Predictive Role of Fluorescein Angiography in Retinopathy of Prematurity
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Grading Parameters
2.3. Statistical Analysis
3. Results
3.1. Demographic Data
3.2. FA Features
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dammann, O.; Hartnett, M.E.; Stahl, A. Retinopathy of prematurity. Dev. Med. Child Neurol. 2023, 65, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Fevereiro-Martins, M.D.R.; Marques-Neves, C.A.M.; Guimarães, H.; Bicho, M.D.P. Retinopathy of prematurity: A review of pathophysiology and signaling pathways. Surv. Ophthalmol. 2023, 68, 175–210. [Google Scholar] [CrossRef]
- Hellström, A.; Smith, L.E.; Dammann, O. Retinopathy of prematurity. Lancet 2013, 382, 1445–1457. [Google Scholar] [CrossRef]
- Quimson, S.K. Retinopathy of Prematurity: Pathogenesis and Current Treatment Options. Neonatal Netw. 2015, 34, 284–287. [Google Scholar] [CrossRef]
- Hans, A.; Narang, S.; Sindhu, M.; Jain, S.; Chawla, D. Fundus fluorescein angiography in retinopathy of prematurity. Eye 2022, 36, 1604–1609. [Google Scholar] [CrossRef] [PubMed]
- Barillà, D.; Guagliano, R.; Bertone, C.; Maffia, A.; Bruttini, C.; Periti, F.; Plaitano, C.; Arpa, C.; Montescani, S.; Tinelli, C.; et al. Screening and Follow-Up of Acute ROP: Reproducibility of Fluorescein Angiography. Adv. Ther. 2020, 37, 860–868. [Google Scholar] [CrossRef]
- Flynn, J.T.; O’Grady, G.E.; Herrera, J.; Kushner, B.J.; Cantolino, S.; Milam, W. Retrolental fibroplasia: I. Clinical observations. Arch. Ophthalmol. 1977, 95, 217–223. [Google Scholar] [CrossRef]
- Flynn, J.T.; Cassady, J.; Essner, D.; Zeskind, J.; Merritt, J.; Flynn, R.; Williams, M.J. Fluorescein angiography in retrolental fibroplasia: Experience from 1969–1977. Ophthalmology 1979, 86, 1700–1723. [Google Scholar] [CrossRef] [PubMed]
- Cantolino, S.; O’Grady, G.E.; Herrera, J.A.; Israel, C.; Justice, J.; Flynn, J.T. Ophthalmoscopic monitoring of oxygen therapy in premature infants. Fluorescein angiography in acute retrolental fibroplasia. Am. J. Ophthalmol. 1971, 72, 322–331. [Google Scholar] [CrossRef]
- Lepore, D.; Ji, M.H.; Ying, G.S.; Orazi, L.; Pagliara, M.M.; Quinn, G.E.; FA Team of the Italian ROP Study Group. Early angiographic signs of retinopathy of prematurity requiring treatment. Eye 2021, 35, 3094–3101. [Google Scholar] [CrossRef]
- Chiang, M.F.; Quinn, G.E.; Fielder, A.R.; Ostmo, S.R.; Chan, R.P.; Berrocal, A.; Binenbaum, G.; Blair, M.; Campbell, J.P.; Capone, A.; et al. International Classification of Retinopathy of Prematurity, Third Edition. Ophthalmology 2021, 128, e51–e68. [Google Scholar] [CrossRef]
- Lepore, D.; Molle, F.; Pagliara, M.M.; Baldascino, A.; Angora, C.; Sammartino, M.; Quinn, G.E. Atlas of fluorescein angiographic findings in eyes undergoing laser for retinopathy of prematurity. Ophthalmology 2011, 118, 168–175. [Google Scholar] [CrossRef]
- Temkar, S.; Azad, S.V.; Chawla, R.; Damodaran, S.; Garg, G.; Regani, H.; Nawazish, S.; Raj, N.; Venkatraman, V. Ultra-widefield fundus fluorescein angiography in pediatric retinal vascular diseases. Indian J. Ophthalmol. 2019, 67, 788–794. [Google Scholar] [CrossRef]
- Mataftsi, A.; Lithoxopoulou, M.; Seliniotaki, A.K.; Talimtzi, P.; Oustoglou, E.; Diamanti, E.; Soubasi, V.; Ziakas, N.; Haidich, A. Avoiding use of lid speculum and indentation reduced infantile stress during retinopathy of prematurity examinations. Acta Ophthalmol. 2022, 100, e128–e134. [Google Scholar] [CrossRef]
- Mangalesh, S.; Sarin, N.; McGeehan, B.; Prakalapakorn, S.G.; Tran-Viet, D.; Cotten, C.M.; Freedman, S.F.; Maguire, M.G.; Toth, C.A.; BabySTEPS Group. Preterm Infant Stress During Handheld Optical Coherence Tomography vs Binocular Indirect Ophthalmoscopy Examination for Retinopathy of Prematurity. JAMA Ophthalmol. 2021, 139, 567–574. [Google Scholar] [CrossRef]
- Moral-Pumarega, M.T.; Caserío-Carbonero, S.; De-La-Cruz-Bértolo, J.; Tejada-Palacios, P.; Lora-Pablos, D.; Pallás-Alonso, C.R. Pain and stress assessment after retinopathy of prematurity screening examination: Indirect ophthalmoscopy versus digital retinal imaging. BMC Pediatr. 2012, 12, 132. [Google Scholar] [CrossRef]
- Kleberg, A.; Warren, I.; Norman, E.; Mörelius, E.; Berg, A.-C.; Mat-Ali, E.; Holm, K.; Fielder, A.; Nelson, N.; Hellström-Westas, L. Lower stress responses after Newborn Individualized Developmental Care and Assessment Program care during eye screening examinations for retinopathy of prematurity: A randomized study. Pediatrics 2008, 121, e1267–e1278. [Google Scholar] [CrossRef]
- Section on Ophthalmology American Academy of Pediatrics; American Academy of Ophthalmology; American Association for Pediatric Ophthalmology and Strabismus. Screening examination of premature infants for retinopathy of prematurity. Pediatrics 2006, 117, 572–576. [Google Scholar] [CrossRef]
- Löfqvist, C.; Andersson, E.; Sigurdsson, J.; Engström, E.; Hård, A.-L.; Niklasson, A.; Smith, L.E.H.; Hellström, A. Longitudinal postnatal weight and insulin-like growth factor I measurements in the prediction of retinopathy of prematurity. Arch. Ophthalmol. 2006, 124, 1711–1718. [Google Scholar] [CrossRef]
- Wu, C.; VanderVeen, D.K.; Hellström, A.; Löfqvist, C.; Smith, L.E. Longitudinal postnatal weight measurements for the prediction of retinopathy of prematurity. Arch. Ophthalmol. 2010, 128, 443–447. [Google Scholar] [CrossRef]
- Wu, C.; Löfqvist, C.; Smith, L.E.H.; VanderVeen, D.K.; Hellström, A.; WINROP Consortium. Importance of early postnatal weight gain for normal retinal angiogenesis in very preterm infants: A multicenter study analyzing weight velocity deviations for the prediction of retinopathy of prematurity. Arch. Ophthalmol. 2012, 130, 992–999. [Google Scholar] [CrossRef]
- Zepeda-Romero, L.C.; Hård, A.-L.; Gomez-Ruiz, L.M.; Gutierrez-Padilla, J.A.; Angulo-Castellanos, E.; Barrera-De-Leon, J.C.; Ramirez-Valdivia, J.M.; Gonzalez-Bernal, C.; Valtierra-Santiago, C.I.; Garnica-Garcia, E.; et al. Prediction of retinopathy of prematurity using the screening algorithm WINROP in a Mexican population of preterm infants. Arch. Ophthalmol. 2012, 130, 720–723. [Google Scholar] [CrossRef]
- Löfqvist, C.; Hansen-Pupp, I.; Andersson, E.; Holm, K.; Smith, L.E.H.; Ley, D.; Hellström, A. Validation of a new retinopathy of prematurity screening method monitoring longitudinal postnatal weight and insulinlike growth factor I. Arch. Ophthalmol. 2009, 127, 622–627. [Google Scholar] [CrossRef]
- Hård, A.-L.; Löfqvist, C.; Filho, J.B.F.; Procianoy, R.S.; Smith, L.; Hellström, A. Predicting proliferative retinopathy in a Brazilian population of preterm infants with the screening algorithm WINROP. Arch. Ophthalmol. 2010, 128, 1432–1436. [Google Scholar] [CrossRef]
- Klufas, M.A.; Patel, S.N.; Ryan, M.C.; Gupta, M.P.; Jonas, K.E.; Ostmo, S.; Martinez-Castellanos, M.A.; Berrocal, A.M.; Chiang, M.F.; Chan, R.P. Influence of Fluorescein Angiography on the Diagnosis and Management of Retinopathy of Prematurity. Ophthalmology 2015, 122, 1601–1608. [Google Scholar] [CrossRef]
- Shats, D.; Balasubramanian, T.; Sidelnikov, D.; Das, U.; Onyekaba, N.-A.; Forbes, H.E.; Lu, N.; Williams, K.; Levin, M.R.; Sundararajan, S.; et al. Association of Speckle-Based Blood Flow Measurements and Fluorescein Angiography in Infants with Retinopathy of Prematurity. Ophthalmol. Sci. 2023, 4, 100463. [Google Scholar] [CrossRef]
- Ruia, S.; Tripathy, K. Fluorescein Angiography. In StatPearls; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Ng, E.Y.J.; Lanigan, B.; O’Keefe, M. Fundus fluorescein angiography in the screening for and management of retinopathy of prematurity. J. Pediatr. Ophthalmol. Strabismus. 2006, 43, 85–90. [Google Scholar] [CrossRef]
- Mansukhani, S.A.; Hutchinson, A.K.; Neustein, R.; Schertzer, J.; Allen, J.C.; Hubbard, G.B. Fluorescein Angiography in Retinopathy of Prematurity: Comparison of Infants Treated with Bevacizumab to Those with Spontaneous Regression. Ophthalmol. Retina. 2019, 3, 436–443. [Google Scholar] [CrossRef]
- Guagliano, R.; Barillà, D.; Bertone, C.; Maffia, A.; Periti, F.; Spallone, L.; Anselmetti, G.; Giacosa, E.; Stronati, M.; Tinelli, C.; et al. Fluorescein angiography-based diagnosis for retinopathy of prematurity: Expert-non expert comparison. Eur. J. Ophthalmol. 2013, 23, 881–886. [Google Scholar] [CrossRef]
- Wallace, D.K.; Quinn, G.E.; Freedman, S.F.; Chiang, M.F. Agreement among pediatric ophthalmologists in diagnosing plus and pre-plus disease in retinopathy of prematurity. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2008, 12, 352–356. [Google Scholar] [CrossRef]
- Chiang, M.F.; Jiang, L.; Gelman, R.; Du, Y.E.; Flynn, J.T. Interexpert agreement of plus disease diagnosis in retinopathy of prematurity. Arch. Ophthalmol. 2007, 125, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Hewing, N.J.; Kaufman, D.R.; Chan, R.V.; Chiang, M.F. Plus disease in retinopathy of prematurity: Qualitative analysis of diagnostic process by experts. JAMA Ophthalmol. 2013, 131, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
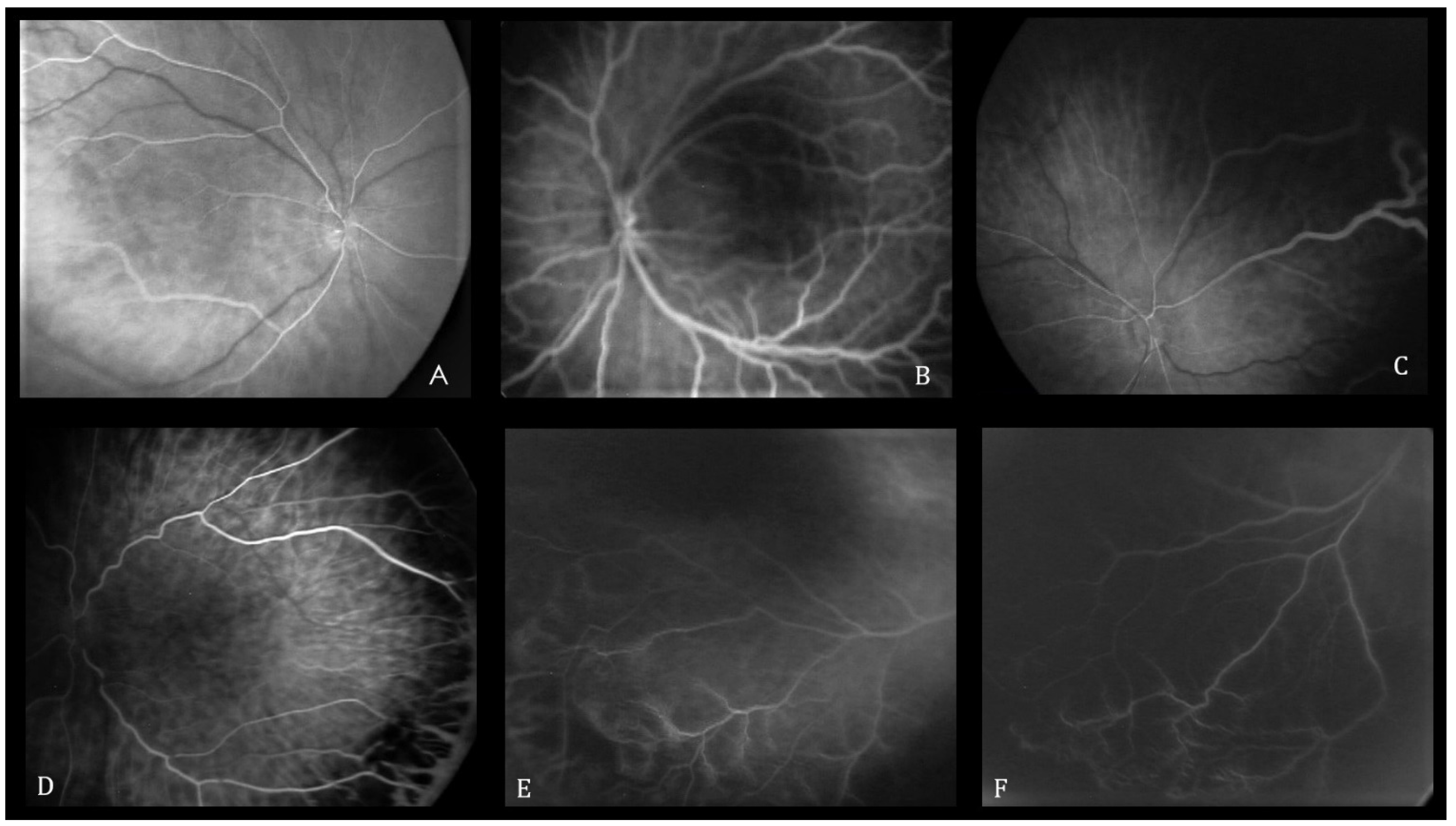
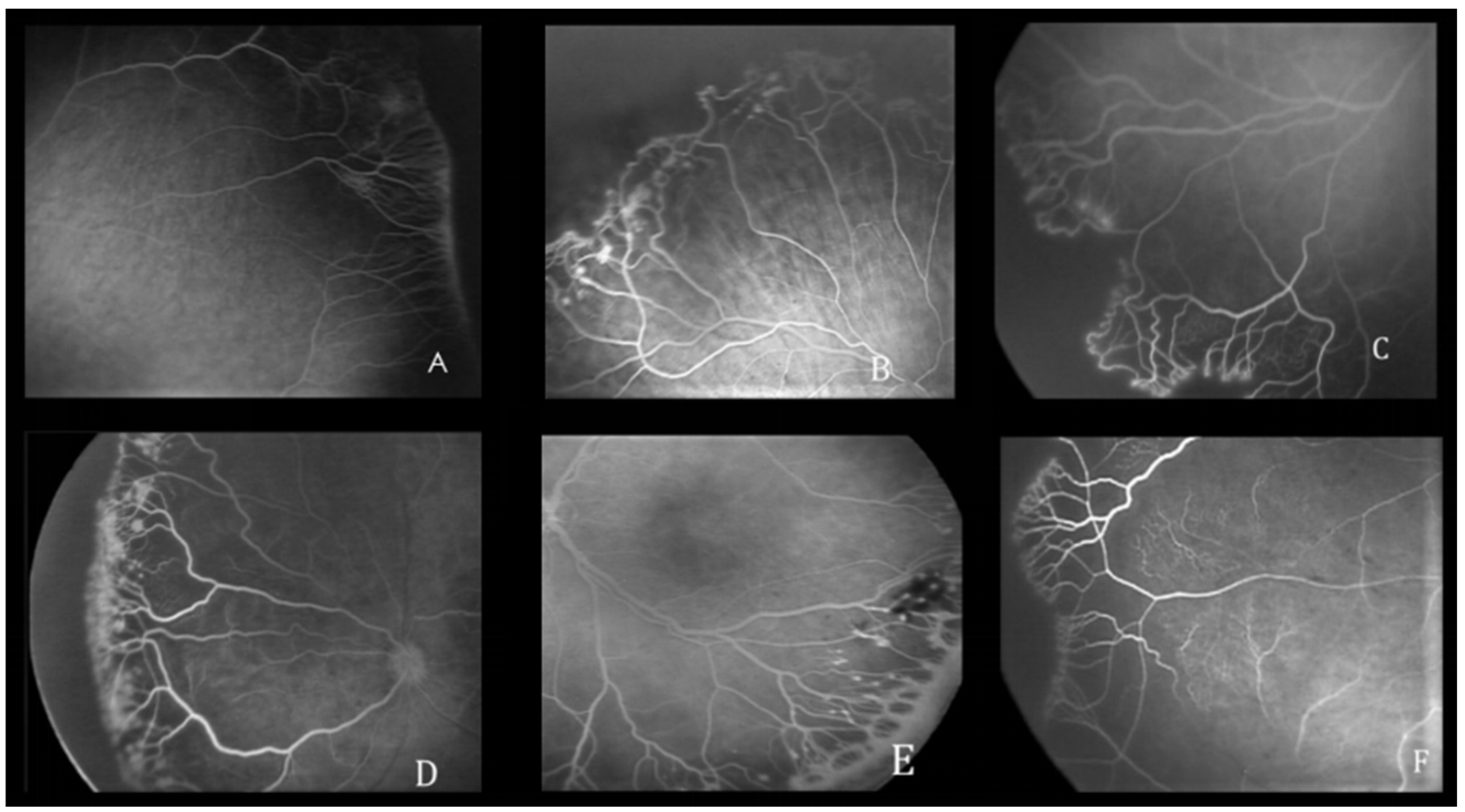
| Characteristics | |
|---|---|
| Birthweight (g) | |
| Mean (SD) | 952 (343.9) |
| Median | 894 |
| Min, max | 500, 2820 |
| Gestational age (weeks) | |
| Mean (SD) | 27.1 (2.3) |
| Median | 27 |
| Min, max | 23, 32 |
| Treatment status | |
| Treated | 47 (58.7%) |
| Not treated | 33 (41.2%) |
| FA Characteristics | N Eyes | Treatment (%) | OR (95% CI) | p Value * |
|---|---|---|---|---|
| Foveal avascular zone | 1 | |||
| Absent | 23 | 14 (60.9%) | 1.05 (0.39, 2.83) | |
| Present | 43 | 24 (55.8%) | 0.68 (0.28, 1.69) | |
| Ungradable | 14 | 9 (64.3%) | 1.84 (0.52, 6.48) | |
| Choroidal vascular pattern | 0.48 | |||
| Linear | 39 | 20 (51.3%) | 0.86 (0.37, 1.99) | |
| Lobular | 34 | 21 (61.8%) | 1.24 (0.5, 3.07) | |
| Ungradable | 7 | 6 (85.7%) | 4.68 (0.54, 40.89) | |
| Hypofluorescent areas WITH Hyperfluorescence | 0.07 | |||
| None | 41 | 20 (48.8%) | 0.42 (0.17, 1.06) | |
| Mild | 26 | 18 (69.2%) | 1.94 (0.72, 5.22) | |
| Moderate | 10 | 7 (70%) | 1.75 (0.42, 7.33) | |
| Severe | 3 | 2 (66.7%) | 1.42 (0.12, 16.36) | |
| Hypofluorescent areas WITHOUT Hyperfluorescence | 1 | |||
| None/mild | 39 | 21 (53.8%) | 0.61 (0.25, 1.48) | |
| Moderate | 35 | 22 (62.8%) | 1.48 (0.6, 3.65) | |
| Severe | 6 | 4 (66.7%) | 1.45 (0.25, 8.43) | |
| Finger-like anastomosis | 0.56 | |||
| None | 3 | 1 (33.3%) | 0.34 (0.03, 3.88) | |
| Mild | 24 | 11 (45.8%) | 0.47 (0.18, 1.24) | |
| Moderate | 39 | 25 (64.1%) | 1.54 (0.63, 3.78) | |
| Severe | 14 | 10 (71.4%) | 1.96 (0.56, 6.89) | |
| Shunts | 0.009 | |||
| None/mild | 52 | 26 (50%) | 0.33 (0.12, 0.92) | |
| Moderate | 21 | 16 (76.2%) | 2.89 (0.94, 8.92) | |
| Severe | 7 | 5 (71.4%) | 1.85 (0.34, 10.14) | |
| Tangles | 0.015 | |||
| None | 19 | 7 (36.8%) | 0.24 (0.08, 0.73) | |
| Mild | 28 | 16 (57.1%) | 0.98 (0.39, 2.48) | |
| Moderate | 27 | 19 (70.4%) | 2.29 (0.85, 6.13) | |
| Severe | 6 | 5 (83.3%) | 1.85 (0.2, 16.84) | |
| Hyperfluorescent lesions | 0.38 | |||
| None | 66 | 37 (56.1%) | 0.51 (0.15, 1.79) | |
| Mild | 4 | 4 (100%) | NA | |
| Moderate/severe | 10 | 6 (60%) | 1.06 (0.27, 4.1) | |
| Leakage | 0.008 | |||
| None | 29 | 11(37.9%) | 0.25 (0.1, 0.67) | |
| Mild | 32 | 22 (68.7%) | 2.02 (0.79, 5.17) | |
| Moderate/severe | 19 | 14 (73.7%) | 2.38 (0.76, 7.42) | |
| Capillary obliteration | 0.48 | |||
| None | 27 | 15 (55.6%) | 0.71 (0.28, 1.8) | |
| Mild | 30 | 22 (73.3%) | 2.98 (1.12, 7.95) | |
| Moderate/severe | 23 | 10 (43.5%) | 0.45 (0.17, 1.2) |
| Study | Number of Patients | Findings Predictive of Treatment-Requiring ROP |
|---|---|---|
| Our study | 40 (80 eyes) | Leakage, shunts, and tangles. |
| Hans et al. [5] | 50 (99 eyes) | Delayed retinal arterial perfusion and popcorn lesions. |
| Lepore et al. [10] | 56 (98 eyes) | Leakage, shunts, and hyperfluorescent lesions at the junction between the vascular and avascular zone. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dini, G.; Beccasio, A.; Della Lena, F.; Verrotti, A.; Cagini, C. Predictive Role of Fluorescein Angiography in Retinopathy of Prematurity. Pediatr. Rep. 2024, 16, 594-601. https://doi.org/10.3390/pediatric16030050
Dini G, Beccasio A, Della Lena F, Verrotti A, Cagini C. Predictive Role of Fluorescein Angiography in Retinopathy of Prematurity. Pediatric Reports. 2024; 16(3):594-601. https://doi.org/10.3390/pediatric16030050
Chicago/Turabian StyleDini, Gianluca, Alfredo Beccasio, Francesco Della Lena, Alberto Verrotti, and Carlo Cagini. 2024. "Predictive Role of Fluorescein Angiography in Retinopathy of Prematurity" Pediatric Reports 16, no. 3: 594-601. https://doi.org/10.3390/pediatric16030050
APA StyleDini, G., Beccasio, A., Della Lena, F., Verrotti, A., & Cagini, C. (2024). Predictive Role of Fluorescein Angiography in Retinopathy of Prematurity. Pediatric Reports, 16(3), 594-601. https://doi.org/10.3390/pediatric16030050







