Multisystemic Inflammatory Syndrome Temporally Associated with COVID-19 in a Regional Pediatric Hospital from México
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
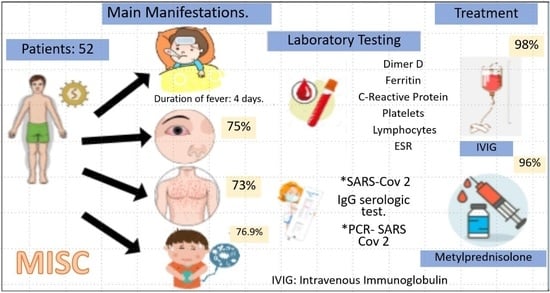
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Royal College of Paediatrics and Child Health. Paediatric Multisystem Inflammatory Syndrome Temporally Associated with COVID-19 (PIMS)—Guidance for Clinicians [Internet]. RCPCH. 2020. Available online: https://www.rcpch.ac.uk/resources/paediatric-multisystem-inflammatory-syndrome-temporally-associated-covid-19-pims-guidance (accessed on 3 March 2022).
- Soma, L.; Shust, F.J.; Ratner. Multisystem Inflammatory Syndrome in children. Infect. Dis. Immun. 2020, 33, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Henderson, L.A.; Canna, S.W.; Friedman, K.G.; Gorelik, M.; Lapidus, S.K.; Bassiri, H.; Behrens, E.M.; Kernan, K.F.; Schulert, G.S.; Seo, P.; et al. American College of Rheumatology Clinical Guidance for Multisystem Inflammatory Syndrome in Children Associated With SARS-CoV-2 and Hyperinflammation in Pediatric COVID-19: Version 3. Arthritis Rheumatol. 2022, 74, e1–e20. [Google Scholar] [CrossRef] [PubMed]
- Rowley, A.H.; Baker, S.C.; Shulman, S.T.; Garcia, F.L.; Guzman-Cottrill, J.A.; Chou, P.; Terai, M.; Kawasaki, T.; Kalelkar, M.B.; Crawford, S.E. Detection of Antigen in Bronchial Epithelium and Macrophages in Acute Kawasaki Disease by Use of Synthetic Antibody. J. Infect. Dis. 2004, 190, 856–865. [Google Scholar] [CrossRef]
- Lutchman, D. PIMS-TS and Kawasaki Disease: The Mystery Deepens. Pediatr. Infect. Dis. 2020, 39, e215–e216. [Google Scholar] [CrossRef] [PubMed]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; Macary, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation, and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef]
- Gustine, J.; Jones, D. Immunopathology of Hyperinflammation in COVID-19. Am. J. Pathol. 2021, 191, 4–17. [Google Scholar] [CrossRef]
- Rosat, C.; Cotugno, N.; Sardh, F.; Pou, C.; Amodio, D.; Rodriguez, L.; Tan, Z.; Zicari, S.; Ruggiero, A.; Rubens, G.; et al. The Immunology of Multisystem Inflammatory Syndrome in Children with COVID-19. Cell 2020, 183, 968–981. [Google Scholar]
- Kaklamanos, A.; Belogiannis, K.; Skendros, P.; Gorgoulis, V.; Vlachoyiannopoulos, P.; Tzioufas, A. COVID-19 Immunobiology: Lessons Learned, New Questions Arise. Front. Immunol. 2021, 12, 719023. [Google Scholar] [CrossRef]
- World Health Organization. Síndrome Inflamatorio Multisistémico en Niños y Adolescentes con COVID-19. Informe Científico 15 de Mayo de 2020. Available online: https://apps.who.int/iris/bitstream/handle/10665/332191/WHO-2019-nCoV-Sci_Brief-Multisystem_Syndrome_Children-2020.1-spa.pdf?sequence=1&isAllowed=y (accessed on 2 March 2022).
- Nakra, N.; Blumberg, D.; Herrera, A.; Lakshminrusimha, S. Multi-system inflammatory Syndrome in Children (MIS-C) Following SARS-CoV-2 Infection: Review of Clinial Presentation, Hypothetical Pathogenesis, and Proposed Management. Children 2020, 7, 69. [Google Scholar] [CrossRef]
- Henderson, L.A.; Canna, S.W.; Friedman, K.G.; Gorelik, M.; Lapidus, S.K.; Bassiri, H.; Behrens, E.M.; Ferris, A.; Kernan, K.F.; Schulert, G.S.; et al. American College of Rheumatology Clinical Guidance for Multisystem Inflammatory Syndrome in Children Associated With SARS-CoV-2 and Hyperinflammation in Pediatric COVID-19: Version 2. Arthritis Rheumatol. 2021, 73, e13–e29. [Google Scholar] [CrossRef]
- Sperotto, F.; Friedman, K.G.; Son, M.; Vanderpluym, C.; Newburger, J.; Dionne, A. Cardiac manifestations in SARS-CoV-2-associated multisystem inflammatory syndrome in children: A comprehensive review and proposed clinical approach. Eur. J. Pediatr. 2021, 180, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, M.; Bridwell, R.; Ravera, J.; Long, B. Multisystem inflammatory syndrome in children with COVID-19. Am. J. Emerg. Med. 2021, 49, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Ricci, Z.; Cruz, D.N.; Ronco, C. Classification and staging of acute kidney injury: Beyond the RIFLE and AKIN criteria. Nat. Rev. Nephrol. 2011, 7, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Abrams, J.Y.; Godfred-Cato, S.E.; Oster, M.E.; Chow, E.J.; Koumans, E.H.; Bryant, B.; Leung, J.W.; Belay, E.D. Multisystem Inflammatory Syndrome in Children Associated with Severe Acute Respiratory Syndrome Coronavirus 2: A Systematic Review. J. Pediatr. 2020, 226, 45–54.e1. [Google Scholar] [CrossRef] [PubMed]
- Radia, T.; Williams, N.; Agrawal, P.; Harman, K.; Weale, J.; Cook, J.; Gupta, A. Multisystem inflammatory syndrome in children and adolescents (MIS-C): A systematic review of clinical features and presentation. Paediatr. Respir. Rev. 2021, 38, 51–57. [Google Scholar] [PubMed]
- Kaushik, S.; Aydin, S.I.; Derespina, K.R.; Bansal, P.B.; Kowalsky, S.; Trachtman, R.; Gillen, J.K.; Perez, M.M.; Soshnick, S.H.; Conway, E.E., Jr.; et al. Multisystem Inflammatory Syndrome in Children Associated with Severe Acute Respiratory Syndrome Coronavirus 2 Infection (MIS-C): A Multi-institutional Study from New York City. J. Pediatr. 2020, 224, 24–29. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Xu, B.W.; Du, J.B. Similarities and differences between multiple inflammatory syndrome in children associated with COVID-19 and Kawasaki disease: Clinical presentations, diagnosis, and treatment. World J. Pediatr. 2021, 17, 335–340. [Google Scholar] [CrossRef]
- Castano-Jaramillo, L.M.; Yamazaki-Nakashimada, M.A.; O’Farrill-Romanillos, P.M.; Muzquiz Zermeño, D.; Scheffler Mendoza, S.C.; Venegas Montoya, E.; García Campos, J.A.; Sánchez-Sánchez, L.M.; Gámez González, L.B.; Ramírez López, J.M.; et al. COVID-19 in the Context of Inborn Errors of Immunity: A Case Series of 31 Patients from Mexico. J. Clin. Immunol. 2020, 41, 1463–1478. [Google Scholar] [CrossRef]
- Bucciol, G.; COVID Human Genetic Effort; Meyts, I. Inherited and acquired errors of type I interferon immunity govern susceptibility to COVID-19 and multisystem inflammatory syndrome in children. J. Allergy Clin. Immunol. 2023, 151, 832–840. [Google Scholar] [CrossRef]
- Sharma, C.; Ganigara, M.; Galeotti, C.; Burns, J.; Berganza, F.; Hayes, D.; Grewal, D.; Bharath, S.; Sajjan, S.; Bayry, J. Multisystem inflammatory syndrome in children and Kawasaki disease: A critical comparison. Nat. Rev. 2021, 17, 731–748. [Google Scholar] [CrossRef]
- Zhou, C.; Zhao, Y.; Wang, X.; Huang, Y.; Tang, X.; Tang, L. Laboratory parameters between multisystem inflammatory syndrome in children and Kawasaki disease. Pediatr. Pulmonol. 2021, 56, 3688–3698. [Google Scholar] [CrossRef]
- Alsaied, T.; Tremoulet, A.H.; Burns, J.C.; Saidi, A.; Dionne, A.; Lang, S.M.; Newburger, J.W.; de Ferranti, S.; Friedman, K.G. Review of Cardiac Involvement in Multisystem Inflammatory Syndrome in Children. Circulation 2021, 143, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Stankovic, C.; DeLaroche, A.; Arora, R.; Ehrman, R. Laboratory trends in severe MIS-C. Acad. Emerg. Med. 2022, 29, 1258–1260. [Google Scholar] [CrossRef] [PubMed]
- Adeyinka, A.; Bailey, K.; Pierre, L.; Kondamudi, N. COVID-19 infection: Pediatric perspectives. J. Am. Coll. Emerg. Physicians Open 2021, 2, e12375. [Google Scholar]
- Chen, M.; Kuo, H.; Lee, Y.; Chi, H.; Li, S.; Lee, H.; Yang, K. Phenotype, Susceptibility, Autoimmunity, and Immunotherapy between Kawasaki Disease and Coronarivurs Disease-19 Associated Multisystem Inflammatory Syndrome in Children. Front. Immunol. 2021, 12, 632890. [Google Scholar] [CrossRef] [PubMed]
- Wessels, P.A.; Bingler, M.A. A comparison of Kawasaki Disease and multisystem inflammatory syndrome in children. Prog. Pediatr. Cardiol. 2022, 65, 101516. [Google Scholar] [CrossRef]
- Jiang, L.; Tang, K.; Irfan, O.; Li, X.; Zhang, E.; Bhutta, Z. Epidemiology, Clinical Features, and Outcomes of multisystem inflammatory syndrome in children (MIS-C) and adolescents- a Live Systematic Review and Metanalysis. Curr. Pediatr. Rep. 2022, 10, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Feldstein, L.R.; Tenforde, M.W.; Friedman, K.G.; Newhams, M.; Billing, E.; Dapul, H.; Soma, V.; Maddux, A.; Mourani, P.; Bowens, C.; et al. Characteristics and Outcomes of US Children and Adolescents with Multisystem Inflammatory Syndrome in Children (MIS-C) Compared with Severe Acute COVID-19. JAMA 2021, 325, 1074–1087. [Google Scholar] [CrossRef] [PubMed]
- Haslak, F.; Barut, K.; Durak, C.; Aliyeva, A.; Yildiz, M.; Guliyeva, V.; Varol, S.E.; Cebeci, S.O.; Aygun, F.; Varli, Y.Z.; et al. Clinical features and outcomes of 76 patients with COVID-19-related multi-system inflammatory syndrome in children. Clin. Rheumatol. 2021, 40, 4167–4178. [Google Scholar] [CrossRef]
- Shimizu, V.; Brodin, P.; Cobat, A.; Biggs, C.; Toubiana, J.; Lucas, C.; Henrickson, S.; Belot, A.; Tangye, S.; Milner, J.; et al. SARS-Cov-2 related MIS-C: A Key to the viral and genetic causes of kawasawki disease. J. Exp. Med. 2021, 218, e20210446. [Google Scholar] [CrossRef]
- Giacalone, M.; Scheier, E.; Shavit, I. Multisystem inflammatory syndrome in children (MIS-C): A mini-review. Int. J. Emerg. Med. 2021, 14, 50. [Google Scholar] [CrossRef]
| Symptoms | Frequency (%) |
|---|---|
| Duration of fever (days) | 4 (min. 3, max. 5) |
| Diarrhea | 30 (57.7) |
| Abdominal pain | 40 (76.9) |
| Emesis | 36 (69.2) |
| Cutaneous rash | 38 (73) |
| Conjunctivitis | 39 (75) |
| Cleft lips/strawberry tongue | 35 (67.3) |
| Lymphadenopathy | 26 (50) |
| Limb edema | 28 (54.9) |
| Headache | 15 (28.8) |
| Mental alteration/Irritability | 15 (28.8) |
| Somnolence | 8 (15.4) |
| Test | Value | Reference Range |
|---|---|---|
| C-Reactive protein (mg/dL) | 18.1 (6.3–26.5) | 0–5 mg/L |
| Procalcitonin (ng/mL) | 2.7 (0.8–9) | <0.1 ng/mL |
| Erythrocyte sedimentation rate (mm/h) | 40 ± 15.3 | 0–10 mm/h |
| D-dimer | 2974 (1457.7–6074) | <0.5 mg/mL |
| Ferritin | 245.7 (175.7–463) | 10–200 ng/mL |
| Platelets | 184 (104.5–303) | 150–400 × 109 cells/L |
| Lymphocytes | 1230 (907–2943) | 3–9.5 × 103 cells/μL |
| Sodium | 135 ± 4.9 | (135–145 mEq/L) |
| Outcomes | Frequency (%) |
|---|---|
| General findings in PICU | |
| Shock | 12 (23.1) |
| Gastrointestinal Bleeding | 1 (1.9) |
| Acute Kidney Injury | 8 (15.4) |
| Vasopressor support | |
| Norepinephrine | 4 (7.7) |
| Epinephrine | 1 (1.9) |
| Dobutamine | 1 (1.9) |
| Norepinephrine + Epinephrine | 2 (3.8) |
| Norepinefrine + Dobutamine | 1 (1.9) |
| Cardiovascular findings | |
| Bilateral opacities | 8 (15.4) |
| Acute pericarditis | 4 (7.7) |
| Pericardia Effusion | 3 (5.8) |
| LV ejection fraction <50% | 1 (1.9) |
| Respiratory support | |
| Mechanic ventilation | 7 (13.46) |
| Oxygen | 3 (5.8) |
| Nasal tips | 1 (1.92) |
| Pharmacologic therapy | |
| Empirical antibiotic treatment | 28 (53.8) |
| Ceftriaxone | 26 (50) |
| Clindamycin | 8 (15.4) |
| Cefepime | 2 (3.85) |
| Intravenous immunoglobulin | 51 (98) |
| Methyl prednisolone | 50 (96.15) |
| Acetylsalicylic acid | 46 (88.4) |
| Antiviral treatment | 4 (7.7) |
| Oseltamivir | 2 (3.9) |
| Acyclovir | 2 (3.9) |
| Mortality | 2 (3.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barroso-Santos, J.; Robledo-Martínez, A.I.; Espinosa-Padilla, S.E.; Hurtado del Ángel, R.G.; Arteaga-García, F.; Langarica-Bulos, M.; Madrid-Gómez-Tagle, J.A.; Sánchez-Reyes, B.A.; Hernández-Cadena, S.E.; Suárez-Soto, J.I.; et al. Multisystemic Inflammatory Syndrome Temporally Associated with COVID-19 in a Regional Pediatric Hospital from México. Pediatr. Rep. 2023, 15, 341-348. https://doi.org/10.3390/pediatric15020030
Barroso-Santos J, Robledo-Martínez AI, Espinosa-Padilla SE, Hurtado del Ángel RG, Arteaga-García F, Langarica-Bulos M, Madrid-Gómez-Tagle JA, Sánchez-Reyes BA, Hernández-Cadena SE, Suárez-Soto JI, et al. Multisystemic Inflammatory Syndrome Temporally Associated with COVID-19 in a Regional Pediatric Hospital from México. Pediatric Reports. 2023; 15(2):341-348. https://doi.org/10.3390/pediatric15020030
Chicago/Turabian StyleBarroso-Santos, Joel, Angelina Ingrid Robledo-Martínez, Sara Elva Espinosa-Padilla, Rubén Genaro Hurtado del Ángel, Felipe Arteaga-García, Mónica Langarica-Bulos, José Antonio Madrid-Gómez-Tagle, Beatriz Adriana Sánchez-Reyes, Sarai Eunice Hernández-Cadena, Jorge Iván Suárez-Soto, and et al. 2023. "Multisystemic Inflammatory Syndrome Temporally Associated with COVID-19 in a Regional Pediatric Hospital from México" Pediatric Reports 15, no. 2: 341-348. https://doi.org/10.3390/pediatric15020030
APA StyleBarroso-Santos, J., Robledo-Martínez, A. I., Espinosa-Padilla, S. E., Hurtado del Ángel, R. G., Arteaga-García, F., Langarica-Bulos, M., Madrid-Gómez-Tagle, J. A., Sánchez-Reyes, B. A., Hernández-Cadena, S. E., Suárez-Soto, J. I., Delgado-Amézquita, C., Godínez-Hernández, B., Otamendi-Canales, O., & Jiménez-Osorio, A. S. (2023). Multisystemic Inflammatory Syndrome Temporally Associated with COVID-19 in a Regional Pediatric Hospital from México. Pediatric Reports, 15(2), 341-348. https://doi.org/10.3390/pediatric15020030