A Comprehensive Methodology for Identifying Pseudomonas aeruginosa Strains Exhibiting Biofilm and Virulence Factor Traits and Assessment of Biofilm Resistance Against Commercial Disinfectant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Evaluation of Biofilm Formation
Quantification Method Using Crystal Violet Microplate Assay
2.2. Assessment of Virulence Factor Production in Selected Biofilm-Forming Strains of Pseudomonas aeruginosa
2.2.1. Evaluation of Polysaccharide Production
2.2.2. Bacterial Motility
2.2.3. Evaluation of Pyocyanin Production
2.2.4. Extraction and Purification of Pyocyanin
2.2.5. Evaluation of LasA Activity (Staphylolytic)
2.2.6. Extraction and Quantification of Rhamnolipids
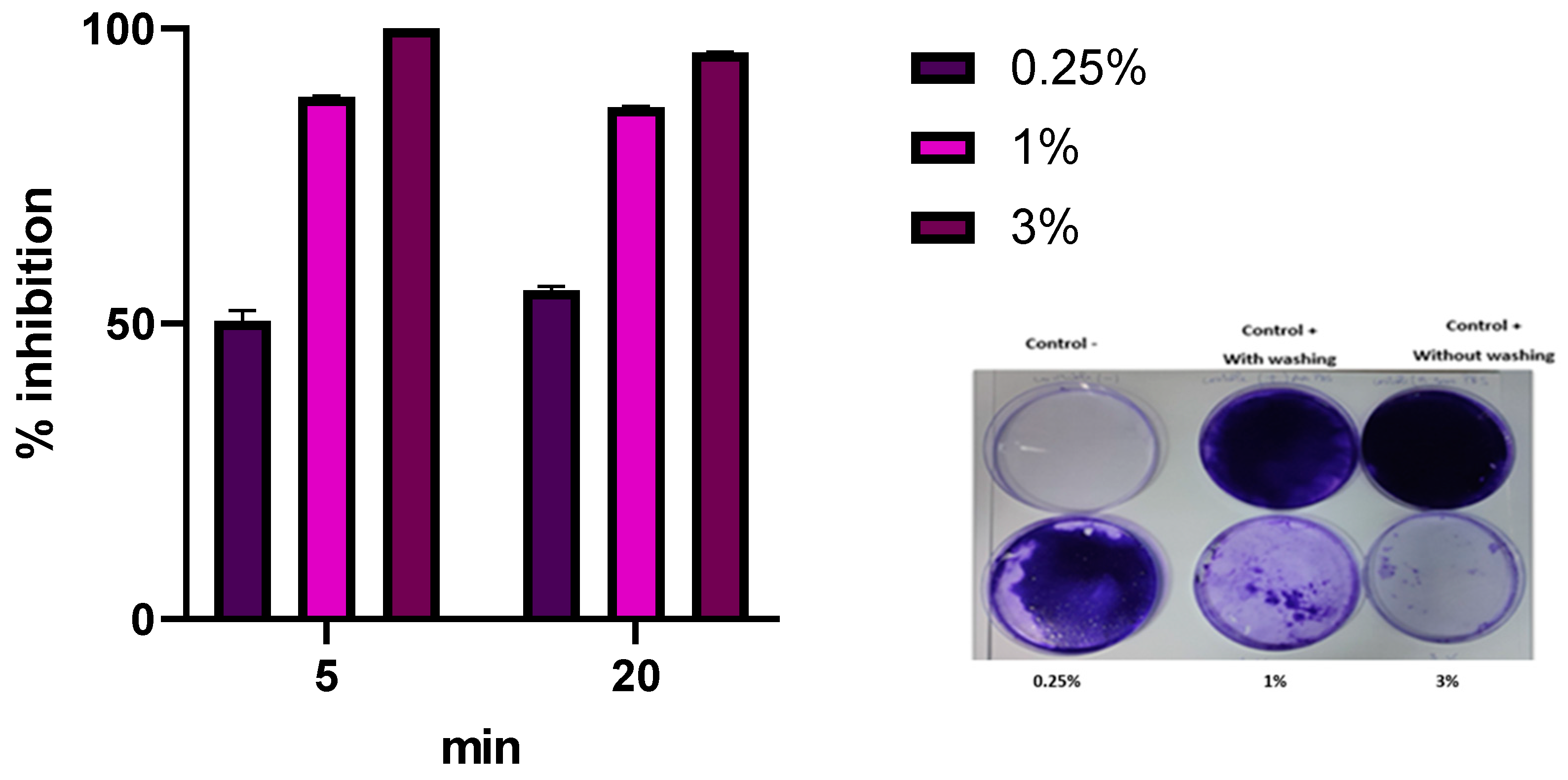
2.2.7. P. aeruginosa 2629 Biofilm Eradication by Commercial Disinfectant Based on Peracetic Acid
2.3. Statistical Analysis
3. Results And Discussion
3.1. Pseudomonas Strains and Their Origins
3.2. Evaluation of Biofilm Formation in Pseudomonas Strains
3.3. Classification of Pseudomonas Strains Based on Biofilm Production
- Selection of Highly Biofilm-Forming Strains
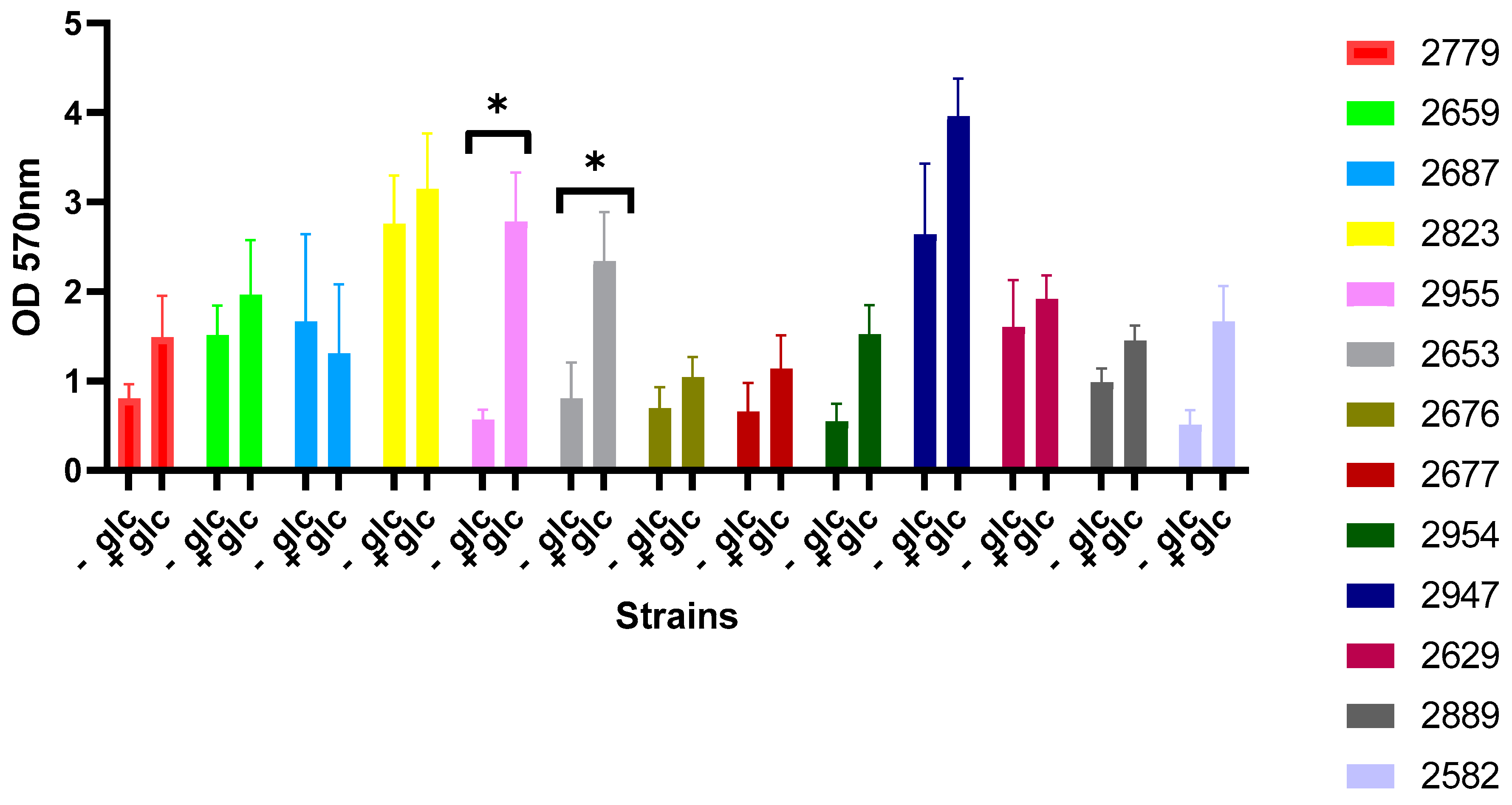
3.4. Effect of Glucose Supplementation on Biofilm Formation by Selected 13 Strains
3.5. Evaluation of the Production of Virulence Factors by Selected Pseudomonas aeruginosa Strains
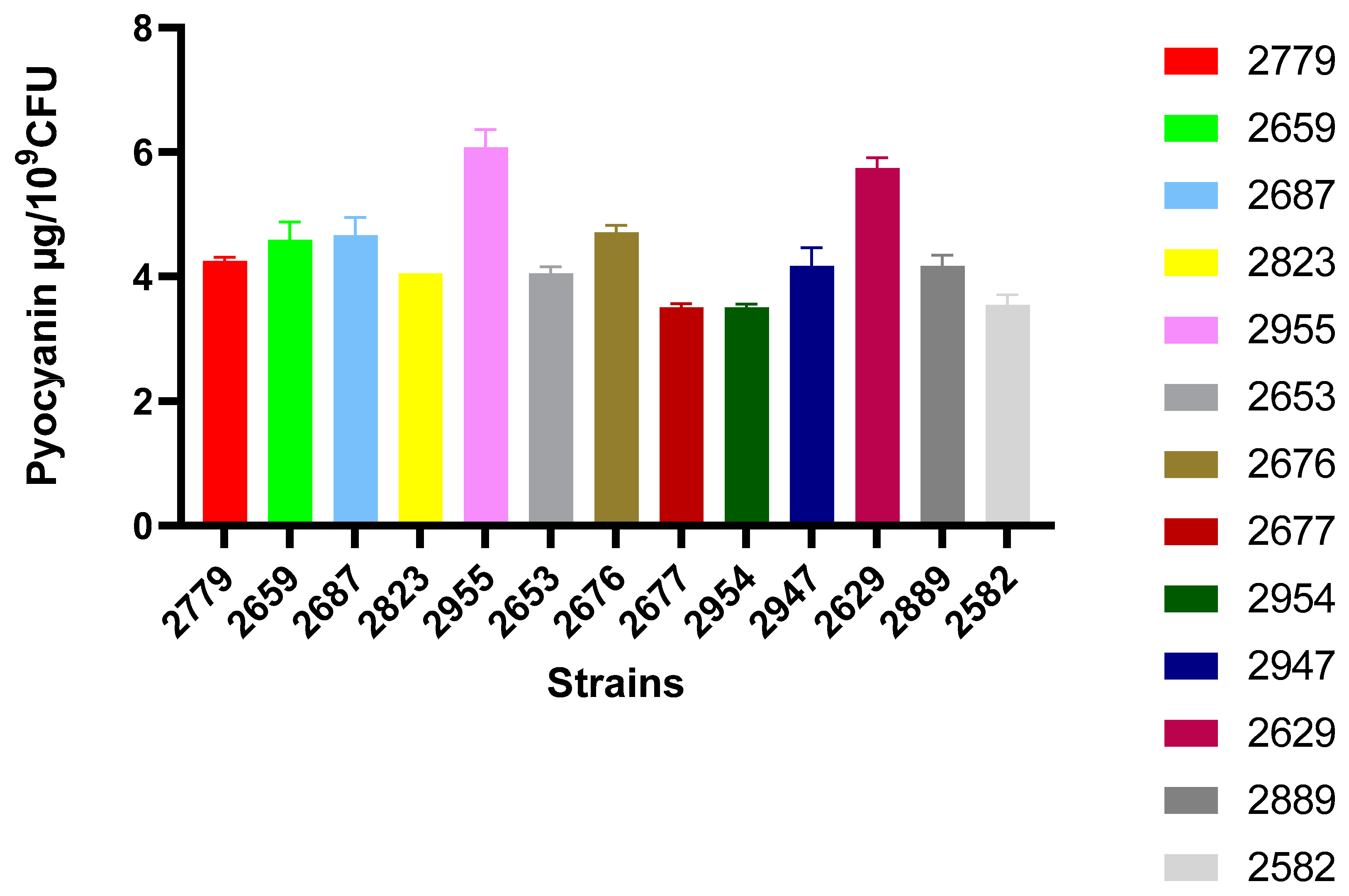
3.5.1. Pyocyanin Production
3.5.2. Production of Exopolysaccharides (EPSs)
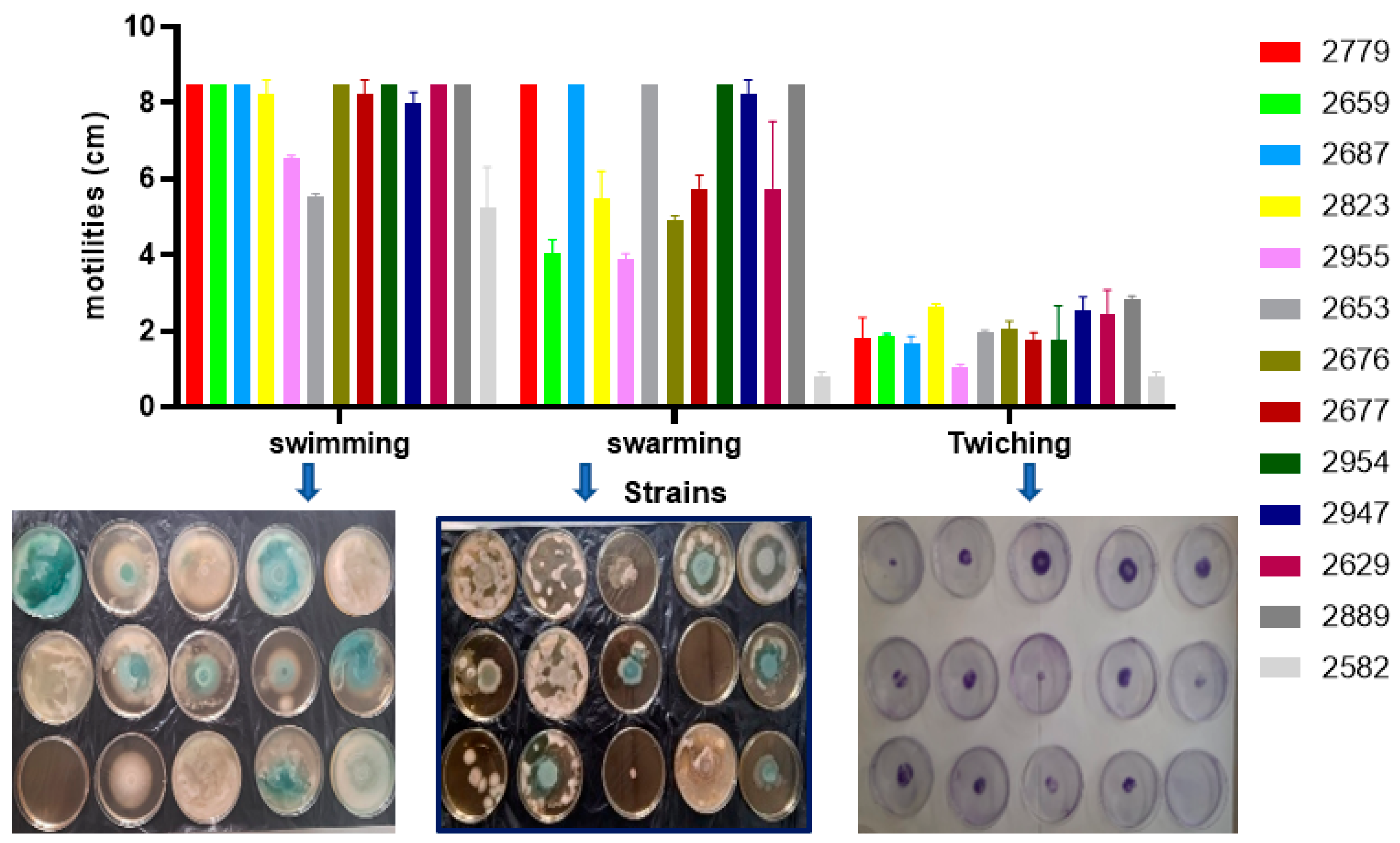
3.5.3. Motility Test
3.5.4. Evaluation of LasA Activity (Staphylolytic Activity)
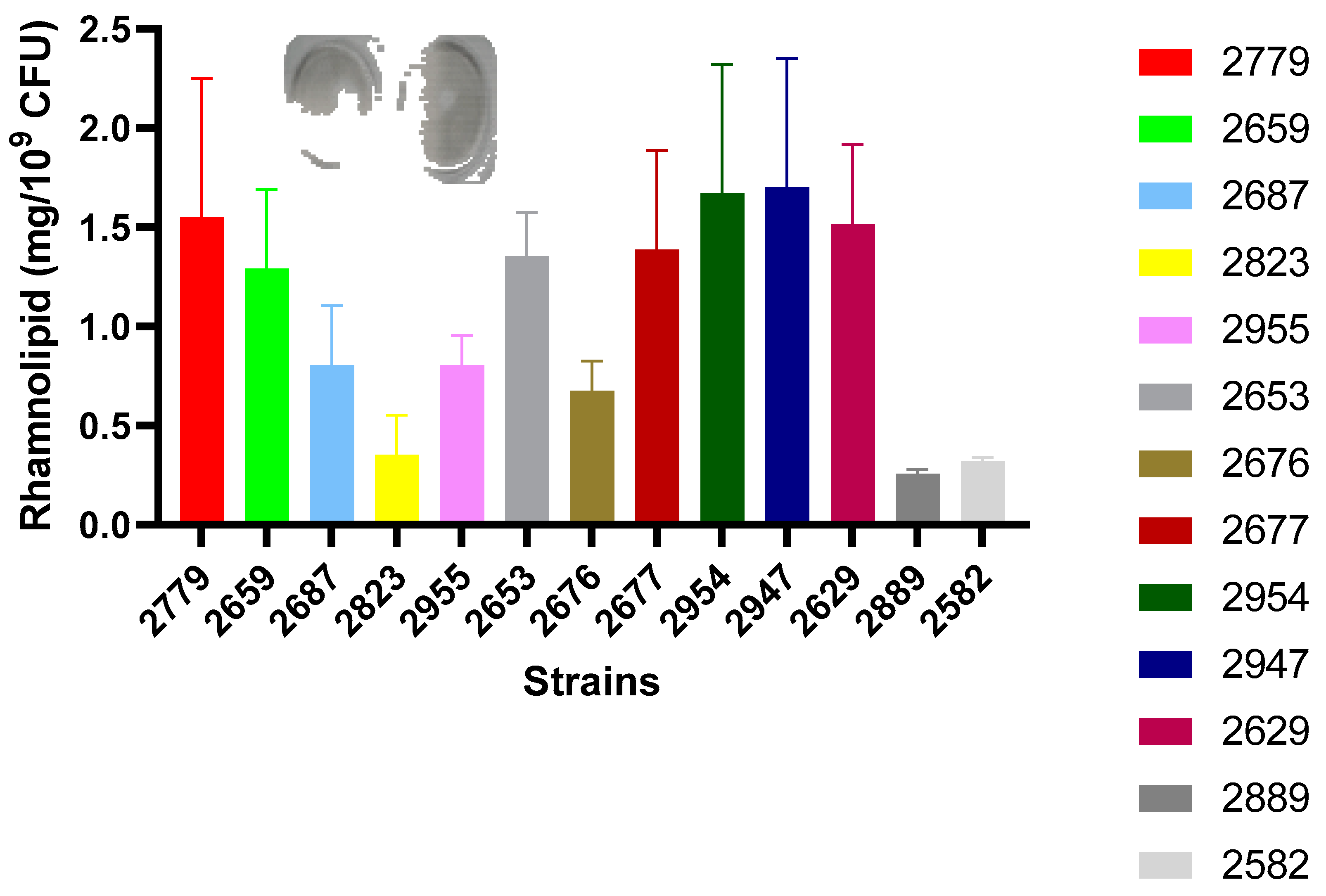
3.5.5. Quantification of Rhamnolipid Production
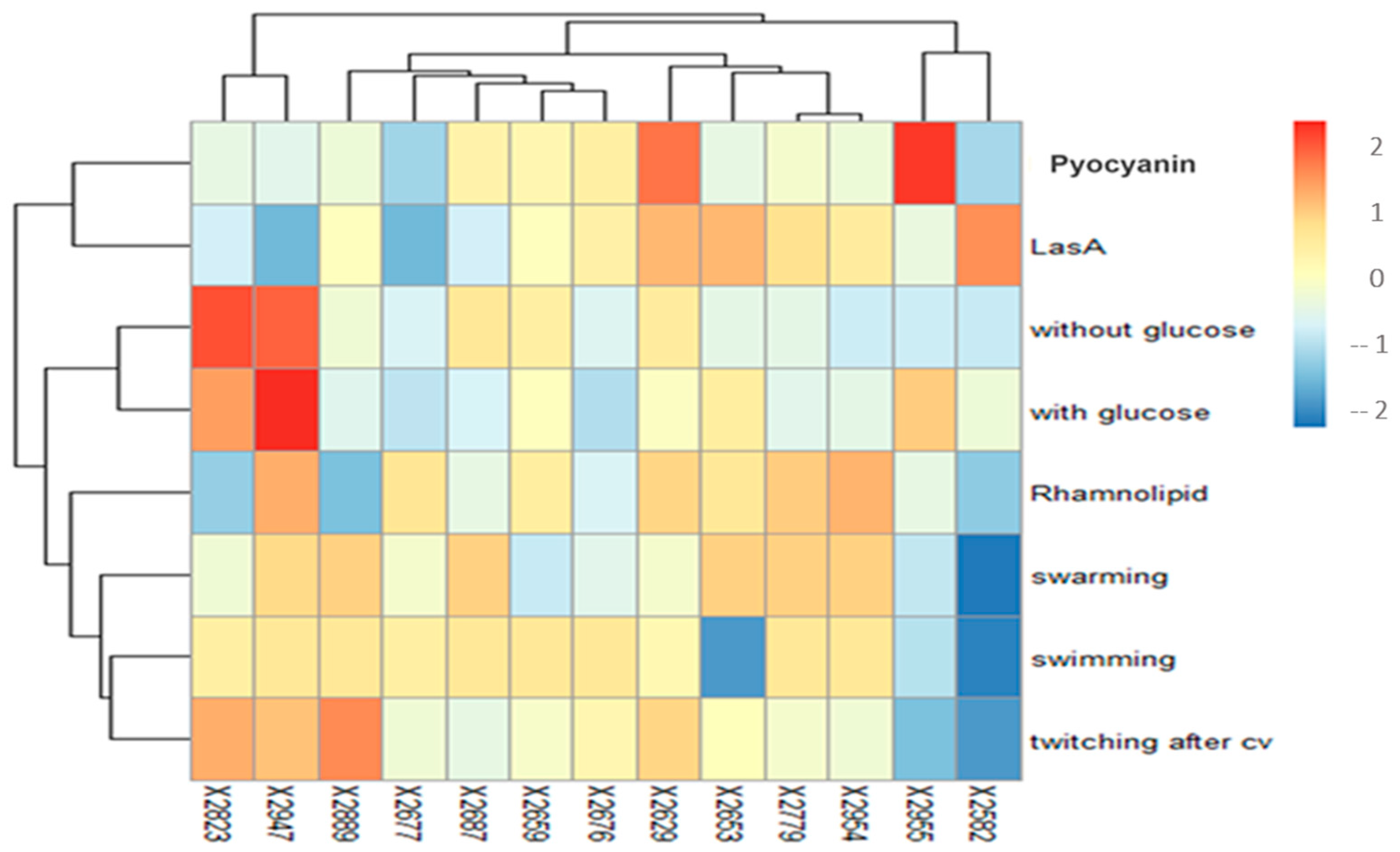
3.5.6. Heatmap: Combining Virulence Factor Production and Biofilm-Forming Capacity to Select a Model of Pseudomonas Strain
3.6. P. aeruginosa 2629 Biofilm Eradication by Commercial Disinfectant Based on Peracetic Acid
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tuon, F.F.; Dantas, L.R.; Sus, P.H.; Ribeiro, V.S.T. Pathogenesis of the Pseudomonas aeruginosa Biofilm: A Review. Pathogens 2022, 11, 300. [Google Scholar] [CrossRef]
- Reynolds, D.; Kollef, M. The Epidemiology and Pathogenesis and Treatment of Pseudomonas aeruginosa Infections: An Update. Drugs 2021, 81, 2117–2131. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms 2023, 11, 1614. [Google Scholar] [CrossRef] [PubMed]
- Ghssein, G.; Ezzeddine, Z. A Review of Pseudomonas aeruginosa Metallophores: Pyoverdine, Pyochelin and Pseudopaline. Biology 2022, 11, 1711. [Google Scholar] [CrossRef]
- Ambreetha, S.; Singh, V. Genetic and Environmental Determinants of Surface Adaptations in Pseudomonas aeruginosa: This Article Is Part of the Environmental Sensing and Cell-Cell Communication Collection. Microbiology 2023, 169, 001335. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Ahn, J. Advances in the Discovery of Efflux Pump Inhibitors as Novel Potentiators to Control Antimicrobial-Resistant Pathogens. Antibiotics 2023, 12, 1417. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Huang, J.; Liu, Z.; Xu, Z.; Zhang, L. Molecular Mechanisms Underlying the Regulation of Biofilm Formation and Swimming Motility by FleS/FleR in Pseudomonas aeruginosa. Front. Microbiol. 2021, 12, 707711. [Google Scholar] [CrossRef]
- Li, X.; Gu, N.; Huang, T.Y.; Zhong, F.; Peng, G. Pseudomonas aeruginosa: A Typical Biofilm Forming Pathogen and an Emerging but Underestimated Pathogen in Food Processing. Front. Microbiol. 2023, 13, 1114199. [Google Scholar] [CrossRef]
- Liao, C.; Huang, X.; Wang, Q.; Yao, D.; Lu, W. Virulence Factors of Pseudomonas aeruginosa and Antivirulence Strategies to Combat Its Drug Resistance. Front. Cell. Infect. Microbiol. 2022, 12, 926758. [Google Scholar] [CrossRef] [PubMed]
- Uzunbayir-Akel, N.; Tekintas, Y.; Yilmaz, F.F.; Ozturk, I.; Okeer, M.; Aydemir, S.S.; Cilli, F.F.; Hosgor-Limoncu, M. Effects of Disinfectants and Ciprofloxacin on Quorum Sensing Genes and Biofilm of Clinical Pseudomonas aeruginosa Isolates. J. Infect. Public Health 2020, 13, 1932–1938. [Google Scholar] [CrossRef]
- Zhou, T.; Huang, J.; Feng, Q.; Liu, Z.; Lin, Q.; Xu, Z.; Zhang, L.-H. A Two-Component System FleS/FleR Regulates Multiple Virulence-Related Traits in Pseudomonas aeruginosa. BioRxiv 2021. Available online: https://www.biorxiv.org/content/10.1101/2021.04.22.441042v1 (accessed on 23 April 2023).
- Botelho, J.; Grosso, F.; Peixe, L. Antibiotic Resistance in Pseudomonas aeruginosa—Mechanisms, Epidemiology and Evolution. Drug Resist. Updates 2019, 44, 100640. [Google Scholar] [CrossRef] [PubMed]
- ISO 16266:2006 (En); Water Quality—Detection and Enumeration of Pseudomonas aeruginosa—Method by Membrane Filtration. ISO: Geneva, Switzerland, 2006.
- Stepanović, S.; Vuković, D.; Hola, V.; Bonaventura, G.D.; Djukić, S.; Ćirković, I.; Ruzicka, F. Quantification of Biofilm in Microtiter Plates: Overview of Testing Conditions and Practical Recommendations for Assessment of Biofilm Production by Staphylococci. APMIS 2007, 115, 891–899. [Google Scholar] [CrossRef]
- Stepanovic, S.; Vukovic, D.; Dakic, I.; Savic, B.; Svabic-Vlahovic, M. A Modified Microtiter-Plate Test for Quantification of Staphylococcal Biofilm Formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef]
- Rajkumari, J.; Borkotoky, S.; Murali, A.; Suchiang, K.; Mohanty, S.K.; Busi, S. Attenuation of Quorum Sensing Controlled Virulence Factors and Biofilm Formation in Pseudomonas aeruginosa by Pentacyclic Triterpenes, Betulin and Betulinic Acid. Microb. Pathog. 2018, 118, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Freeman, D.J.; Falkiner, F.R.; Keane, C.T. New Method for Detecting Slime Production by Coagulase Negative Staphylococci. J. Clin. Pathol. 1989, 42, 872–874. [Google Scholar] [CrossRef]
- Barakat, R. Etude Des Propriétés Biologiques et Antimicrobiennes de la Pyocyanine, Pigment Redox-Actif Produit Par Pseudomonas aeruginosa. Ph.D. Thesis, Université de La Rochelle, La Rochelle, France, 2012. [Google Scholar]
- Feghali, P.; Na’was, T. Pyocyanin: A Powerful Inhibitor of Bacterial Growth and Biofilm Formation. Madridge J. Case Rep. Stud. 2018, 3, 101–107. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, Y.; Hu, X.; Huang, X. How to Simply and Efficiently Screen Microbial Strains Capable of Anaerobic Biosynthesis of Biosurfactants: Method Establishment, Influencing Factors and Application Example Evaluation. Front. Microbiol. 2022, 13, 989998. [Google Scholar] [CrossRef]
- Roulová, N.; Mot’ková, P.; Brožková, I.; Pejchalová, M. Antibiotic Resistance of Pseudomonas aeruginosa Isolated from Hospital Wastewater in the Czech Republic. J. Water Health 2022, 20, 692–701. [Google Scholar] [CrossRef]
- Mann, E.E.; Wozniak, D.J. Pseudomonas Biofilm Matrix Composition and Niche Biology. FEMS Microbiol. Rev. 2012, 36, 893–916. [Google Scholar] [CrossRef]
- Park, S.; Dingemans, J.; Gowett, M.; Sauer, K. Glucose-6-Phosphate Acts as an Extracellular Signal of SagS To Modulate Pseudomonas aeruginosa c-Di-GMP Levels, Attachment, and Biofilm Formation. mSphere 2021, 6, e01231-20. [Google Scholar] [CrossRef]
- Li, Z.C. Study on Biofilm Formation Process Based on Image Structure Analyzer Software Analysis. Indian J. Pharm. Sci. 2021, 83, 204–212. [Google Scholar] [CrossRef]
- She, P.; Wang, Y.; Liu, Y.; Tan, F.; Chen, L.; Luo, Z.; Wu, Y. Effects of Exogenous Glucose on Pseudomonas aeruginosa Biofilm Formation and Antibiotic Resistance. Microbiologyopen 2019, 8, e933. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Pradhan, A.; Das, R.; Panigrahi, L.; Arakha, M. Pyocyanin Is the Microbial Blue-Green Pigment: A Review on Its History, Virulence, and Therapeutic Use. Curr. Bioact. Compd. 2022, 19, 62–76. [Google Scholar] [CrossRef]
- Mahgoub, Y.; Arif, R.; Zughaier, S. Pyocyanin Pigment from Pseudomonas aeruginosa Modulates Innate Immune Defenses in Macrophages. In Proceedings of the Qatar University Annual Research Forum and Exhibition (QUARFE 2021), Doha, Qatar, 20 October 2021. [Google Scholar] [CrossRef]
- Rashid, M.I.; Rashid, H.; Andleeb, S.; Ali, A. Evaluation of Blood-Brain-Barrier Permeability, Neurotoxicity, and Potential Cognitive Impairment by Pseudomonas aeruginosa’s Virulence Factor Pyocyanin. Oxidative Med. Cell. Longev. 2022, 2022, 3060579. [Google Scholar] [CrossRef] [PubMed]
- Bonneau, A.; Roche, B.; Schalk, I.J. Iron acquisition in Pseudomonas aeruginosa by the siderophore pyoverdine: An intricate interacting network including periplasmic and membrane proteins. Sci. Rep. 2020, 10, 120. [Google Scholar] [CrossRef]
- Lhospice, S.; Gomez, N.O.; Ouerdane, L.; Brutesco, C.; Ghssein, G.; Hajjar, C.; Liratni, A.; Wang, S.; Richaud, P.; Bleves, S.; et al. Pseudomonas aeruginosa zinc uptake in chelating environment is primarily mediated by the metallophore pseudopaline. Sci. Rep. 2017, 7, 17132. [Google Scholar] [CrossRef]
- Vélez, J.M.B.; Martínez, J.G.; Ospina, J.T.; Agudelo, S.O. Bioremediation Potential of Pseudomonas Genus Isolates from Residual Water, Capable of Tolerating Lead through Mechanisms of Exopolysaccharide Production and Biosorption. Biotechnol. Rep. 2021, 32, e00685. [Google Scholar] [CrossRef]
- Jali, J.N.; Naik, S.S.; Mishra, R.; Soren, N.; Sardar, K.K. Study of Antimicrobial Diversity Profile of Both Planktonic and Sessile Phenotypes during Chronic Infections of Bovine. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 452–459. [Google Scholar] [CrossRef]
- de Sousa, T.; Hébraud, M.; Alves, O.; Costa, E.; Maltez, L.; Pereira, J.E.; Martins, Â.; Igrejas, G.; Poeta, P. Study of Antimicrobial Resistance, Biofilm Formation, and Motility of Pseudomonas aeruginosa Derived from Urine Samples. Microorganisms 2023, 11, 1345. [Google Scholar] [CrossRef]
- Palma, V.; Gutiérrez, M.S.; Vargas, O.; Parthasarathy, R.; Navarrete, P. Methods to Evaluate Bacterial Motility and Its Role in Bacterial–Host Interactions. Microorganisms 2022, 10, 563. [Google Scholar] [CrossRef]
- Song, P.; Zhang, X.; Wang, S.; Xu, W.; Wang, F.; Fu, R.; Wei, F. Microbial Proteases and Their Applications. Front. Microbiol. 2023, 14, 1236368. [Google Scholar] [CrossRef]
- Islamieh, D.I.; Afshar, D.; Esmaeili, D. Effect of Satureja Khuzistanica Essential Oil (SKEO) Extract on Expression of lasA and lasB Genes in Pseudomonas aeruginosa. Iran. J. Microbiol. 2019, 11, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Kessler, E.; Safrin, M.; Gustin, J.K.; Ohman, D.E. Elastase and the LasA Protease of Pseudomonas aeruginosa Are Secreted with Their Propeptides. J. Biol. Chem. 1998, 273, 30225–30231. [Google Scholar] [CrossRef] [PubMed]
- Soberón-Chávez, G.; González-Valdez, A.; Soto-Aceves, M.P.; Cocotl-Yañez, M. Rhamnolipids Produced by Pseudomonas: From Molecular Genetics to the Market. Microb. Biotechnol. 2021, 14, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Mangiola, S.; Papenfuss, A. tidyHeatmap: An R Package for Modular Heatmap Production Based on Tidy Principles. J. Open Source Softw. 2020, 5, 2472. [Google Scholar] [CrossRef]
- Chino, T.; Nukui, Y.; Morishita, Y.; Moriya, K. Morphological Bactericidal Fast-Acting Effects of Peracetic Acid, a High-Level Disinfectant, against Staphylococcus Aureus and Pseudomonas aeruginosa Biofilms in Tubing. Antimicrob. Resist. Infect. Control 2017, 6, 122. [Google Scholar] [CrossRef]
- Mudgil, U.; Khullar, L.; Chadha, J.; Prerna; Harjai, K. Beyond Antibiotics: Emerging Antivirulence Strategies to Combat Pseudomonas aeruginosa in Cystic Fibrosis. Microb. Pathog. 2024, 193, 106730. [Google Scholar] [CrossRef]

| Isolation Date | Strain Code | Hospital | Service | Origin | Biofilm-Forming Capacities |
|---|---|---|---|---|---|
| 23 June 2022 | 2501 | Hédi Chaker University Hospital Sfax | Gastro endoscopy unit | Softened water (filtered) | 0 |
| 29 June 2022 | 2560 | Hédi Chaker University Hospital Sfax | Hemodialysis: water treatment room | Machine-treated water | ++ |
| 29 June 2022 | 2582 | Military Hospital | Healthcare room | Tap water | +++ |
| 5 July 2022 | 2629 | Hossine Bouzayen Regional Hospital Gafsa | Stomatology service | Dental chair water | +++ |
| 8 July 2022 | 2642 | Hédi Chaker University Hospital Sfax | Gastro endoscopy unit | Tap 1 filtered softened water | +++ |
| 8 July 2022 | 2643 | Hédi Chaker University Hospital Sfax | Gastro endoscopy unit | Tap 2 filtered softened water | + |
| 12 July 2022 | 2653 | Hédi Chaker University Hospital Sfax | Gastro endoscopy unit | Tap 1 softened water | +++ |
| 12 July 2022 | 2654 | Hédi Chaker University Hospital Sfax | Gastro endoscopy unit | Tap 2 softened water | 0 |
| 12 July 2022 | 2659 | Jebéniana Regional Hospital | Hemodialysis: water treatment room | Post-softener water | +++ |
| 14 July 2022 | 2676 | Mahres Regional Hospital | Intensive care unit | Tap water | +++ |
| 14 July 2022 | 2677 | Mahres Regional Hospital | Hemodialysis: water treatment room | Tap water | +++ |
| 14 July 2022 | 2687 | Military Hospital | Intensive care unit | Tap water | +++ |
| 19 July 2022 | 2712 | Hédi Chaker University Hospital Sfax | Gastro endoscopy unit | Tap 1 softened water | ++ |
| 19 July 2022 | 2713 | Hédi Chaker University Hospital Sfax | Gastro endoscopy unit | Tap 2 softened water | 0 |
| 21 July 2022 | 2778 | Hédi Chaker University Hospital Sfax | Gastro endoscopy unit | Softened water tap 1 with thermal filter | +++ |
| 21 July 2022 | 2779 | Hédi Chaker University Hospital Sfax | Gastro endoscopy unit | Softened water tap 2 with thermal filter | +++ |
| 21 July 2022 | 2784 | Jebéniana Regional Hospital | Hemodialysis: water treatment room | Loop inlet water | ++ |
| 23 July 2022 | 2822 | Hédi Chaker University Hospital Sfax | Gastro endoscopy unit | Softened water without thermal filter (+UV) tap 2 | ++ |
| 23 July 2022 | 2823 | Hédi Chaker University Hospital Sfax | Gastro endoscopy unit | Softened water without thermal filter (without UV) tap 2 | +++ |
| 28 July 2022 | 2887 | Hédi Chaker University Hospital Sfax | Gastro endoscopy unit | Softened water without thermal filter tap 1 | ++ |
| 28 July 2022 | 2888 | Hédi Chaker University Hospital Sfax | Gastro endoscopy unit | Softened water without thermal filter tap 1 | 0 |
| 28 July 2022 | 2889 | Hédi Chaker University Hospital Sfax | Gastro endoscopy unit | Softened water without thermal tap filter 2 | +++ |
| 28 July 2022 | 2890 | Hédi Chaker University Hospital Sfax | Gastro endoscopy unit | Softened water without thermal tap filter 2 | +++ |
| 29 July 2022 | 2946 | Hédi Chaker University Hospital Sfax | Gastro endoscopy unit | Softened water tap 1 | +++ |
| 29 July 2022 | 2947 | Hédi Chaker University Hospital Sfax | Gastro endoscopy unit | Softened water tap 2 | +++ |
| 29 July 2022 | 2952 | Hédi Chaker University Hospital Sfax | Gastro endoscopy unit | Post-softener water | +++ |
| 29 July 2022 | 2953 | Hédi Chaker University Hospital Sfax | Gastro endoscopy unit | Post-filtration water | ++ |
| 29 July 2022 | 2954 | Hédi Chaker University Hospital Sfax | Gastro endoscopy unit | Pre-UV water | +++ |
| 29 July 2022 | 2955 | Hédi Chaker University Hospital Sfax | Gastro endoscopy unit | Pre-UV water | +++ |
| 29 July 2022 | 2956 | Hédi Chaker University Hospital Sfax | Gastro endoscopy unit | Tap water 1 | + |
| 29 July 2022 | 2957 | Hédi Chaker University Hospital Sfax | Gastro endoscopy unit | Tap water 2 | +++ |
| 2 August 2022 | 2965 | Hédi Chaker University Hospital Sfax | Hemodialysis: water treatment room | Start of treatment cycle | + |
| 2 August 2022 | 2966 | Hédi Chaker University Hospital Sfax | Hemodialysis: water treatment room | Start of treatment cycle | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guesmi, M.; Ben Hmida, M.; Smaoui, S.; Ayadi, M.; Maalej, S.; Toumi, S.; Aifa, S.; Kammoun, K.; Messadi-Akrout, F.; Mnif, S. A Comprehensive Methodology for Identifying Pseudomonas aeruginosa Strains Exhibiting Biofilm and Virulence Factor Traits and Assessment of Biofilm Resistance Against Commercial Disinfectant. Microbiol. Res. 2025, 16, 62. https://doi.org/10.3390/microbiolres16030062
Guesmi M, Ben Hmida M, Smaoui S, Ayadi M, Maalej S, Toumi S, Aifa S, Kammoun K, Messadi-Akrout F, Mnif S. A Comprehensive Methodology for Identifying Pseudomonas aeruginosa Strains Exhibiting Biofilm and Virulence Factor Traits and Assessment of Biofilm Resistance Against Commercial Disinfectant. Microbiology Research. 2025; 16(3):62. https://doi.org/10.3390/microbiolres16030062
Chicago/Turabian StyleGuesmi, Maha, Mohamed Ben Hmida, Salma Smaoui, Mariem Ayadi, Salma Maalej, Salma Toumi, Sami Aifa, Khawla Kammoun, Férièle Messadi-Akrout, and Sami Mnif. 2025. "A Comprehensive Methodology for Identifying Pseudomonas aeruginosa Strains Exhibiting Biofilm and Virulence Factor Traits and Assessment of Biofilm Resistance Against Commercial Disinfectant" Microbiology Research 16, no. 3: 62. https://doi.org/10.3390/microbiolres16030062
APA StyleGuesmi, M., Ben Hmida, M., Smaoui, S., Ayadi, M., Maalej, S., Toumi, S., Aifa, S., Kammoun, K., Messadi-Akrout, F., & Mnif, S. (2025). A Comprehensive Methodology for Identifying Pseudomonas aeruginosa Strains Exhibiting Biofilm and Virulence Factor Traits and Assessment of Biofilm Resistance Against Commercial Disinfectant. Microbiology Research, 16(3), 62. https://doi.org/10.3390/microbiolres16030062







