Comparative Genomics of Three Hybrid-Pathogen Multidrug-Resistant Escherichia coli Strains Isolated from Healthy Donors’ Feces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Genome Sequencing
2.2. Assembly and Annotation
2.3. Bioinformatic Analysis
3. Results and Discussion
3.1. General Features of the Hybrid Strains
3.2. Resistance and Virulence Features
3.3. Mobilizable Genetic Elements (MGEs)
3.4. Phylogeny
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erjavec, M.S. The Universe of Escherichia coli; IntechOpen: London, UK, 2019; Available online: https://www.intechopen.com/books/6970 (accessed on 25 February 2024).
- Águila Sánchez, A.; Rodríguez, A.; Fernández Abreu, A.; Cruz Infante, Y.; Bravo Fariñas, L.; Hernández Martínez, J.L. Escherichia coli diarreogénicos, identificación de patotipos y fenotipos de resistencia antimicrobiana en aislados cubanos. Rev. Cuba. Med. Trop. 2020, 72, 1–19. [Google Scholar]
- Torres, A.G. Escherichia coli in the Americas; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Farfán-García, A.E.; Ariza-Rojas, S.C.; Vargas-Cárdenas, F.A.; Vargas-Remolina, L.V. Mecanismos de virulencia de Escherichia coli enteropatógena. Rev. Chil. Infectología 2016, 33, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Geurtsen, J.; De Been, M.; Weerdenburg, E.; Zomer, A.; McNally, A.; Poolman, J. Genomics and pathotypes of the many faces of Escherichia coli. FEMS Microbiol. Rev. 2022, 46, fuac031. [Google Scholar] [CrossRef] [PubMed]
- De Mello Santos, A.C.; Fernandez Santos, F.; Silva, R.M.; Gomes, T.A.T. Diversity of hybrid-and hetero-pathogenic Escherichia coli and their potential implication in more severe diseases. Front. Cell. Infect. Microbiol. 2020, 10, 339. [Google Scholar] [CrossRef]
- Nascimento, J.A.S.; Santos, F.F.; Valiatti, T.B.; Santos-Neto, J.F.; Santos, A.C.M.; Cayô, R.; Gales, A.C.; Gomes, T.A.T. Frequency and diversity of hybrid Escherichia coli strains isolated from urinary tract infections. Microorganisms 2021, 9, 693. [Google Scholar] [CrossRef]
- Tanabe, R.H.S.; Dias, R.C.B.; Orsi, H.; de Lira, D.R.P.; Vieira, M.A.; dos Santos, L.F.; Ferreira, A.M.; Rall, V.L.M.; Mondelli, A.L.; Gomes, T.A.T.; et al. Characterization of Uropathogenic Escherichia coli Reveals Hybrid Isolates of Uropathogenic and Diarrheagenic (UPEC/DEC) E. coli. Microorganisms 2022, 10, 645. [Google Scholar] [CrossRef]
- Nunes, K.O.; Santos, A.C.P.; Bando, S.Y.; Silva, R.M.; Gomes, T.A.T.; Elias, W.P. Enteroaggregative Escherichia coli with uropathogenic characteristics are present in feces of diarrheic and healthy children. Pathog. Dis. 2017, 75, ftx106. [Google Scholar] [CrossRef] [PubMed]
- Salmani, H.; Azarnezhad, A.; Fayazi, M.R.; Hosseini, A. Pathotypic and Phylogenetic Study of Diarrheagenic Escherichia coli and Uropathogenic E. coli Using Multiplex Polymerase Chain Reaction. Jundishapur J. Microbiol. 2016, 9, e28331. [Google Scholar] [CrossRef]
- Frank, C.; Werber, D.; Cramer, J.P.; Askar, M.; Faber, M.; an der Heiden, M.; Krause, G. Epidemic profile of Shiga-toxin–producing Escherichia coli O104: H4 outbreak in Germany. N. Engl. J. Med. 2011, 365, 1771–1780. [Google Scholar] [CrossRef]
- Bai, X.; Zhang, J.; Ambikan, A.; Jernberg, C.; Ehricht, R.; Scheutz, F.; Matussek, A. Molecular characterization and comparative genomics of clinical hybrid Shiga toxin-producing and enterotoxigenic Escherichia coli (STEC/ETEC) strains in Sweden. Sci. Rep. 2019, 9, 5619. [Google Scholar] [CrossRef]
- Lee, W.; Kim, M.H.; Sung, S.; Kim, E.; An, E.S.; Kim, S.H.; Kim, S.H.; Kim, H.Y. Genome-Based Characterization of Hybrid Shiga Toxin-Producing and Enterotoxigenic Escherichia coli (STEC/ETEC) Strains Isolated in South Korea, 2016–2020. Microorganisms 2023, 11, 1285. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Moreno, E.; Caporal-Hernandez, L.; Méndez-Pfeiffer, P.; Enciso-Martínez, Y.; De la Rosa López, R.; Valencia, D. Characterization of Diarreaghenic Escherichia coli Strains Isolated from Healthy Donors, including a Triple Hybrid Strain. Antibiotics 2022, 11, 833. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Enríquez, J.Z.; Arenas-Hernández, M.M.; Barrios-Villa, E. Draft genome sequence of a triple hybrid Escherichia coli strain isolated from a healthy donor feces. Microbiol. Resour. Announc. 2014, 13, e00113-24. [Google Scholar] [CrossRef] [PubMed]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid annotations using subsystems technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Tatusova, T.; DiCuccio, M.; Badretdin, A.; Chetvernin, V.; Nawrocki, E.P.; Zaslavsky, L.; Lomsadze, A.; Pruitt, K.D.; Borodovsky, M.; Ostell, J. NCBI Prokaryotic Genome Annot. Pipeline. Nucleic Acids Res. 2016, 44, 6614–6624. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, C.; Laird, M.R.; Williams, K.P.; Simon Fraser University Research Computing Group; Lau, B.Y.; Hoad, G.; Winsor, G.L.; Brinkman, F.S.L. IslandViewer 4: Expanded prediction of genomic islands for larger-scale datasets. Nucleic Acids Res. 2017, 45, W30–W35. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.R.; Enns, E.; Marinier, E.; Mandal, A.; Herman, E.K.; Chen, C.Y.; Graham, M.; Van Domselaar, G.; Stothard, P. Proksee: In-depth characterization and visualization of bacterial genomes. Nucleic Acids Res. 2023, 51, W484–W492. [Google Scholar] [CrossRef] [PubMed]
- Jain, C.; Rodriguez-R, L.M.; Phillippy, A.M.; Konstantinidis, K.T.; Aluru, S. High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat. Commun. 2018, 9, 5114. [Google Scholar] [CrossRef]
- Joensen, K.G.; Tetzschner, A.M.; Iguchi, A.; Aarestrup, F.M.; Scheutz, F. Rapid and easy in silico serotyping of Escherichia coli using whole genome sequencing (WGS) data. J. Clin. Microbiol. 2015, 53, 2410–2426. [Google Scholar] [CrossRef]
- Roer, L.; Tchesnokova, V.; Allesøe, R.; Muradova, M.; Chattopadhyay, S.; Ahrenfeldt, J.; Thomsen, M.C.F.; Lund, O.; Hansen, F.; Hammerum, A.M.; et al. Development of a Web Tool for Escherichia coli Subtyping Based on fimH Alleles. J. Clin. Microbiol. 2017, 55, 2538–2543. [Google Scholar] [CrossRef] [PubMed]
- Bortolaia, V.; Kaas, R.S.; Ruppe, E.; Roberts, M.C.; Schwarz, S.; Cattoir, V.; Philippon, A.; Allesoe, R.L.; Rebelo, A.R.; Florensa, A.F.; et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J. Antimicrob. Chemother. 2020, 75, 3491–3500. [Google Scholar] [CrossRef] [PubMed]
- Zankari, E.; Allesøe, R.; Joensen, K.G.; Cavaco, L.M.; Lund, O.; Aarestrup, F.M. PointFinder: A novel web tool for WGS-based detection of antimicrobial resistance associated with chromosomal point mutations in bacterial pathogens. J. Antimicrob. Chemother. 2020, 72, 2764–2768. [Google Scholar] [CrossRef] [PubMed]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Alcock, B.P.; Huynh, W.; Chalil, R.; Smith, K.W.; Raphenya, A.R.; A Wlodarski, M.; Edalatmand, A.; Petkau, A.; A Syed, S.; Tsang, K.K.; et al. CARD 2023: Expanded curation, support for machine learning, and resistome prediction at the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res. 2023, 51, D690–D699. [Google Scholar] [CrossRef] [PubMed]
- Joensen, K.G.; Scheutz, F.; Lund, O.; Hasman, H.; Kaas, R.S.; Nielsen, E.M.; Aarestrup, F.M. Real-time whole-genome sequencing for routine typing, surveillance, and outbreak detection of verotoxigenic Escherichia coli. J. Clin. Microbiol. 2014, 52, 1501–1510. [Google Scholar] [CrossRef]
- Malberg Tetzschner, A.M.; Johnson, J.R.; Johnston, B.D.; Lund, O.; Scheutz, F. In Silico Genotyping of Escherichia coli Isolates for Extraintestinal Virulence Genes by Use of Whole-Genome Sequencing Data. J. Clin. Microbiol. 2020, 58, e01269-20. [Google Scholar] [CrossRef] [PubMed]
- Johansson MH, K.; Bortolaia, V.; Tansirichaiya, S.; Aarestrup, F.M.; Roberts, A.P.; Petersen, T.N. Detection of mobile genetic elements associated with antibiotic resistance in Salmonella enterica using a newly developed web tool: MobileElementFinder. J. Antimicrob. Chemother. 2021, 76, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Carattoli, A.; Zankari, E.; Garcia-Fernandez, A.; Voldby Larsen, M.; Lund, O.; Villa, L.; Aarestrup, F.M.; Hasman, H. PlasmidFinder and pMLST: In silico detection and typing of plasmids. Antimicrob. Agents Chemother. 2014, 58, 3895–3903. [Google Scholar] [CrossRef]
- Larsen, M.; Cosentino, S.; Rasmussen, S.; Rundsten, C.; Hasman, H.; Marvig, R.; Jelsbak, L.; Sicheritz-Pontón, T.; Ussery, D.; Aarestrup, F.; et al. Multilocus Sequence Typing of Total Genome Sequenced Bacteria. J. Clin. Microbiol. 2021, 50, 1355–1361. [Google Scholar] [CrossRef]
- Bartual, S.; Seifert, H.; Hippler, C.; Luzon, M.; Wisplinghoff, H.; Rodríguez-Valera, F. Development of a multilocus sequence typing scheme for characterization of clinical isolates of Acinetobacter baumannii. J. Clin. Microbiol. 2005, 43, 4382–4390. [Google Scholar] [CrossRef]
- Griffiths, D.; Fawley, W.; Kachrimanidou, M.; Bowden, R.; Crook, D.; Fung, R.; Golubchik, T.; Harding, R.; Jeffery, K.; Jolley, K.; et al. Multilocus sequence typing of Clostridium difficile. J. Clin. Microbiol. 2010, 48, 770–778. [Google Scholar] [CrossRef]
- Lemee, L.; Dhalluin, A.; Pestel-Caron, M.; Lemeland, J.; Pons, J. Multilocus sequence typing analysis of human and animal Clostridium difficile isolates of various toxigenic types. J. Clin. Microbiol. 2004, 42, 2609–2617. [Google Scholar] [CrossRef]
- Wirth, T.; Falush, D.; Lan, R.; Colles, F.; Mensa, P.; Wieler, L.; Karch, H.; Reeves, P.; Maiden, M.; Ochman, H.; et al. Sex and virulence in Escherichia coli: An evolutionary perspective. Mol. Microbiol. 2006, 60, 1136–1151. [Google Scholar] [CrossRef]
- Jaureguy, F.; Landraud, L.; Passet, V.; Diancourt, L.; Frapy, E.; Guigon, G.; Carbonnelle, E.; Lortholary, O.; Clermont, O.; Denamur, E.; et al. Phylogenetic and genomic diversity of human bacteremic Escherichia Coli Strains. BMC Genom. 2008, 9, 560. [Google Scholar] [CrossRef]
- Kaas, R.S.; Leekitcharoenphon, P.; Aarestrup, F.M.; Lund, O. Solving the Problem of Comparing Whole Bacterial Genomes across Different Sequencing Platforms. PLoS ONE 2014, 9, e104984. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Doumith, M.; Day, M.; Ciesielczuk, H.; Hope, R.; Underwood, A.; Reynolds, R.; Wain, J.; Livermore, D.M.; Woodford, N. Rapid identification of major Escherichia coli sequence types causing urinary tract and bloodstream infections. J. Clin. Microbiol. 2015, 53, 160–166. [Google Scholar] [CrossRef]
- Matsui, Y.; Hu, Y.; Rubin, J.; de Assis, R.S.; Suh, J.; Riley, L.W. Multilocus sequence typing of Escherichia coli isolates from urinary tract infection patients and from fecal samples of healthy subjects in a college community. MicrobiologyOpen 2021, 9, 1225–1233. [Google Scholar] [CrossRef]
- Guerra, J.A.; Romero-Herazo, Y.C.; Arzuza, O.; Gómez-Duarte, O.G. Phenotypic and genotypic characterization of enterotoxigenic Escherichia coli clinical isolates from northern Colombia, South America. BioMed Res. Int. 2014, 2014, 236260. [Google Scholar] [CrossRef]
- Liu, C.M.; Stegger, M.; Aziz, M.; Johnson, T.J.; Waits, K.; Nordstrom, L.; Gauld, L.; Weaver, B.; Rolland, D.; Statham, S.; et al. Escherichia coli ST131-H22 as a Foodborne Uropathogen. mBio 2018, 9, e00470-18. [Google Scholar] [CrossRef] [PubMed]
- Barrios-Villa, E.; Martínez de la Peña, C.F.; Lozano-Zaraín, P.; Cevallos, M.A.; Torres, C.; Torres, A.G.; del Carmen Rocha-Gracia, R. Comparative genomics of a subset of Adherent/Invasive Escherichia coli strains isolated from individuals without inflammatory bowel disease. Genomics 2020, 112, 1813–1820. [Google Scholar] [CrossRef] [PubMed]
- Yasir, M.; Farman, M.; Shah, M.W.; Jiman-Fatani, A.A.; Othman, N.A.; Almasaudi, S.B.; Alawi, M.; Shakil, S.; Al-Abdullah, N.; Ismaeel, N.A.; et al. Genomic and antimicrobial resistance genes diversity in multidrug-resistant CTX-M-positive isolates of Escherichia coli at a health care facility in Jeddah. J. Infect. Public Health 2020, 13, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Manageiro, V.; Jones-Dias, D.; Ferreira, E.; Caniça, M. Plasmid-Mediated Colistin Resistance (mcr-1) in Escherichia coli from Non-Imported Fresh Vegetables for Human Consumption in Portugal. Microorganisms 2020, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Jiménez, D.; García-Meniño, I.; Herrera, A.; García, V.; López-Beceiro, A.M.; Alonso, M.P.; Blanco, J.; Mora, A. Genomic Characterization of Escherichia coli Isolates Belonging to a New Hybrid aEPEC/ExPEC Pathotype O153:H10-A-ST10 eae-beta1 Occurred in Meat, Poultry, Wildlife and Human Diarrheagenic Samples. Antibiotics 2020, 9, 192. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, S.L.; Stegger, M.; Kudirkiene, E.; Lilje, B.; Poulsen, L.L.; Ronco, T.; Dos Santos, T.P.; Kiil, K.; Bisgaard, M.; Pedersen, K.; et al. Diversity and Population Overlap between Avian and Human Escherichia coli Belonging to Sequence Type 95. mSphere 2019, 4, e00333-18. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, W.; Tanaka, H.; Taniguchi, Y.; Iimura, M.; Soga, E.; Kubo, R.; Matsuo, N.; Kawamura, K.; Arakawa, Y.; Nagano, Y.; et al. Acquisition of mcr-1 and Cocarriage of Virulence Genes in Avian Pathogenic Escherichia coli Isolates from Municipal Wastewater Influents in Japan. Appl. Environ. Microbiol. 2019, 85, e01661-19. [Google Scholar] [CrossRef]
- Barrera, S.; Vázquez-Flores, S.; Needle, D.; Rodríguez-Medina, N.; Iglesias, D.; Sevigny, J.L.; Gordon, L.M.; Simpson, S.; Thomas, W.K.; Rodulfo, H.; et al. Serovars, Virulence and Antimicrobial Resistance Genes of Non-Typhoidal Salmonella Strains from Dairy Systems in Mexico. Antibiotics 2023, 12, 1662. [Google Scholar] [CrossRef]
- Jibril, A.H.; Okeke, I.N.; Dalsgaard, A.; Menéndez, V.G.; Olsen, J.E. Genomic analysis of antimicrobial resistance and resistance plasmids in Salmonella serovars from poultry in Nigeria. Antibiotics 2021, 10, 99. [Google Scholar] [CrossRef]
- Shelenkov, A.; Mikhaylova, Y.; Voskanyan, S.; Egorova, A.; Akimkin, V. Whole-Genome Sequencing Revealed the Fusion Plasmids Capable of Transmission and Acquisition of Both Antimicrobial Resistance and Hypervirulence Determinants in Multidrug-Resistant Klebsiella pneumoniae Isolates. Microorganisms 2023, 11, 1314. [Google Scholar] [CrossRef]
- Mousavi, Z.E.; Koolman, L.; Macori, G.; Fanning, S.; Butler, F. Comprehensive Genomic Characterization of Cronobacter sakazakii Isolates from Infant Formula Processing Facilities Using Whole-Genome Sequencing. Microorganisms 2023, 11, 2749. [Google Scholar] [CrossRef] [PubMed]
- Rozwandowicz, M.; Brouwer MS, M.; Fischer, J.; Wagenaar, J.A.; Gonzalez-Zorn, B.; Guerra, B.; Mevius, D.J.; Hordijk, J. Plasmids carrying antimicrobial resistance genes in Enterobacteriaceae. J. Antimicrob. Chemother. 2018, 73, 1121–1137. [Google Scholar] [CrossRef] [PubMed]
- Balbuena-Alonso, M.G.; Cortés-Cortés, G.; Kim, J.W.; Lozano-Zarain, P.; Camps, M.; Del Carmen Rocha-Gracia, R. Genomic analysis of plasmid content in food isolates of E. coli strongly supports its role as a reservoir for the horizontal transfer of virulence and antibiotic resistance genes. Plasmid 2022, 123–124, 102650. [Google Scholar] [CrossRef]
- Yang, X.; Liu, X.; Xu, Y.; Chan, E.W.; Zhang, R.; Chen, S. An IncB/O/K/Z conjugative plasmid encodes resistance to azithromycin and mediates transmission of virulence plasmid in Klebsiella pneumoniae. Int. J. Antimicrob. Agents 2022, 60, 106683. [Google Scholar] [CrossRef] [PubMed]
- Vale, F.F.; Lehours, P.; Yamaoka, Y. The role of Mobile genetic elements in bacterial evolution and their adaptability. Front. Microbiol. 2022, 13, 849667. [Google Scholar] [CrossRef] [PubMed]
- Heieck, K.; Brück, T. Localization of Insertion Sequences in Plasmids for L-Cysteine Production in E. coli. Genes 2023, 14, 1317. [Google Scholar] [CrossRef] [PubMed]
- Loftsdóttir, H.; Söderlund, R.; Jinnerot, T.; Eriksson, E.; Bongcam-Rudloff, E.; Aspán, A. Dynamics of insertion sequence element IS629 inactivation of verotoxin 2 genes in Escherichia coli O157:H7. FEMS Microbiol. Lett. 2017, 364, fnx074. [Google Scholar] [CrossRef] [PubMed]
- Fordham, S.M.E.; Mantzouratou, A.; Sheridan, E. Prevalence of insertion sequence elements in plasmids relating to mgrB gene disruption causing colistin resistance in Klebsiella pneumoniae. MicrobiologyOpen 2022, 11, e1262. [Google Scholar] [CrossRef] [PubMed]
- Shepard, S.M.; Danzeisen, J.L.; Isaacson, R.E.; Seemann, T.; Achtman, M.; Johnson, T.J. Genome sequences and phylogenetic analysis of K88- and F18-positive porcine enterotoxigenic Escherichia coli. J. Bacteriol. 2012, 194, 395–405. [Google Scholar] [CrossRef]
- Minnick, M.F. Functional Roles and Genomic Impact of Miniature Inverted-Repeat Transposable Elements (MITEs) in Prokaryotes. Genes 2024, 15, 328. [Google Scholar] [CrossRef]
- Fattash, I.; Rooke, R.; Wong, A.; Hui, C.; Luu, T.; Bhardwaj, P.; Yang, G. Miniature inverted-repeat transposable elements: Discovery, distribution, and activity. Genome 2013, 56, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Klai, K.; Zidi, M.; Chénais, B.; Denis, F.; Caruso, A.; Casse, N.; Mezghani Khemakhem, M. Miniature Inverted-Repeat Transposable Elements (MITEs) in the Two Lepidopteran Genomes of Helicoverpa armigera and Helicoverpa Zea. Insects 2022, 13, 313. [Google Scholar] [CrossRef] [PubMed]
- Momose, M.; Abe, Y.; Ozeki, Y. Miniature inverted-repeat transposable elements of Stowaway are active in potato. Genetics 2010, 186, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Lyu, N.; Li, Z.; Ma, S.; Cao, D.; Zhu, B. The temporal dynamics of antimicrobial-resistant Salmonella enterica and predominant serovars in China. Natl. Sci. Rev. 2023, 10, nwac269. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Samudio, V.; Pimentel-Peralta, G.; De La Cruz, A.; Landires, I. Genetic Diversity and New Sequence Types of Escherichia coli Coharboring β-Lactamases and PMQR Genes Isolated from Domestic Dogs in Central Panama. Genes 2022, 14, 73. [Google Scholar] [CrossRef] [PubMed]
- Magaña-Lizárraga, J.A.; Ahumada-Santos, Y.P.; Parra-Unda, J.R.; de Jesús Uribe-Beltrán, M.; Vega-López, I.F.; Prieto-Alvarado, R.; Báez-Flores, M.E. Draft genome sequence of Escherichia coli M51-3: A multidrug-resistant strain assigned as ST131-H30 recovered from infant diarrheal infection in Mexico. J. Glob. Antimicrob. Resist. 2019, 19, 311–312. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Samudio, V.; Pecchio, M.; Pimentel-Peralta, G.; Quintero, Y.; Herrera, M.; Landires, I. Molecular Epidemiology of Escherichia coli Clinical Isolates from Central Panama. Antibiotics 2021, 10, 899. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Malviya, N.; Gaddy, J.A.; Zhang, C.; Seier, A.J.; Haley, K.P.; Doster, R.S.; Farfán-García, A.E.; Gómez-Duarte, O.G. Enteroinvasive Escherichia coli O96:H19 is an Emergent Biofilm-Forming Pathogen. J. Bacteriol. 2022, 204, e0056221. [Google Scholar] [CrossRef]
- Vogt, N.A.; Hetman, B.M.; Pearl, D.L.; Vogt, A.A.; Reid-Smith, R.J.; Parmley, E.J.; Janecko, N.; Bharat, A.; Mulvey, M.R.; Ricker, N.; et al. Using whole-genome sequence data to examine the epidemiology of Salmonella, Escherichia coli and associated antimicrobial resistance in raccoons (Procyon lotor), swine manure pits, and soil samples on swine farms in southern Ontario, Canada. PLoS ONE 2021, 16, e0260234. [Google Scholar] [CrossRef]
- Vasconcelos, P.C.; Leite, E.L.; Araújo, W.J.; Silva, N.M.V.; Saraiva, M.M.S.; Santos Filho, L.; Freitas Neto, O.C.; Givisiez, P.E.N.; Oliveira, C.J.B. Draft genome sequence of mcr-1-mediated colistin-resistant Escherichia coli ST359 from chicken carcasses in Northeastern Brazil. J. Glob. Antimicrob. Resist. 2020, 23, 135–136. [Google Scholar] [CrossRef]
- Boyd, E.; Trmcic, A.; Taylor, M.; Shyng, S.; Hasselback, P.; Man, S.; Tchao, C.; Stone, J.; Janz, L.; Hoang, L.; et al. Escherichia coli O121 outbreak associated with raw milk Gouda-like cheese in British Columbia, Canada, 2018. Can. Commun. Dis. Rep. 2021, 47, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Papa-Ezdra, R.; Grill Diaz, F.; Vieytes, M.; García-Fulgueiras, V.; Caiata, L.; Ávila, P.; Brasesco, M.; Christophersen, I.; Cordeiro, N.F.; Algorta, G.; et al. First three Escherichia coli isolates harbouring mcr-1 in Uruguay. J. Glob. Antimicrob. Resist. 2020, 20, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Orsi, H.; Guimarães, F.F.; Leite, D.S.; Guerra, S.T.; Joaquim, S.F.; Pantoja, J.C.F.; Hernandes, R.T.; Lucheis, S.B.; Ribeiro, M.G.; Langoni, H.; et al. Characterization of mammary pathogenic Escherichia coli reveals the diversity of Escherichia coli isolates associated with bovine clinical mastitis in Brazil. J. Dairy Sci. 2023, 106, 1403–1413. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.E.V.; Chalmers, G.; Murray, R.; Mataseje, L.; Pearl, D.L.; Mulvey, M.; Topp, E.; Boerlin, P. Characterization of Escherichia coli and Other Enterobacterales Resistant to Extended-Spectrum Cephalosporins Isolated from Dairy Manure in Ontario, Canada. Appl. Environ. Microbiol. 2023, 89, e0186922. [Google Scholar] [CrossRef] [PubMed]
- Loayza-Villa, F.; Salinas, L.; Tijet, N.; Villavicencio, F.; Tamayo, R.; Salas, S.; Rivera, R.; Villacis, J.; Satan, C.; Ushiña, L.; et al. Diverse Escherichia coli lineages from domestic animals carrying colistin resistance gene mcr-1 in an Ecuadorian household. J. Glob. Antimicrob. Resist. 2020, 22, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Fillor, R.E.; Brilhante, M.; Marrero-Moreno, C.M.; Baez, M.; Espinosa, I.; Perreten, V. Characterization of Third-Generation Cephalosporin-Resistant Escherichia coli Isolated from Pigs in Cuba Using Next-Generation Sequencing. Microb. Drug Resist. 2021, 27, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Silva-Sánchez, J.; Duran-Bedolla, J.; Lozano, L.; Reyna-Flores, F.; Bacterial Resistance Consortium; Barrios-Camacho, H. Molecular characterization of Escherichia coli producing extended-spectrum β-lactamase CTX-M-14 and CTX-M-28 in Mexico. Braz. J. Microbiol. 2024, 55, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Liao, N.; Borges, C.A.; Rubin, J.; Hu, Y.; Ramirez, H.A.; Chen, J.; Zhou, B.; Zhang, Y.; Zhang, R.; Jiang, J.; et al. Prevalence of β-Lactam Drug-Resistance Genes in Escherichia coli Contaminating Ready-to-Eat Lettuce. Foodborne Pathog. Dis. 2020, 17, 739–742. [Google Scholar] [CrossRef]
- Espinoza, L.L.; Huamán, D.C.; Cueva, C.R.; Gonzales, C.D.; León, Y.I.; Espejo, T.S.; Monge, G.M.; Alcántara, R.R.; Hernández, L.M. Genomic analysis of multidrug-resistant Escherichia coli strains carrying the mcr-1 gene recovered from pigs in Lima-Peru. Comp. Immunol. Microbiol. Infect. Dis. 2023, 99, 102019. [Google Scholar] [CrossRef]
- Aworh, M.K.; Thakur, S.; Gensler, C.; Harrell, E.; Harden, L.; Fedorka-Cray, P.J.; Jacob, M. Characteristics of antimicrobial resistance in Escherichia coli isolated from retail meat products in North Carolina. PLoS ONE 2024, 19, e0294099. [Google Scholar] [CrossRef]
- Weiler, N.; Martínez, L.J.; Campos, J.; Poklepovich, T.; Orrego, M.V.; Ortiz, F.; Alvarez, M.; Putzolu, K.; Zolezzi, G.; Miliwebsky, E.; et al. First molecular characterization of Escherichia coli O157:H7 isolates from clinical samples in Paraguay using whole-genome sequencing. Rev. Argent. Microbiol. 2023, 55, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Furlan, J.P.R.; Savazzi, E.A.; Stehling, E.G. Widespread high-risk clones of multidrug-resistant extended-spectrum β-lactamase-producing Escherichia coli B2-ST131 and F-ST648 in public aquatic environments. Int. J. Antimicrob. Agents 2020, 56, 106040. [Google Scholar] [CrossRef] [PubMed]
- Ballash, G.A.; Mollenkopf, D.F.; Diaz-Campos, D.; van Balen, J.C.; Cianciolo, R.E.; Wittum, T.E. Pathogenomics and clinical recurrence influence biofilm capacity of Escherichia coli isolated from canine urinary tract infections. PLoS ONE 2022, 17, e0270461. [Google Scholar] [CrossRef]
- Calero-Cáceres, W.; Tadesse, D.; Jaramillo, K.; Villavicencio, X.; Mero, E.; Lalaleo, L.; Welsh, C.; Villacís, J.E.; Quentin, E.; Parra, H.; et al. Characterization of the genetic structure of mcr-1 gene among Escherichia coli isolates recovered from surface waters and sediments from Ecuador. Sci. Total Environ. 2022, 806 Pt 2, 150566. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Bergholz, P.; Wiedmann, M. Adjacent Terrestrial Landscapes Impact the Biogeographical Pattern of Soil Escherichia coli Strains in Produce Fields by Modifying the Importance of Environmental Selection and Dispersal. Appl. Environ. Microbiol. 2021, 87, e02516-20. [Google Scholar] [CrossRef] [PubMed]
- Aristizábal-Hoyos, A.M.; Rodríguez, E.A.; Arias, L.; Jiménez, J.N. High clonal diversity of multidrug-resistant and extended spectrum beta-lactamase-producing Escherichia coli in a wastewater treatment plant. Lett. Appl. Microbiol. 2019, 69, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Rasko, D.A.; Del Canto, F.; Luo, Q.; Fleckenstein, J.M.; Vidal, R.; Hazen, T.H. Comparative genomic analysis and molecular examination of the diversity of enterotoxigenic Escherichia coli isolates from Chile. PLoS Neglected Trop. Dis. 2019, 13, e0007828. [Google Scholar] [CrossRef]
- Martins, J.C.L.; Pintor-Cora, A.; Alegría, Á.; Santos, J.A.; Herrera-Arias, F. Characterization of ESBL-producing Escherichia spp. and report of an mcr-1 colistin-resistance Escherichia fergusonni strain from minced meat in Pamplona, Colombia. Int. J. Food Microbiol. 2023, 394, 110168. [Google Scholar] [CrossRef]
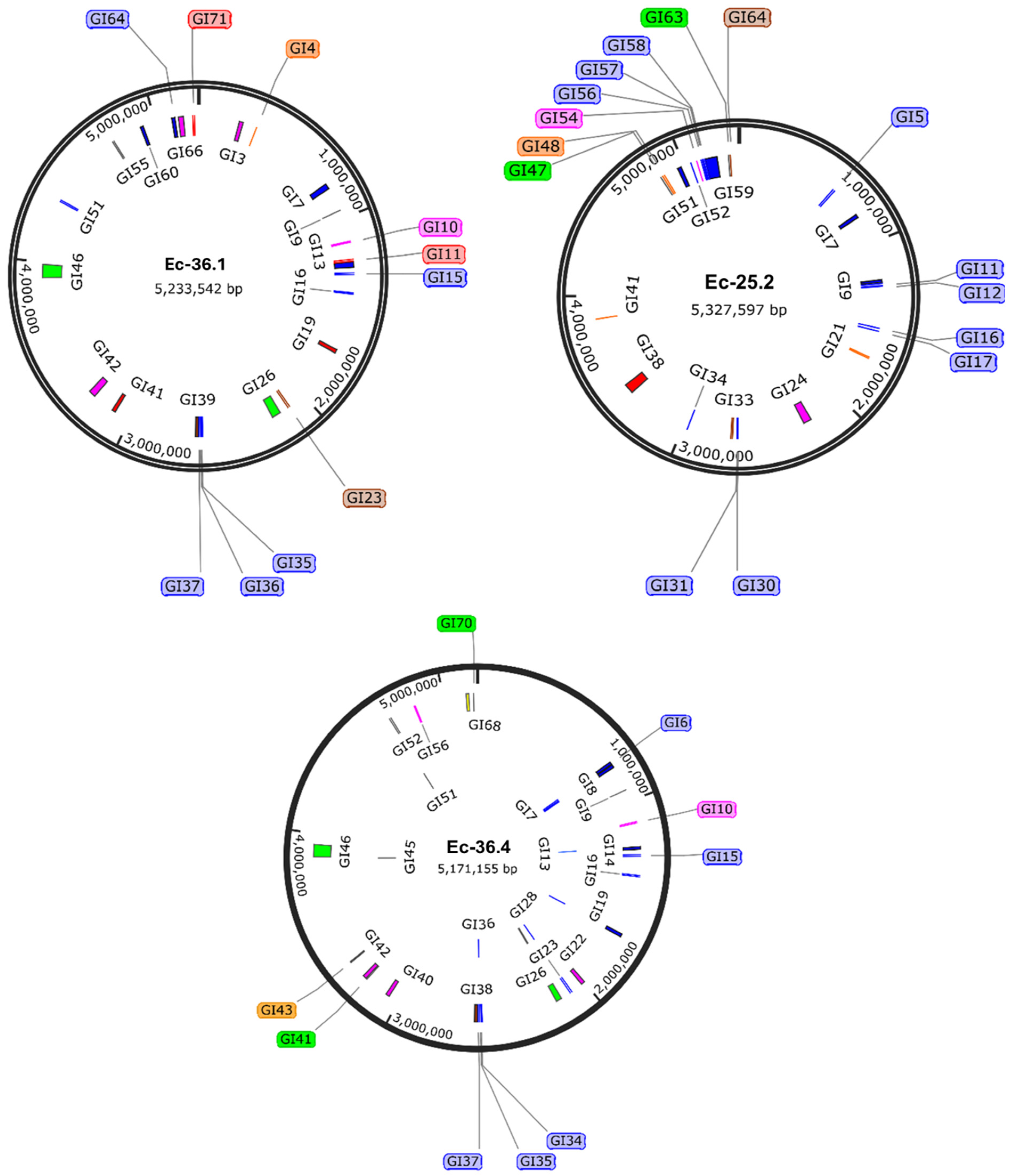
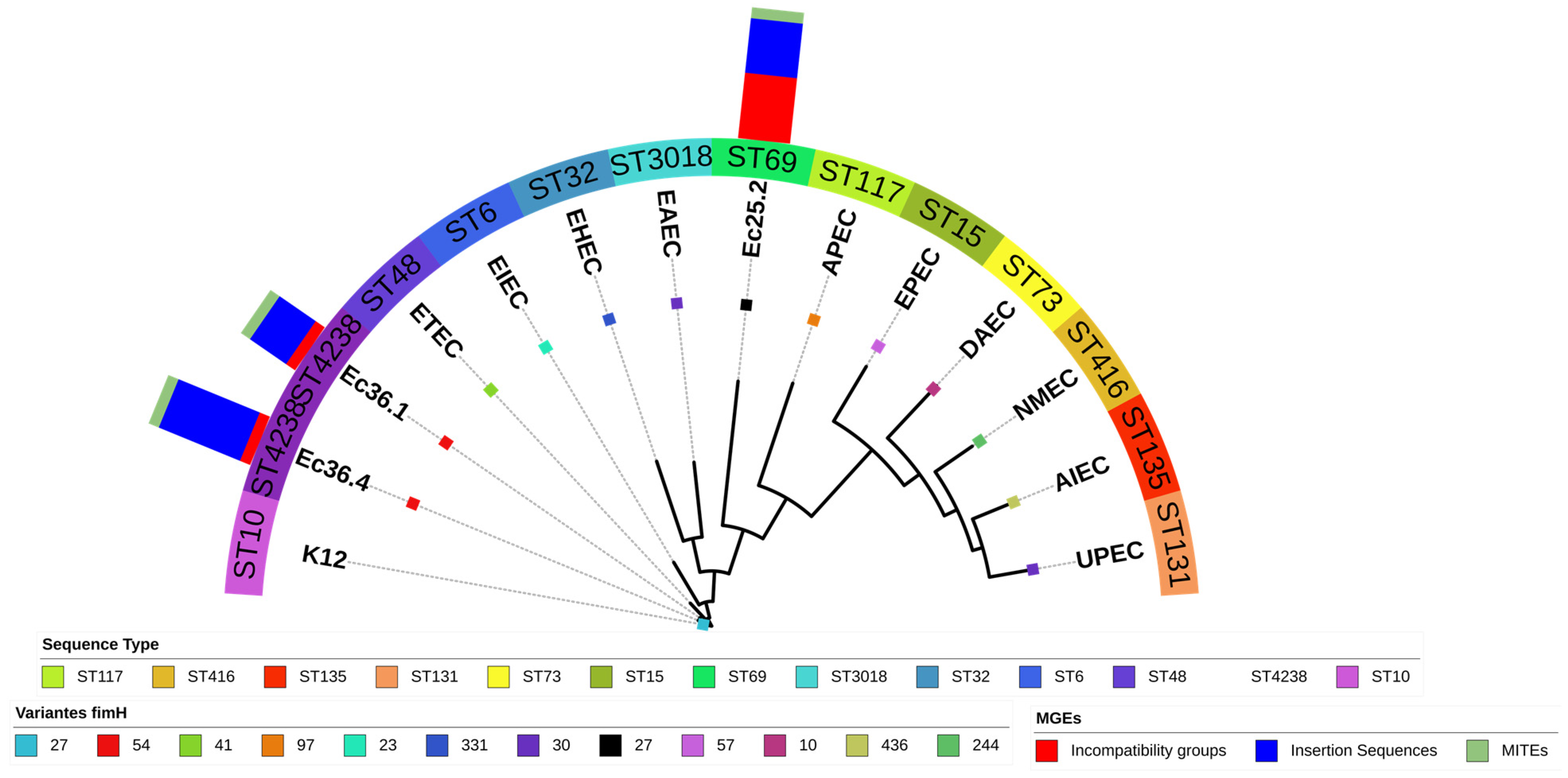
| Strain | Ec-25.2 | Ec-36.1 | Ec-36.4 |
|---|---|---|---|
| fimH variant | fimH27 | fimH54 | fimH54 |
| Resistance genes | Fluoroquinolones (qnrB19); Aminoglycosides (aadA5; aac (6′)- lb-cr); Sulfonamides (sul1); Sulfamethoxazole-Trimethoprim (, dfrA17); | Aminoglycosides (aac (6′)- lb-cr); Fluoroquinolones (qnrB) | Aminoglycosides (aac (6′)- lb-cr); Fluoroquinolones (qnrB) |
| Disinfectant | sitABCD (hydrogen peroxide), qacE (benzylconium chloride, ethidium bromide, chlorhexidine, cetylpyridinium chloride) | NF | NF |
| Efflux pumps associated with antibiotic resistance | Fluoroquinolones (evgA, mdtH, marA, emrR, emrB); Tetracycline (emrY, evgA, marA); Macrolide (evgA); Monobactams (marA); Carbapenems (marA); Cephalosporins (marA). | Macrolides (tolC, evgA,, gadX, mdtE); Tetracyclines (evgA, emrY); Nitroimidazole (msbA); Fluoroquinolones (tolC, acrA, acrB, mdtH, emrR, emrB, evgA, acrS, marA, gadX, mdtE, kdpDE, cpxA); Aminoglycosides (tolC, acrA, acrB, mdtH, emrR, emrB, evgA, acrS, marA, gadX, mdtE, kdpDE, cpxA); Carbapenems (tolC, acrA, acrB, mdtH, emrR,, emrB, evgA, AcrS, marA, gadX, mdtE, kdpDE, cpxA); Cephalosporins (tolC, acrA, acrB, mdtH, emrR, emrA, emrB, evgS, evgA, acrS, acrE, marA, gadX, mdtE, kdpDE, cpxA); Phenicols (tolC, acrA, acrB, mdtH, emrR, emrA, emrB, evgS, evgA, acrS, acrE, marA, gadX, mdtE, kdpDE, cpxA) | Macrolides (msr(A), mph(C)); Tetracyclines (evgA, emrY); Streptogramin b (msrA); Nitroimidazole (msbA); Fluoroquinolones (tolC, acrA, acrB, mdtH, emrR,, emrB, evgA, acrS, marA, gadX, mdtE, kdpDE, cpxA); Aminoglycosides (tolC, acrA, acrB, mdtH, emrR,, emrB, evgA, acrS, marA, gadX, mdtE, kdpDE, cpxA); Carbapenems (tolC, acrA, acrB, mdtH, emrR,, emrB, evgA, AcrS, marA, gadX, mdtE, kdpDE, cpxA); Cephalosporins (tolC, acrA, acrB, mdtH, emrR, emrA, emrB, evgS, evgA, acrS, acrE, marA, gadX, mdtE, kdpDE, cpxA); Phenicols (tolC, acrA, acrB, mdtH, emrR,emrA, emrB, evgS, evgA, acrS, acrE, marA, gadX, mdtE, kdpDE, cpxA) |
| Virulence genes | chuA, cia, eilA, fimH, fyuA, gad, hlyE, iha, irp2, iss, iucC, iutA, kpsE, kpsMII_K52, lpfA, ompT, papA, papC, sat, senB, sitA, terC, traJ, traT, yehA, yehB, yehC, yehD | afaD, capU, cea, colE5, csgA, fimH, hlyE, iha, ireA, iucC, iutA, shiB, sigA, traT, yehA, yehB, yehC, yehD | afaD, capU, cea, colE5, csgA, fimH, hlyE, iha, ireA, iucC, iutA, shiB, sigA, traT, yehA, yehB, yehC, yehD |
| MGE | Plasmids: Col156, Col440I, Col(pHAD28), IncFIB, IncF11, IncI1-l. Insertion Sequences: IS629, ISEc46, ISEc38, ISKpn26, ISEc45. Miniature Inverted Repeat: MITEEc1. | Plasmids: IncB/O/K/Z. Insertion Sequence: IS609, IS5, ISEc38, ISSfl3. Miniature Inverted Repeat: MITEEc1 | Plasmids: IncB/O/K/Z. Insertion Sequences: ISEc18, IS609, IS5, ISSso4, ISEc38, IS256, ISSfl3, ISSha1. Miniature Inverted Repeat: MITEEc1. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega-Enríquez, J.Z.; Martínez-de la Peña, C.; Lara-Ochoa, C.; Rocha-Gracia, R.d.C.; Barrios-Villa, E.; Arenas-Hernández, M.M.P. Comparative Genomics of Three Hybrid-Pathogen Multidrug-Resistant Escherichia coli Strains Isolated from Healthy Donors’ Feces. Microbiol. Res. 2024, 15, 1412-1424. https://doi.org/10.3390/microbiolres15030095
Ortega-Enríquez JZ, Martínez-de la Peña C, Lara-Ochoa C, Rocha-Gracia RdC, Barrios-Villa E, Arenas-Hernández MMP. Comparative Genomics of Three Hybrid-Pathogen Multidrug-Resistant Escherichia coli Strains Isolated from Healthy Donors’ Feces. Microbiology Research. 2024; 15(3):1412-1424. https://doi.org/10.3390/microbiolres15030095
Chicago/Turabian StyleOrtega-Enríquez, Judith Z., Claudia Martínez-de la Peña, Cristina Lara-Ochoa, Rosa del Carmen Rocha-Gracia, Edwin Barrios-Villa, and Margarita M. P. Arenas-Hernández. 2024. "Comparative Genomics of Three Hybrid-Pathogen Multidrug-Resistant Escherichia coli Strains Isolated from Healthy Donors’ Feces" Microbiology Research 15, no. 3: 1412-1424. https://doi.org/10.3390/microbiolres15030095






