A predicts protection from infection with the Lyme disease spirochete, Borrelia burgdorferi. Infect. Immun. 1997, 65, 882–889. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liekniņa, I.; Kozlova, A.; Šaško, M.; Akopjana, I.; Brangulis, K.; Tārs, K. Evaluation of Outer Surface Protein Vaccine Candidates of Borrelia burgdorferi for Lyme Disease. Microbiol. Res. 2023, 14, 2022-2033. https://doi.org/10.3390/microbiolres14040136
Liekniņa I, Kozlova A, Šaško M, Akopjana I, Brangulis K, Tārs K. Evaluation of Outer Surface Protein Vaccine Candidates of Borrelia burgdorferi for Lyme Disease. Microbiology Research. 2023; 14(4):2022-2033. https://doi.org/10.3390/microbiolres14040136
Chicago/Turabian StyleLiekniņa, Ilva, Anna Kozlova, Marina Šaško, Ināra Akopjana, Kalvis Brangulis, and Kaspars Tārs. 2023. "Evaluation of Outer Surface Protein Vaccine Candidates of Borrelia burgdorferi for Lyme Disease" Microbiology Research 14, no. 4: 2022-2033. https://doi.org/10.3390/microbiolres14040136
APA StyleLiekniņa, I., Kozlova, A., Šaško, M., Akopjana, I., Brangulis, K., & Tārs, K. (2023). Evaluation of Outer Surface Protein Vaccine Candidates of Borrelia burgdorferi for Lyme Disease. Microbiology Research, 14(4), 2022-2033. https://doi.org/10.3390/microbiolres14040136




