Tahiti Lemon Juice: A Natural Alternative to Reduce Bacteria from Eggshells
Abstract
1. Introduction
2. Materials and Methods
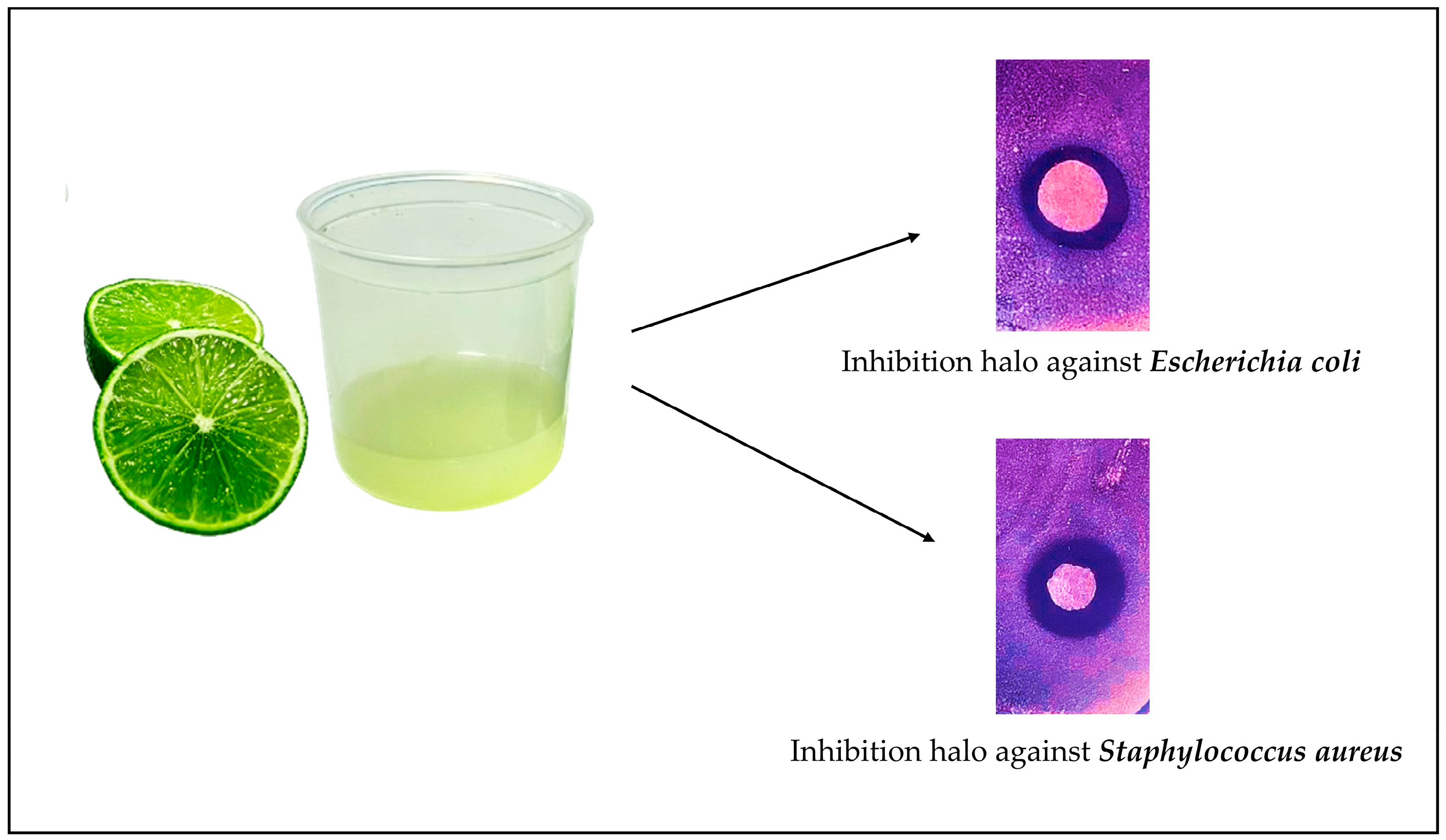
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wen, C.; Li, Q.; Lan, F.; Li, X.; Li, G.; Yan, Y.; Wu, G.; Yang, N.; Sun, C. Microbiota Continuum Along the Chicken Oviduct And Its Association With Host Genetics And Egg Formation. Poult. Sci. 2021, 100, 101104. [Google Scholar] [CrossRef] [PubMed]
- Cader, S.; Goburdhun, D.; Neetoo, H. Assessment of the Microbial Safety and Quality of Eggs from Small and Large-Scale Hen Breeders. J. World’s Poult. Res. 2014, 4, 75–81. [Google Scholar]
- Chan, H.Y.; Meor Hussin, A.S.; Ahmad, N.H.; Rukayadi, Y.; Farouk, A.E. Effectiveness of Quaternary Ammonium in Reducing Microbial Load on Eggs. Molecules 2021, 26, 5259. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.d.S.; McManus, C.; Salgado, C.B.; dos Santos, V.M. Effects of Sanitizers on Microbiological Control of Hatching Eggshells and Poultry Health during Embryogenesis and Early Stages after Hatching in the Last Decade. Animals 2022, 12, 2826. [Google Scholar] [CrossRef] [PubMed]
- Batkowska, J.; Wlazlo, L.; Drabik, K.; Nowakowicz-Debek, B.; Al-Shammari, K.I.A.; Gryzinska, M. Evaluation of Grapefruit Juice (Citrus paradisi) as an Alternative Disinfectant for Hatching Eggs. Pak. J. Zool. 2018, 50, 647–653. [Google Scholar] [CrossRef]
- Taşdemir, A.N.; Onbaşılar, E.E.; Yalçın, S.; Boyalı, B.; Aygören, H.; Tülek, E.; Sarıçam, S.; Akan, M. Effects of Oregano Juice on Eggshell Microbial Load, Layer Embryo Development, Hatching Results, and Growth at the First 2 Weeks after Hatch. Trop. Anim. Health Prod. 2021, 53, 404. [Google Scholar] [CrossRef] [PubMed]
- Enejoh, O.S.; Ogunyemi, I.O.; Bala, M.S.; Oruene, I.S.; Suleiman, M.M.; Ambali, S.F. Ethnomedical Importance of Citrus Aurantifolia (Christm) Swingle. Pharm. Innov. J. 2015, 4, 1–6. [Google Scholar]
- Coban, H.B. Organic Acids as Antimicrobial Food Agents: Applications and Microbial Productions. Bioprocess Biosyst. Eng. 2020, 43, 569–591. [Google Scholar] [CrossRef] [PubMed]
- Mohd-Hanif, H.; Shamsudin, R.; Adzahan, N.M. UVC Dosage Effects on The Physico-Chemical Properties of Lime (Citrus aurantifolia) juice. Food Sci. Biotechnol. 2016, 25, 63–67. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic Susceptibility Testing by a Standardized Single Disk Method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Abdulwahid, A.T.; Nasear, H.A.; Ghazi, S.S. Disinfection of Table Eggs Using Lemon Juice as a Natural Biocide. Basrah J. Vet. Res. 2020, 19, 177–189. [Google Scholar]
- Oliveira, G.D.S.; McManus, C.; Pires, P.G.D.S.; dos Santos, V.M. Combination of Cassava Starch Biopolymer and Essential Oils for Coating Table Eggs. Front. Sustain. Food Syst. 2022, 6, 957229. [Google Scholar] [CrossRef]
- Oliveira, G.d.S.; McManus, C.; Salgado, C.B.; Pires, P.G.d.S.; de Figueiredo Sousa, H.A.; da Silva, E.R.; dos Santos, V.M. Antimicrobial Coating Based on Tahiti Lemon Essential Oil and Green Banana Flour to Preserve the Internal Quality of Quail Eggs. Animals 2023, 13, 2123. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.d.S.; McManus, C.; Vale, I.R.R.; Dos Santos, V.M. Obtaining Microbiologically Safe Hatching Eggs from Hatcheries: Using Essential Oils for Integrated Sanitization Strategies in Hatching Eggs, Poultry Houses and Poultry. Pathogens 2024, 13, 260. [Google Scholar] [CrossRef]
- Jesumirhewe, C.; Omeke, N.M.; Ogunlowo, O.P. Comparison of Phytochemicals, Antioxidant and Antimicrobial Activity of Peels and Juices of Citrus Species. Fudma J. Sci. 2019, 3, 258–265. [Google Scholar]
- Ugwu, C.C.; Mbah-Omeje, K.N.; Ezeugwu, R.I.; Onuorah, S.C.; Agbo, M.C. Antimicrobial Activities and Phytochemical Screening of Citrus Aurantifoila (Lime) Leaf Extracts and Fruit Juice on Some Microorganisms. Int. J. Innov. Res. Dev. 2018, 7, 135–142. [Google Scholar]
- Shakya, A.; Luitel, B.; Kumari, P.; Devkota, R.; Dahal, P.R.; Chaudhary, R. Comparative study of Antibacterial Activity of Juice and Peel Extract of Citrus Fruits. Tribhuvan Univ. J. Microbiol. 2019, 6, 82–88. [Google Scholar] [CrossRef]
- Izah, S.C.; Odubo, T.C. Effects of Citrus aurantifolia Fruit Juice on Selected Pathogens of Public Health Importance. ES Food Agrofor. 2023, 11, 829. [Google Scholar] [CrossRef]
- Álvarez-Ordóñez, A.; Carvajal, A.; Arguello, H.; Martínez-Lobo, F.J.; Naharro, G.; Rubio, P. Antibacterial Activity and Mode of Action of a Commercial Citrus Fruit Extract. J. Appl. Microbiol. 2013, 115, 50–60. [Google Scholar] [CrossRef]
- Li, X.; Anderson, D.; Rathgeber, B.; McLean, N.; MacIsaac, J. Fumigating Broiler Hatching Eggs with Lysozyme Product (Inovapure) to Reduce Eggshell Microbial Load. Poult. Sci. 2018, 97, 4252–4261. [Google Scholar] [CrossRef]
- Oliveira, G.S.; dos Santos, V.M.; Nascimento, S.T.; Rodrigues, J.C. Alternative Sanitizers to Paraformaldehyde for Incubation of Fertile Eggs. Poult. Sci. 2020, 99, 2001–2006. [Google Scholar] [CrossRef] [PubMed]

| Treatments | Total Aerobic Mesophilic Bacteria (log10 CFU/mL) | Enterobacteriaceae (log10 CFU/mL) |
|---|---|---|
| Non-sanitized eggs | 5.49 ± 0.12 a | 2.57 ± 0.51 a |
| Quaternary ammonium | 1.02 ± 0.29 c | 0.00 ± 0.00 b |
| Tahiti lemon juice | 2.07 ± 0.18 b | 0.65 ± 0.56 b |
| p value | <0.0001 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Jesus, L.M.; Oliveira, G.d.S.; Vale, I.R.R.; McManus, C.; Sousa, H.A.d.F.; dos Santos, V.M. Tahiti Lemon Juice: A Natural Alternative to Reduce Bacteria from Eggshells. Microbiol. Res. 2024, 15, 1406-1411. https://doi.org/10.3390/microbiolres15030094
de Jesus LM, Oliveira GdS, Vale IRR, McManus C, Sousa HAdF, dos Santos VM. Tahiti Lemon Juice: A Natural Alternative to Reduce Bacteria from Eggshells. Microbiology Research. 2024; 15(3):1406-1411. https://doi.org/10.3390/microbiolres15030094
Chicago/Turabian Stylede Jesus, Luana Maria, Gabriel da Silva Oliveira, Igor Rafael Ribeiro Vale, Concepta McManus, Heloisa Alves de Figueiredo Sousa, and Vinícius Machado dos Santos. 2024. "Tahiti Lemon Juice: A Natural Alternative to Reduce Bacteria from Eggshells" Microbiology Research 15, no. 3: 1406-1411. https://doi.org/10.3390/microbiolres15030094
APA Stylede Jesus, L. M., Oliveira, G. d. S., Vale, I. R. R., McManus, C., Sousa, H. A. d. F., & dos Santos, V. M. (2024). Tahiti Lemon Juice: A Natural Alternative to Reduce Bacteria from Eggshells. Microbiology Research, 15(3), 1406-1411. https://doi.org/10.3390/microbiolres15030094







