Characteristics of Root Cells during In Vitro Rhizogenesis under Action of NaCl in Two Tomato Genotypes Differing in Salt Tolerance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Obtaining of Tomato Seedlings
2.2. Light and Transmission Electron Microscopy (TEM)
2.3. Immunofluorescent Staining with Antibodies to Tubulin
2.4. Cytophotometric Analysis
2.5. Statistical Analysis
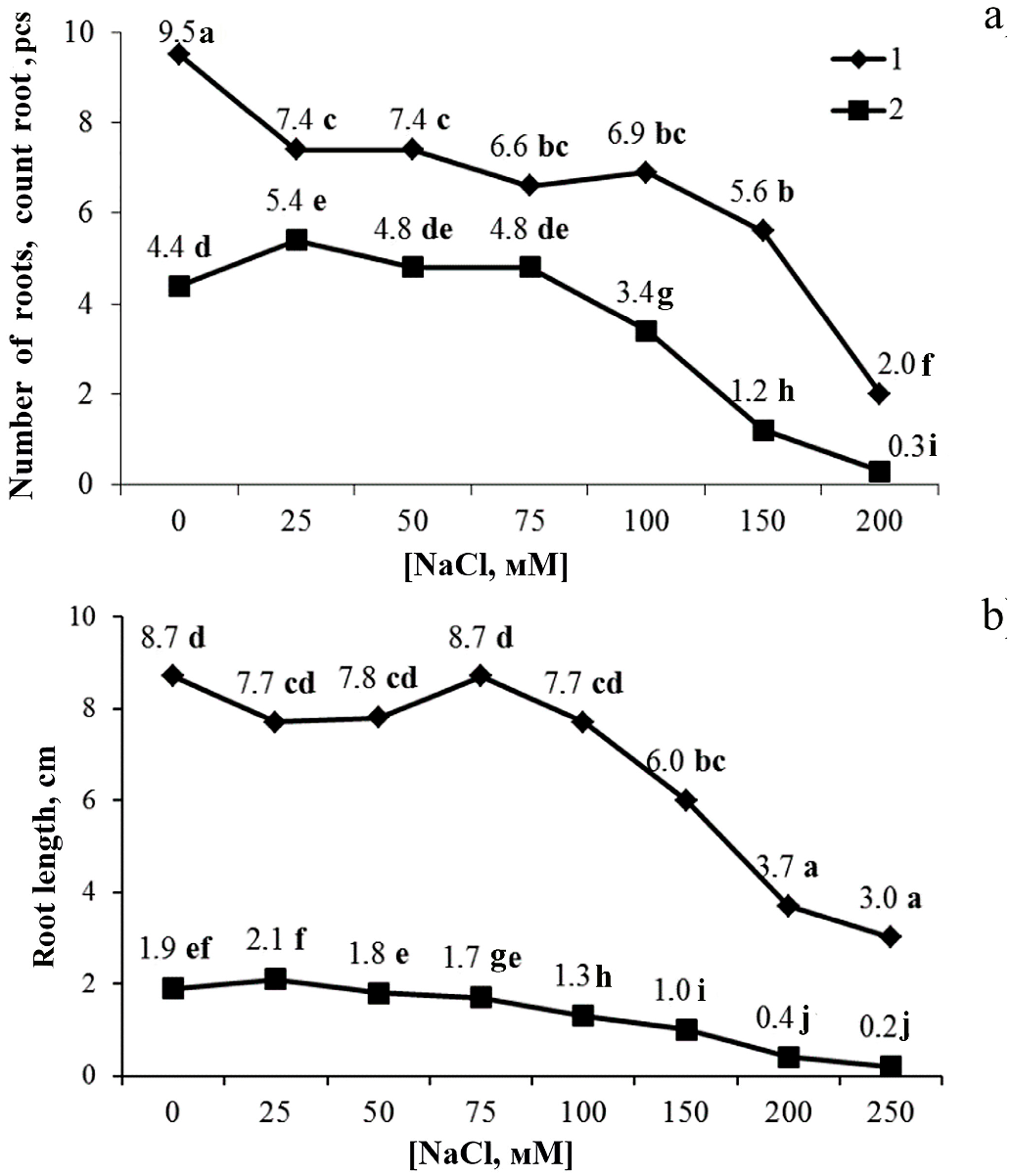
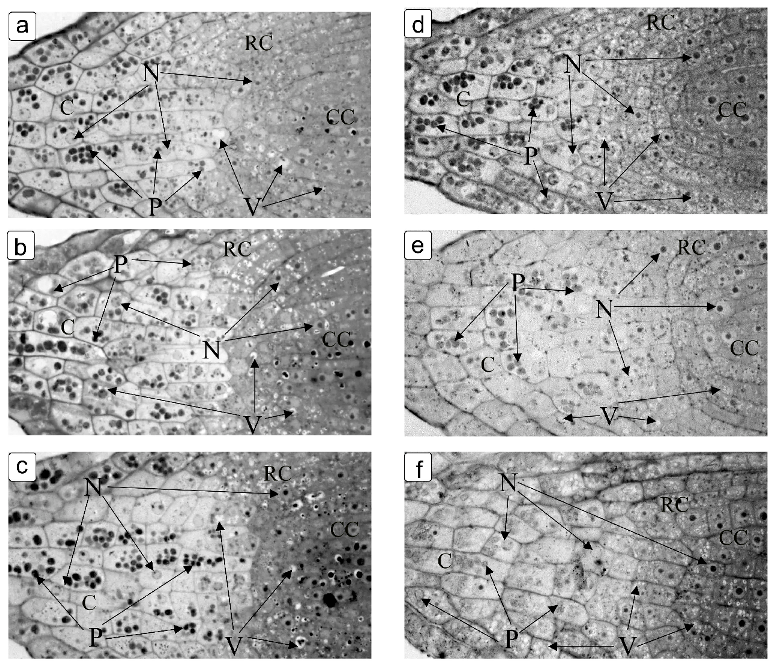
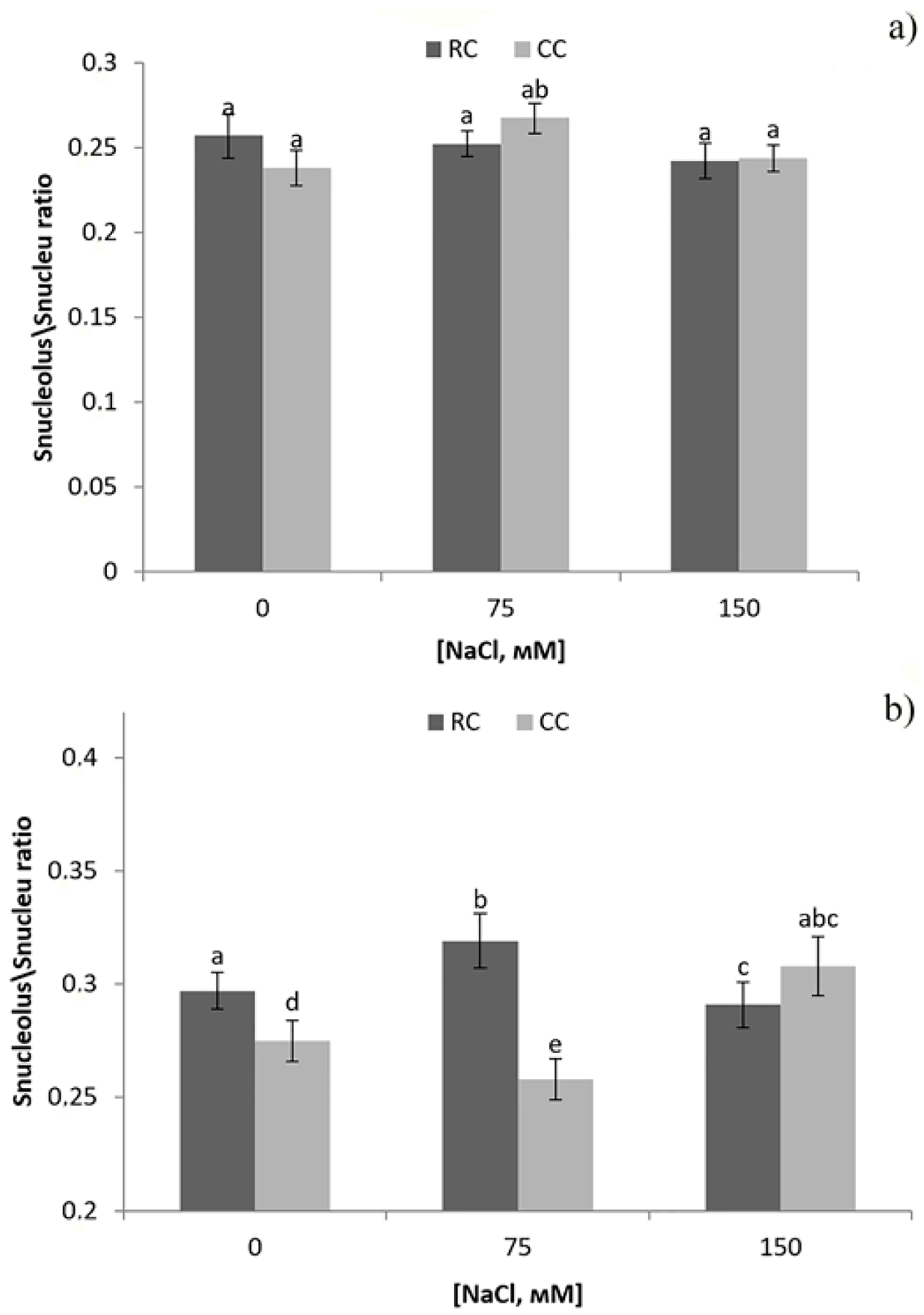
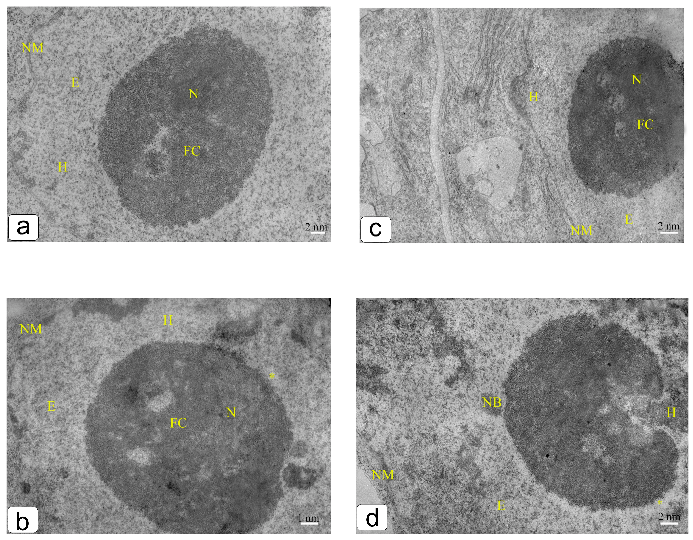
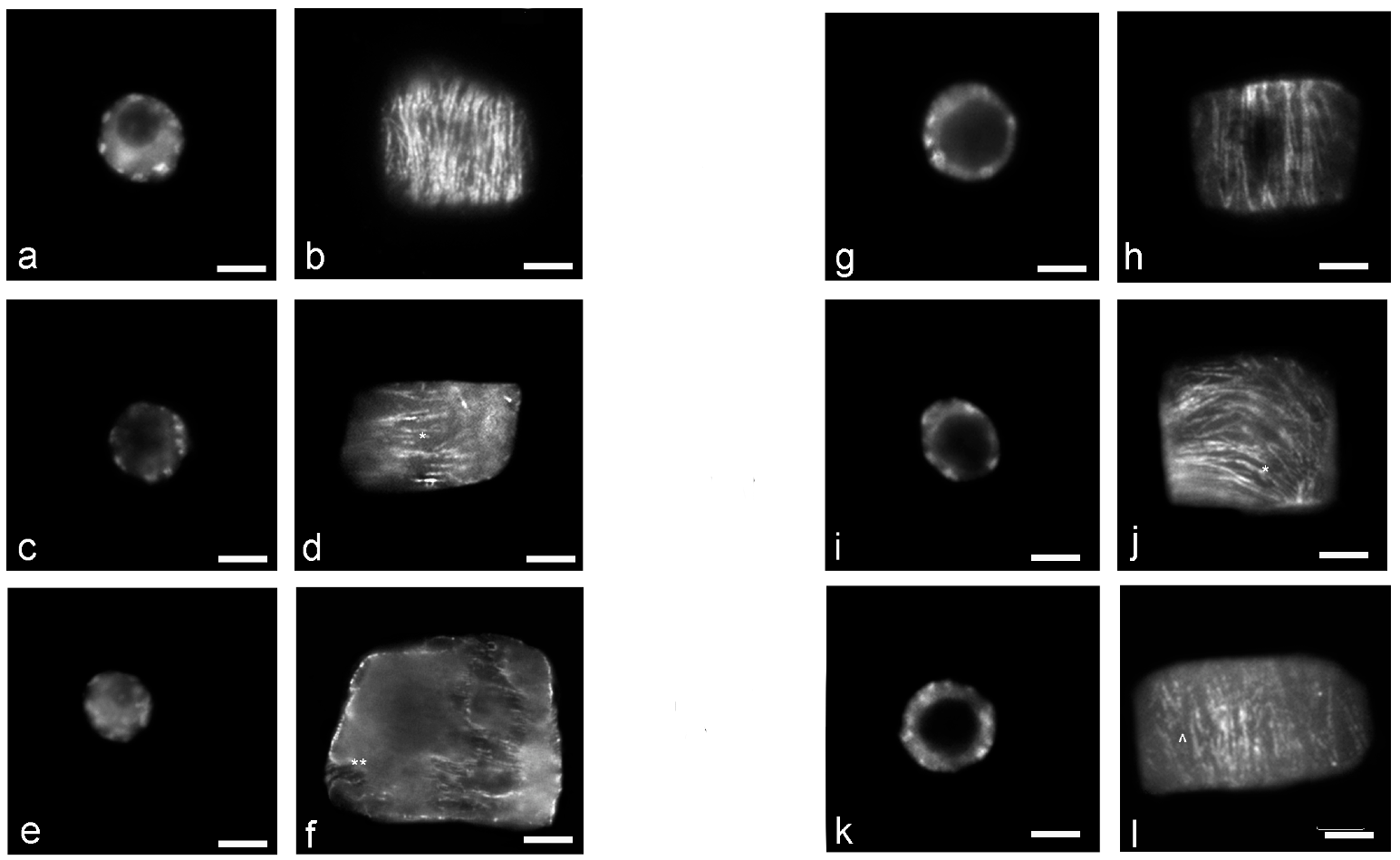
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Flowers, T.J. Improving crop salt tolerance. J. Exp. Bot. 2004, 55, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Safdar, H.; Amin, A.; Shafiq, Y.; Ali, A.; Yasin, R.; Shoukat, A.; Sarwar, M.I. A review: Impact of salinity on plant growth. Nat. Sci. 2019, 17, 34. [Google Scholar]
- Baetz, U.; Eisenach, C.; Tohge, T.; Martinoia, E.; De Angeli, A. Vacuolar chloride fluxes impact ion content and distribution during early salinity stress. Plant Physiol. 2016, 172, 1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, F.; Yasmeen, T.; Arif, M.S.; Ali, S.; Ali, B.; Hameed, S.; Zhou, W. Plant growth promoting bacteria confer salt tolerance in Vigna radiata by up-regulating antioxidant defense and biological soil fertility. Plant Growth Regul. 2016, 80, 23. [Google Scholar] [CrossRef]
- Baranova, E.N.; Gulevich, A.A. Asymmetry of plant cell divisions under salt stress. Symmetry 2021, 13, 1811. [Google Scholar] [CrossRef]
- Civelek, C.; Yildirim, E. Effects of exogenous glycine betaine treatments on growth and some physiological characteristics of tomato under salt stress condition. Atatürk Üniv. Ziraat Fakült. Dergisi. 2019, 50, 153. [Google Scholar]
- Ghorbani, A.; Omran, V.O.G.; Razavi, S.M.; Pirdashti, H.; Ranjbar, M. Piriformospora indica confers salinity tolerance on tomato (Lycopersicon esculentum Mill.) through amelioration of nutrient accumulation, K+/Na+ homeostasis and water status. Plant Cell Rep. 2019, 38, 1151. [Google Scholar] [CrossRef]
- Malcolm, C.V.; Lindley, V.A.; O’Leary, J.W.; Runciman, H.V.; Barrett-Lennard, E.G. Halophyte and glycophyte salt tolerance at germination and the establishment of halophyte shrubs in saline environments. Plant Soil 2003, 253, 171–185. [Google Scholar] [CrossRef]
- Zhu, G.Y.; Kinet, J.M.; Lutts, S. Characterization of rice (Oryza sativa L.) F3 populations selected for salt resistance. I. Physiological behavior during vegetative growth. Euphytica 2001, 121, 251–263. [Google Scholar] [CrossRef]
- Qadir, M.; Quillerou, E.; Nangia, V.; Murtaza, G.; Singh, M.; Thomas, R.J.; Drechsel, P.; Noble, A.D. Economics of salt-induced land degradation and restoration. Nat. Res. Forum 2014, 38, 282–295. [Google Scholar] [CrossRef]
- Wafa’a, A. Comparative effects of drought and salt stress on germination and seedling growth of Pennisetum divisum (Gmel.) Henr. Amer. J. Appl. Sci. 2010, 7, 640–646. [Google Scholar]
- Saeed, A.; Shahid, M.Q.; Anjum, S.A.; Khan, A.A.; Shakeel, A.; Saleem, M.F.; Saeed, N. Genetic analysis of NaCl tolerance in tomato. Genet. Mol. Res. 2011, 10, 1754–1776. [Google Scholar] [CrossRef]
- Zaki, H.E.; Yokoi, S. A comparative in vitro study of salt tolerance in cultivated tomato and related wild species. Plant Biotechnol. 2016, 33, 361–372. [Google Scholar] [CrossRef]
- Sultana, N.; Sultana, I.; Sultana, S. Effect of salinity on in vitro performance of tomato (Lycopersicon esculentum L.). Bangladesh J. 2019, 36, 43–46. [Google Scholar]
- Cano, E.A.; Pérez-Alfocea, F.; Moreno, V.; Bolarin, M.C. Responses to NaCl stress of cultivated and wild tomato species and their hybrids in callus cultures. Plant Cell Rep. 1996, 15, 791–794. [Google Scholar] [CrossRef]
- Tahir, M.; Zafar, M.M.; Imran, A.; Hafeez, M.A.; Rasheed, M.S.; Mustafa, H.S.B.; Ullah, A. Response of tomato genotypes against salinity stress at germination and seedling stage. Nat. Sci. 2018, 16, 10–17. [Google Scholar]
- Bogoutdinova, L.R.; Baranova, G.B.; Baranova, E.N.; Khaliluev, M.R. Comparative anatomical and morphological studies of the epidermal and cortical parenchyma hypocotyl cells of two tomato genotypes (Solanum lycopersicum L.) under sodium chloride stress in vitro. Sel’skokhozyaistvennaya Biol. 2016, 51, 318–326. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Khaliluev, M.R.; Bogoutdinova, L.R.; Raldugina, G.N.; Baranova, E.N. A simple and effective bioassay method suitable to comparative in vitro study of tomato salt tolerance at early development stages. Methods Protoc. 2022, 5, 11. [Google Scholar] [CrossRef]
- Baranova, E.N.; Gulevich, A.A.; Kalinina-Turner, E.B.; Koslov, N.N. Effects of NaCl, Na2SO4 and mannitol on utilization of storage protein and transformation of vacuoles in the cotyledons and seedling roots of alfalfa (Medicago sativa L.). Russ. Agric. Sci. 2011, 37, 11–19. [Google Scholar] [CrossRef]
- Reynolds, E.S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 1963, 17, 208–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogoutdinova, L.R.; Baranova, E.N.; Baranova, G.B.; Kononenko, N.V.; Lazareva, E.M.; Smirnova, E.A.; Khaliluev, M.R. Morpho-biological and cytological characterization of tomato roots (Solanum lycopersicum L.; cv. Rekordsmen) regenerated under NaCl salinity in vitro. Cell Tissue Biol. 2020, 14, 228–242. [Google Scholar] [CrossRef]
- Sharma, S.; Dietz, K.; Mimura, T. Vacuolar compartmentalization as an indispensable component of heavy metal detoxification in plants. Plant Cell Environ. 2016, 39, 1112–1126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinoia, E.; Maeshima, M.; Neuhaus, H.E. Vacuolar transporters and their essential role in plant metabolism. J. Exp. Bot. 2007, 83, 83–102. [Google Scholar] [CrossRef] [PubMed]
- Baranova, E.N.; Gulevich, A.A.; Polyakov, V.Y. Effects of NaCl, Na2SO4, and mannitol on storage lipid mobilization in the cotyledons and roots of purple alfalfa seedlings. Russ. J. Plant Physiol. 2006, 53, 779–784. [Google Scholar] [CrossRef]
- Baranova, E.N.; Gulevich, A.A.; Polyakov, V.Y. Effect of NaCl, Na2SO4, and mannitol on utilization of storage starch and formation of plastids in the cotyledons and roots of alfalfa seedlings. Russ. J. Plant Physiol. 2007, 54, 50–57. [Google Scholar] [CrossRef]
- Ratnakar, A.; Rai, A. Effect of sodium chloride salinity on seed germination and early seedling growth of Trigonella foenum-graecum L. Var. Peb. Octa J. Environ. Res. 2013, 1, 4. [Google Scholar]
- Whitmore, A.P.; Whalley, W.R. Physical effects of soil drying on roots and crop growth. J. Exp. Bot. 2009, 60, 2845–2857. [Google Scholar] [CrossRef] [Green Version]
- Javot, H.; Maurel, C. The role of aquaporins in root water uptake. Ann. Bot. 2002, 90, 301–313. [Google Scholar] [CrossRef] [Green Version]
- Flexas, J.; Diaz-Espejo, A.; Galmes, J.; Kaldenhoff, R.; Medrano, H.; Ribas-Carbo, M. Rapid variations of mesophyll conductance in response to changes in CO2 concentration around leaves. Plant Cell Environ. 2007, 30, 1284–1298. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Cornic, G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ. 2002, 25, 275–294. [Google Scholar] [CrossRef] [Green Version]
- Alsafari, S.A.; Galal, H.K.; Bafeel, S.O. Growth and anatomy of tomato (Solanum lycopersicum Mill.) cultivars Marmande and Oria under salinity stress. Pak. J. Bot. 2019, 51, 1199–1207. [Google Scholar] [CrossRef]
- De Veylder, L.; Larkin, J.C.; Schnittger, A. Molecular control and function of endoreplication in development and physiology. Trends Plant Sci. 2011, 16, 624–634. [Google Scholar] [CrossRef]
- Bourdon, M.; Pirrello, J.; Cheniclet, C.; Coriton, O.; Bourge, M.; Brown, S.; Frangne, N. Evidence for karyoplasmic homeostasis during endoreduplication and a ploidy-dependent increase in gene transcription during tomato fruit growth. Development 2012, 139, 3817–3826. [Google Scholar] [CrossRef] [Green Version]
- Katagiri, Y.; Hasegawa, J.; Fujikura, U.; Hoshino, R.; Matsunaga, S.; Tsukaya, H. The coordination of ploidy and cell size differs between cell layers in leaves. Development 2016, 143, 1120–1125. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Phillips, T.E.; Rogers, S.W.; Rogers, J.C. Biogenesis of the protein storage vacuole crystalloid. J. Cell Biol. 2000, 150, 755–770. [Google Scholar] [CrossRef]
- Muntz, K.; Belozersky, M.A.; Dunaevsky, Y.E.; Schlereth, A.; Tiedemann, J. Stored proteinases and the Initiation of storage protein mobilization in seeds during germination and seedling growth. J. Exp. Bot. 2001, 52, 1741–1752. [Google Scholar] [CrossRef] [Green Version]
- Di Sansebastiano, G.P.; Paris, N.; MarcMartin, S.; Neuhaus, J.M. Regeneration of a lytic central vacuole and of neutral peripheral vacuoles can be visualized by green fluorescent proteins targeted to either type of vacuoles. Plant Physiol. 2001, 126, 78–86. [Google Scholar] [CrossRef] [Green Version]
- Mimura, T.; Kura-Hotta, M.; Tsujimura, T.; Ohnishi, M.; Miura, M.; Okazaki, Y.; Mimura, M.; Maeshima, M.; Washitani-Nemoto, S. Rapid increase of vacuolar volume in response to salt stress. Planta 2003, 216, 397–402. [Google Scholar] [CrossRef]
- Hamaji, K.; Nagira, M.; Yoshida, K.; Ohnishi, M.; Oda, Y.; Uemura, T. Dynamic aspects of ion accumulation by vesicle traffic under salt stress in Arabidopsis. Plant Cell Physiol. 2009, 50, 2023–2033. [Google Scholar] [CrossRef] [Green Version]
- Dermendjiev, G.; Schnurer, M.; Weiszmann, J.; Wilfinger, S.; Ott, E.; Gebert, C. Tissue-Specific Proteome and Subcellular Microscopic Analyses Reveal the Effect of High Salt Concentration on Actin Cytoskeleton and Vacuolization in Aleurone Cells during Early Germination of Barley. Int. J. Mol. Sci. 2021, 22, 9642. [Google Scholar] [CrossRef] [PubMed]
- El-Banna, Y.; Attia, T. Root tip meristematic cell and leaf chloroplast structure in three barley (Hordeum vulgare L.) genotypes exposed to salinity stress. Cytologia 1999, 64, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, W.; Qiu, C.; Zhang, C.; Cao, F.; Shuiji, Z.; Wu, F. Comparative physiological analysis in the tolerance to salinity and drought individual and combination in two cotton genotypes with contrasting salt tolerance. Physiol. Plant. 2019, 165, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotox. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.; Yasmeen, T.; Ali, S.; Ali, B.; Farooq, M.A.; Gill, R.A. Priming-induced antioxidative responses in two wheat cultivars under saline stress. Acta Physiol. Plant. 2015, 37, 153. [Google Scholar] [CrossRef]
- Li, J.; Liu, L.; Bai, Y.; Zhang, P.; Finkers, R.; Du, Y.; Visser, R.G.F.; van Heusden, A.W. Seedling salt tolerance in tomato. Euphytica 2011, 178, 403–414. [Google Scholar] [CrossRef]
- Vriet, C.; Hennig, L.; Laloi, C. Stress-induced chromatin changes in plants: Of memories, metabolites and crop improvement. Cell Mol. Life Sci. 2015, 72, 1261–1273. [Google Scholar] [CrossRef]
- Lämke, J.; Bäurle, I. Epigenetic and chromatin-based mechanisms in environmental stress adaptation and stress memory in plants. Genome Biol. 2017, 18, 124. [Google Scholar] [CrossRef]
- Kim, J.H. Multifaceted chromatin structure and transcription changes in plant stress response. Int. J. Mol. Sci. 2021, 22, 2013. [Google Scholar] [CrossRef]
- Baranova, E.N.; Chaban, I.A.; Kononenko, N.V.; Khaliluev, M.R.; Christov, N.K.; Gulevich, A.A.; Todorovska, E.G. Ultrastructural organization of the domains in the cell nucleus of dicotyledonous and monocotyledonous plants under abiotic stress. Russ. Agric. Sci. 2017, 43, 199–206. [Google Scholar] [CrossRef]
- Jabeen, Z.; Hussain, N.; Han, Y.; Shah, M.J.; Zeng, F.; Zeng, J.; Zhang, G. The differences in physiological responses, ultrastructure changes, and Na+ subcellular distribution under salt stress among the barley genotypes differing in salt tolerance. Acta Physiol. Plant. 2014, 36, 2397. [Google Scholar] [CrossRef]
- Zeeshan, M.; Lu, M.; Sehar, S.; Holford, P.; Wu, F. Comparison of biochemical, anatomical, morphological, and physiological responses to salinity stress in wheat and barley genotypes deferring in salinity tolerance. Agronomy 2020, 10, 127. [Google Scholar] [CrossRef] [Green Version]
- Goswami, R.; Asnacios, A.; Milani, P.; Graindorge, S.; Houlné, G.; Mutterer, J. Mechanical shielding in plant nuclei. Curr. Biol. 2020, 30, 2013–2025. [Google Scholar] [CrossRef]
- Irianto, J.; Swift, J.; Martins, R.; McPhail, G.; Knight, M.; Discher, D.; Lee, D. Osmotic drives challenge rapid and reversible chromatin condensation in chondrocytes. Biophys. J. 2013, 104, 759–769. [Google Scholar] [CrossRef] [Green Version]
- Lovett, D.; Shekhar, N.; Nickerson, J.; Roux, K.; Lele, T. Modulation of nuclear shape by substrate rigidity. Cell Mol. Bioeng. 2013, 6, 230–238. [Google Scholar] [CrossRef] [Green Version]
- Finan, J.; Guilak, F. The effects of osmotic stress on the structure and function of the cell nucleus. J. Cell Biochem. 2010, 109, 460–467. [Google Scholar] [CrossRef]
- Čiamporová, M.; Mistrík, I. The ultrastructural response of root cells to stressful conditions. Environ. Exp. Bot. 1993, 33, 11–26. [Google Scholar] [CrossRef]
- Probst, A.V.; Scheid, O.M. Stress-induced structural changes in plant chromatin. Curr. Opin. Plant Biol. 2015, 27, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.; Soni, P. Unraveling the epigenetic landscape for salt tolerance in plants. Int. J. Plant Biol. 2022, 13, 443–462. [Google Scholar] [CrossRef]
- Onelli, E.; Scali, M.; Caccianiga, M.; Stroppa, N.; Morandini, P.; Pavesi, G.; Moscatelli, A. Microtubules play a role in trafficking prevacuolar compartments to vacuoles in tobacco pollen tubes. R. Soc. Open Biol. 2018, 8, 180078. [Google Scholar] [CrossRef] [Green Version]
- Bogoutdinova, L.R.; Lazareva, E.M.; Chaban, I.A.; Kononenko, N.V.; Dilovarova, T.A.; Khaliluev, M.R.; Kurenina, L.V.; Gulevich, A.A.; Smirnova, E.A.; Baranova, E.N. Salt stress-induced structural changes are mitigated in transgenic tomato plants over-expressing superoxide dismutase. Biology 2020, 9, 297. [Google Scholar] [CrossRef] [PubMed]
- Giordano, M.; Petropoulos, S.A.; Rouphael, Y. Response and defence mechanisms of vegetable crops against drought, heat and salinity stress. Agriculture 2021, 11, 463. [Google Scholar] [CrossRef]
- Baranova, E.N.; Christov, N.K.; Kurenina, L.V.; Khaliluev, M.R.; Todorovska, E.G.; Smirnova, E.A. Formation of atypical tubulin structures in plant cells as a nonspecific response to abiotic stress. Bulg. J. Agric. Sci. 2016, 22, 987–992. [Google Scholar]
- Chun, H.J.; Baek, D.; Jin, B.J.; Cho, H.M.; Park, M.S.; Lee, S.H.; Lim, L.H.; Cha, Y.J.; Bae, D.-W.; Kim, S.T.; et al. Microtubule dynamics plays a vital role in plant adaptation and tolerance to salt stress. Int. J. Mol. Sci. 2021, 22, 5957. [Google Scholar] [CrossRef]
- Yang, P.; Jin, J.; Zhang, J.; Wang, D.; Bai, X.; Xie, W.; Hu, T.; Zhao, X.; Mao, T.; Qin, T. MDP25 mediates the fine—Tuning of microtubule organization in response to salt stress. J. Integr. Plant Biol. 2022, 64, 1181–1195. [Google Scholar] [CrossRef]



| YaLF | Recordsmen | |||
|---|---|---|---|---|
| Cell Length Relative to Control, % | Cell Length Relative to Control, % | |||
| NaCl Concentration in the Medium (mM) | Columella | Epidermis | Columella | Epidermis |
| 0 | 100 | 100 | 100 | 100 |
| 75 | 98.61 | 95.87 | 118.9 | 81.97 |
| 150 | 130.55 | 102.48 | 155.11 | 74.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogoutdinova, L.R.; Baranova, E.N.; Kononenko, N.V.; Chaban, I.A.; Konovalova, L.N.; Gulevich, A.A.; Lazareva, E.M.; Khaliluev, M.R. Characteristics of Root Cells during In Vitro Rhizogenesis under Action of NaCl in Two Tomato Genotypes Differing in Salt Tolerance. Int. J. Plant Biol. 2023, 14, 104-119. https://doi.org/10.3390/ijpb14010010
Bogoutdinova LR, Baranova EN, Kononenko NV, Chaban IA, Konovalova LN, Gulevich AA, Lazareva EM, Khaliluev MR. Characteristics of Root Cells during In Vitro Rhizogenesis under Action of NaCl in Two Tomato Genotypes Differing in Salt Tolerance. International Journal of Plant Biology. 2023; 14(1):104-119. https://doi.org/10.3390/ijpb14010010
Chicago/Turabian StyleBogoutdinova, Liliya R., Ekaterina N. Baranova, Neonila V. Kononenko, Inna A. Chaban, Ludmila N. Konovalova, Alexander A. Gulevich, Elena M. Lazareva, and Marat R. Khaliluev. 2023. "Characteristics of Root Cells during In Vitro Rhizogenesis under Action of NaCl in Two Tomato Genotypes Differing in Salt Tolerance" International Journal of Plant Biology 14, no. 1: 104-119. https://doi.org/10.3390/ijpb14010010
APA StyleBogoutdinova, L. R., Baranova, E. N., Kononenko, N. V., Chaban, I. A., Konovalova, L. N., Gulevich, A. A., Lazareva, E. M., & Khaliluev, M. R. (2023). Characteristics of Root Cells during In Vitro Rhizogenesis under Action of NaCl in Two Tomato Genotypes Differing in Salt Tolerance. International Journal of Plant Biology, 14(1), 104-119. https://doi.org/10.3390/ijpb14010010








