Abstract
Western flower thrips, Frankliniella occidentalis (WFT), is one of the most destructive insect pests of vegetables and ornamental crops globally. Ultraviolet-C (UV-C) exposure has been shown to reduce populations of arthropod pests, including whiteflies and two-spotted spider mites, but has not been fully assessed for WFT. The goal of this study was to determine if UV-C radiance could be a viable strategy for inclusion in integrated pest management (IPM) programs for WFT. The objectives were to (1) assess the relationship among UV-C dose (irradiance × duration) and mortality of WFT adults and second instar larvae, (2) determine the effect of UV-C on WFT fecundity and egg hatch, and (3) assess the effect of the WFT lethal dose of UV-C on three WFT-prone ornamental plants. A UV-C dose is measured in Joules, which equals power (watts) × exposure time. A dose-dependent relationship between UV-C exposure and mortality of WFT larvae and adults was observed. At the doses of 0.98 and 0.68 J/cm2 (5 and 4 min exposure, respectively), 50% of the larvae died within 24 and 48 h, respectively. The UV-C dose needed to achieve 50% mortality was higher for adults than larvae, occurring at 5.2 and 4.4 J/cm2 (35 min and 25 min exposure, respectively) within 72 and 120 h, respectively. The number of eggs laid by surviving WFT subjected to UV-C treatment was less than by those that were untreated, and the egg-laying period was significantly shorter among those treated with UV-C. When leaves containing WFT eggs were exposed to UV-C doses known to cause 30–40% mortality in adults, 86–98% fewer eggs hatched compared to untreated controls. Ornamental plants exposed to UV-C doses lethal to eggs, second instars, and adult WFT either showed no damage, or when damage occurred, plants recovered within 14–30 days. Additional studies under controlled greenhouse conditions are needed to elucidate the effectiveness of UV-C radiance against WFT over time and its compatibility with biological control and other IPM practices.
1. Introduction
Western flower thrips (WFT), Frankliniella occidentalis (Thysanoptera: Thripidae), is one of the most important insect herbivores in the world [1]. It is an economically important pest of many vegetables and ornamental plants, causing damage by direct feeding of plant sap that scars or deforms foliage, flowers, and fruits, reducing their aesthetic value and crop yield, as well as disrupting plant growth and physiology. Equally important, WFT transmits several destructive plant viruses, including tomato spotted wilt virus (TSWV), impatiens necrotic spot virus (INSV), chrysanthemum stem necrosis virus, groundnut ringspot virus, and tomato chlorotic spot virus [2,3]. It was reported in 1998 that TSWV caused over USD 1 billion in losses annually on a global basis [4]. In the United States, losses over a 10 year period were estimated at USD 1.4 billion [5]. This estimate does not include direct cosmetic damage caused by WFT. This combination of damage and virus infection can render plants unsalable or kills them outright and threatens growers with severe economic loss. WFT are extremely difficult to manage. They are tiny and crawl deep into plant crevices where chemical sprays do not penetrate. In many greenhouses, crops are grown year-round and WFT becomes a resident pest. Their mobility, high reproductive rate, and wide host range contribute to their success, and growers are left with few management options [2,3]. Due to national and international quarantine restrictions, plants and cut flowers shipped between states and countries must be free of this pest. In the past, growers have relied heavily on agricultural chemicals for WFT management, but due to resistance, many are no longer effective. Additionally, customers prefer crops that are grown with few or no chemical insecticides. IPM tactics for WFT are needed because current chemical, cultural, and biological control options are not sufficient, and thus novel, alternative management approaches must be developed.
Ultraviolet energy has been used widely in an array of settings for the disinfection of microbial contaminants by preventing their growth and replication. The potential of UV for pest management has not been studied extensively because of the lack of knowledge about how to effectively apply it to insect pests. UV irradiation is divided into three classes: UV-A, UV-B, and UV-C. The sunlight that reaches the Earth’s surface has no UV-C, only UV-A and UV-B. There are limited reports on the lethal effects of UV-A or UV-B (315–400 nm) on insects. However, it has been documented that UV-A irradiation slightly reduces adult longevity in certain insect species [6,7]. UV-C radiation is highly energetic (λ ≤ 280 nm) and is strongly absorbed by oxygen and ozone in the stratosphere, being absent in terrestrial sunlight. It penetrates the outer membrane of organisms and causes chemical damage, mutations, and body lesions. UV radiation induces various biological effects, such as changes in protein and DNA structures, as well as alterations in other biologically significant molecules. It can cause chronic depression in essential physiological processes and acute physiological stress, resulting in reduced growth, cell division, pigment bleaching, impaired N2 fixation, decreased energy production, or the inhibition of photosynthesis in numerous organisms [8,9,10].
In insects, UV radiation interferes with their orientation, navigation, feeding, and interactions between the sexes [11,12]. The smaller the insect, the more susceptible they appear to be. Plant diseases, such as powdery mildew, can be controlled by a variety of spectral wavelengths, including UV-C. However, limited information is available on the detrimental effects of UV on insect pests [13,14]. The effect of UV-C has been studied on the growth, development, and reproduction of house dust mites (Dermatophagoides farina), stored product beetles (Tribolium castaneum), and mite pests (Tyrophagus putrescentiae) [15,16,17]. Research demonstrated that UV-C exposure significantly diminished egg hatching and adult emergence, leading to a prolonged development time in grain beetles [17]. Studies on the impact of UV irradiance on two-spotted spider mite, Tetranychus urticae, revealed that exposure to both UV-C and UV-B significantly increased mortality and inhibited oviposition in non-diapausing females [18,19,20,21]. Similarly, nightly UV-C treatments have been shown to significantly reduce populations of greenhouse whiteflies, Trialeurodes vaporariorum, offering a possible non-chemical means of management on tomatoes [22].
It is important that the application of the UV-C energy designed to kill the pest does not kill or damage the host plant. Therefore, it is necessary to deliver the lethal dose for the pest precisely in terms of spectrum, intensity, duration, and timing while simultaneously maintaining plant host health. Early research on the biological activity of UV light suggested detrimental effects on plants. UV exposure can influence the quality of plant tissue for herbivores, through either change in leaf chemistry or reinforcement of the plant cell wall [23,24,25,26]. For example, solar UV-B reduced damage by the thrips Caliothrips phaseoli on soybean (Glycine max) leaves in field and laboratory choice experiments [27]. Thrips not only preferred leaves from plants that were not exposed to UV-B over those that were in choice experiments but they also appeared to directly sense and avoid exposure to UV-B. The anti-herbivory effect induced by UV-B is most likely due to the accumulation of phenylpropanoid derivatives in plants similar to their response to insect herbivory [28]. Biochemical changes such as variation in the level of flavonoids, leaf nitrogen, hemicellulose, and lignin, induced by UV exposure, are also likely to affect plant-insect interaction. It is reported that the activation of phytohormones such as jasmonic acid (JA) increased plant resistance against piercing-sucking arthropods such as spider mites and thrips [29,30].
The primary objective of this study was to assess the potential of UV-C exposure as a component within a comprehensive integrated pest management (IPM) program. The initial step involved investigating the relationship among UV-C doses (irradiance x duration) and WFT mortality. The study also investigated the impact of UV-C energy on WFT fecundity, egg hatchability, and the effect of lethal UV-C doses on selected ornamental crops. We used UV-C optical radiation at 254 nm because it is known to be very effective for pest suppression and it is readily and inexpensively available from commercially produced germicidal lamps. Once effective doses for controlling WFT have been established in the laboratory, engineering solutions to deliver them in various applications can be developed.
2. Materials and Methods
2.1. UV-C Chamber
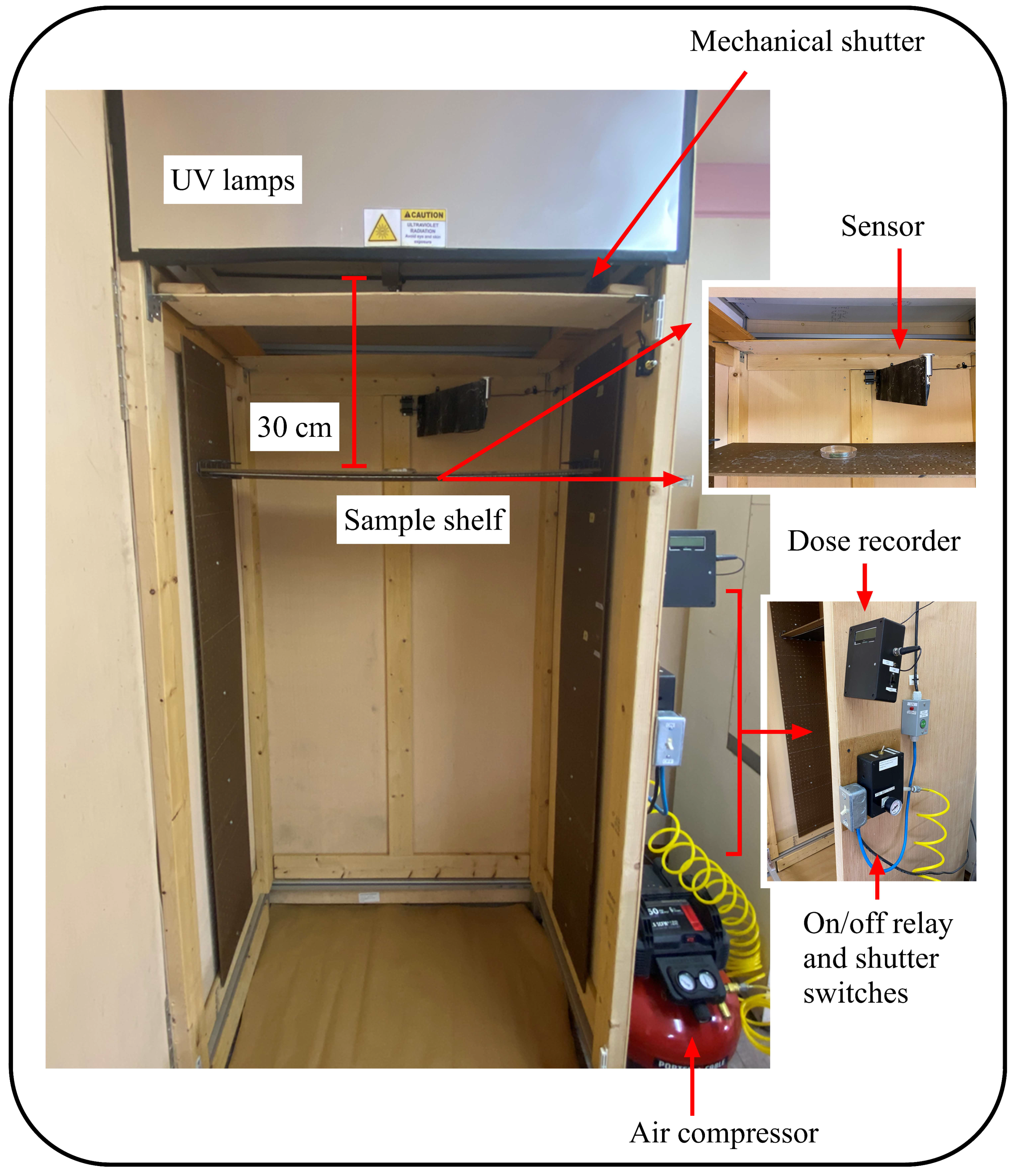
A specially designed chamber (2.3 m tall, 0.94 m wide, 0.76 m deep) was used for UV-C exposures of WFT and plant samples (Lighting Research Center, Rensselaer Polytechnic Institute, Troy, NY, USA) (Figure 1). The chamber was equipped with two UV-C lamps (OSRAM Puritec low pressure lamps, HNS-55W-G13, Munich, Germany) and a shelf for samples positioned 30 cm from the light source. Prior to testing, the irradiance level at the center of the shelf had been calibrated with a spectroradiometer (Gigahertz-Optik BTS2048-UV-S, Munich, Germany) and was directly related with the (arbitrary) response of the UV sensor at the top of the chamber. This sensor ensured that the calibrated irradiance level at the center of the shelf was maintained throughout the UV-C exposure. After stabilization of the lamps, test samples were placed at the center of the shelf where the irradiance level delivered to the samples was always 28.33 W/m2. The air compressor was used to move the mechanical shutter automatically in response to the shutter switch. The shutter sensor measured the duration of UV-C exposure, which was varied by the experimenter with a stopwatch, stopping the exposure with the on/off relay. The timer recorded the duration of UV-C exposure at the sensor so the prescribed energy, in Joules (Ws/m2), was recorded. Different test dosages were achieved by changing the length of the exposure time.
2.2. Effect of UV-C on WFT Adults and Second Instars
A laboratory colony of WFT maintained at University of Vermont Entomology Research Laboratory (Burlington, VT, USA, 44°27′22″ N; 73°11′37″ W) for >35 years was used for all trials. The species of several individuals from the colony were confirmed using molecular analysis. WFT were reared on primary leaves of green beans (Phaseolus vulgaris) in water-filled glass flasks held within a clear plastic Ziploc container (24 × 24 × 13 cm). All containers received fresh plant material twice per week. The beans were grown under florescent lights in a separate room to prevent infestation by thrips or other insect pests. In each container, 5 × 9 cm holes were cut into two parallel sides of the container and fitted with thrips-proof mesh for air circulation. The rearing containers were placed inside an incubator (62 × 54 × 81 cm) that provided stable light (7.71-watt LED bulb, 629 lumens), 24:0 (L/D); temperature (22 °C); and RH (≈75%) conditions.
For each experiment, ten even-aged WFT, either adults or second instars depending on the experiment, were transferred from the rearing containers to a Petri dish (9 cm diameter) using a fine paintbrush. WFT were treated in the dishes without lids. A thin layer of Vaseline was added to the top rim of the Petri dishes to prevent WFT from escaping during exposure.
Figure 1.
Test chamber (2.3 m tall, 0.94 m wide, 0.76 m deep) used for UV-C exposures of WFT and plant samples, equipped with two UV-C lamps (OSRAM Puritec low-pressure lamps, HNS-55W-G13).
Figure 1.
Test chamber (2.3 m tall, 0.94 m wide, 0.76 m deep) used for UV-C exposures of WFT and plant samples, equipped with two UV-C lamps (OSRAM Puritec low-pressure lamps, HNS-55W-G13).
The UV-C doses used against second instars were 0.08, 0.17, 0.34, 0.68, and 0.98 J/cm2 (30 s, 60 s, 2 min, 4 min, and 5 min, respectively). For adults, the doses were 1.70, 2.58, 3.50, 4.40, and 5.20 J/cm2 (10, 15, 20, 25, and 30 min, respectively). An untreated control that was not subjected to UV-C treatment was used in each set of experiments. The UV-C doses tested were determined for adults and second instars based on preliminary tests to estimate the effect and variance of the UV-C exposure on each of these life stages. In preliminary trials, we found that doses that killed larvae were not enough to kill adults. This is likely because adults have a thicker exoskeleton and greater mobility. Therefore, we tested different doses for adults and larvae.
Immediately after UV-C exposure, a green bean leaf disc (6 cm diameter) was placed on filter paper moistened with 100 μL of sterile distilled water and added to each Petri dish to provide food and humidity for the insects. Petri dishes were covered with lids, sealed with parafilm to maintain humidity, and incubated at 25 °C and 16:8 (L/D). Each sample was inspected under a microscope with 40× magnification 1, 24, 48, 72, and 120 h post-treatment and the number of dead WFT was recorded. Adults were considered dead if they were immobile on the bottom of the dish. This provided data on both immediate and delayed effects of UV-C on the test insects. For each UV-C dose, three Petri dishes were prepared, including the untreated controls, and each experiment was repeated three times.
2.3. Effect of UV-C on WFT Fecundity and Egg Survival
2.3.1. Fecundity Trials
A series of trials were conducted to assess the secondary effects of UV-C on egg laying as this would reduce WFT populations overtime. In these trials, a one-day-old WFT female was placed in a 6 cm diameter Petri dish as described for the bioassays and exposed to UV-C at doses of 0.5, 1.5, 2.5, and 3.5 J/cm2 (2.9, 8, 15, and 22 min, respectively). Immediately after exposure, a green bean leaf disc on moist filter paper was added to each dish for food and a substrate for egg laying. Petri dishes were incubated at 25 °C and 16:8 (L/D) and inspected after 5, 6, 7, 8, 9, and 10 days, and the number of larvae that emerged was recorded daily. Ten WFT were tested at each UV-C dose, and the entire experiment was repeated five times. Each treatment was replicated three times within a trial and included an untreated control. The entire experiment was repeated three times.
2.3.2. Egg Trials
WFT generally lay eggs on the underside of the leaf, between the upper and lower epidermal layers. Experiments were conducted to determine the impact of UV-C radiation on WFT eggs laid inside bean and marigold (Tagetes sp.) leaf tissue. Ten one-day-old WFT females were placed on leaves and allowed to lay eggs. For beans, a 6 cm diameter leaf disc was used, whereas for marigolds, entire leaflets (≈4 cm long) were used. After 24 h, WFT were removed, and the leaf samples were exposed to UV-C at 0.5, 1.5, 2.5, and 3.5 J/cm2 doses (2.9, 8, 15, and 22 min, respectively). Leaves were then placed on a moist filter paper in a covered Petri dish and incubated at 25 °C and 16:8 (L/D) for 7 days, after which the number of larvae that emerged from the eggs and were visible on the bean disc were counted. Each treatment, including an untreated control, was replicated three times within a trial, and the entire experiment was repeated three times over a 2 month period.
2.4. Effect of UV-C Exposure on Plants
WFT is a major pest of ornamental plants. It is therefore critical that the level of radiation used to kill WFT does not also negatively impact the appearance of the host plant. The effect of four UV-C doses (0.5, 1.5, 2.5, and 3.5 J/cm2 (2.9, 8, 15, and 22 min, respectively)) were tested on immature and flowering stages of Portulaca (Happy HR Fuchsia), African marigold (Tagetes sp., African Taishan Gold), and Calibrachoa (var. Cabaret orange) (Ball Horticultural Co., Chicago, IL, USA). Untreated plants were used as controls. Three plants were treated for each UV-C dose, and the entire test was repeated three times. After exposure, plants were inspected for signs of damage daily for 14 days (non-flowering) and 30 days (flowering) for evidence of recovery from UV-C exposure.
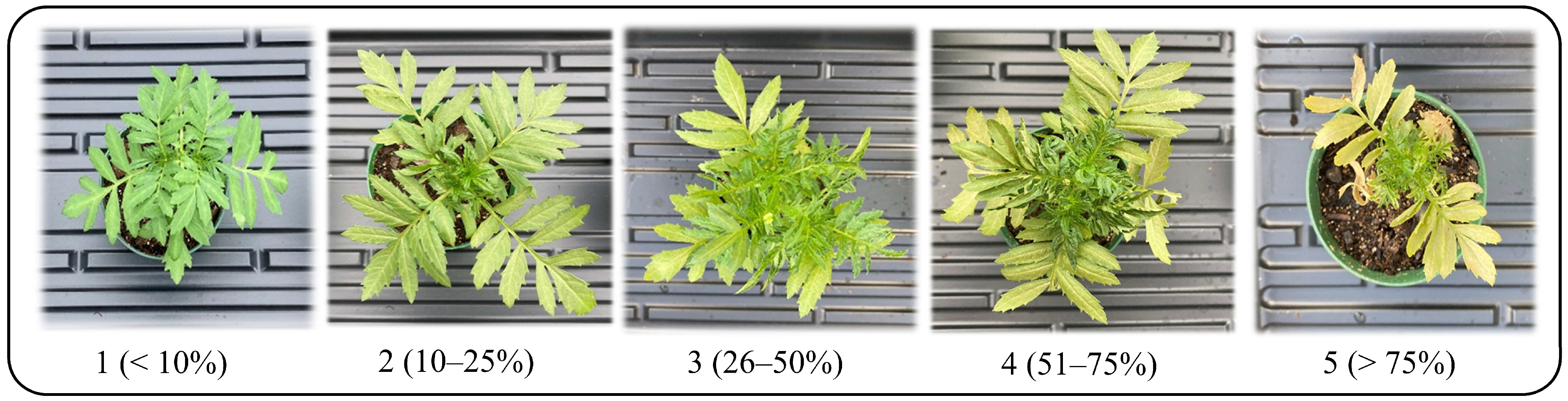
A rating system was developed to assess UV-C damage, which was modified from a method commonly used by the authors to assess foliar feeding damage by WFT [31]. The level of damage characteristic of UV-C damage (defoliation, discoloration, and leaf curling) on each plant was assessed visually (Figure 2) using the following criteria:

Figure 2.
Generic example of foliar damage ratings on marigold plants after exposure to UV-C.
1—(<10%) no leaf curl/distortion–slight discoloration–no dieback.
2—(10–25%) slight leaf curl/distortion–slight discoloration–no dieback.
3—(26–50%) moderate leaf curl/distortion–moderate discoloration–slight dieback.
4—(51–75%) severe leaf curl/distortion–moderate discoloration–moderate dieback.
5—(>75%) severe leaf curl/distortion–heavy discoloration–heavy dieback.
2.5. Statistical Analysis
Data from all experiments on the number of WFT (adults, larvae, and eggs) and the foliar damage ratings were analyzed using a general linear model with repeated measures and/or univariate procedures, followed by Tukey’s honestly significant difference (HSD) tests (α = 0.05). The data were not transformed and were assessed for any substantial deviations from normality before proceeding with the analyses. For repeated measures, if Mauchly’s test of sphericity was violated, Greenhouse–Geisser or Huynh–Feldt corrected F- and p-values were reported depending on if the estimated epsilon value was below or above 0.75, respectively. Analyses were conducted using IBM-SPSS v.26 (IBM, Armonk, NY, USA), and figures were produced with Prism v.10 (GraphPad Software, Boston, MA, USA).
3. Results
3.1. Effect of UV-C Exposure on WFT Mortality
3.1.1. Larvae
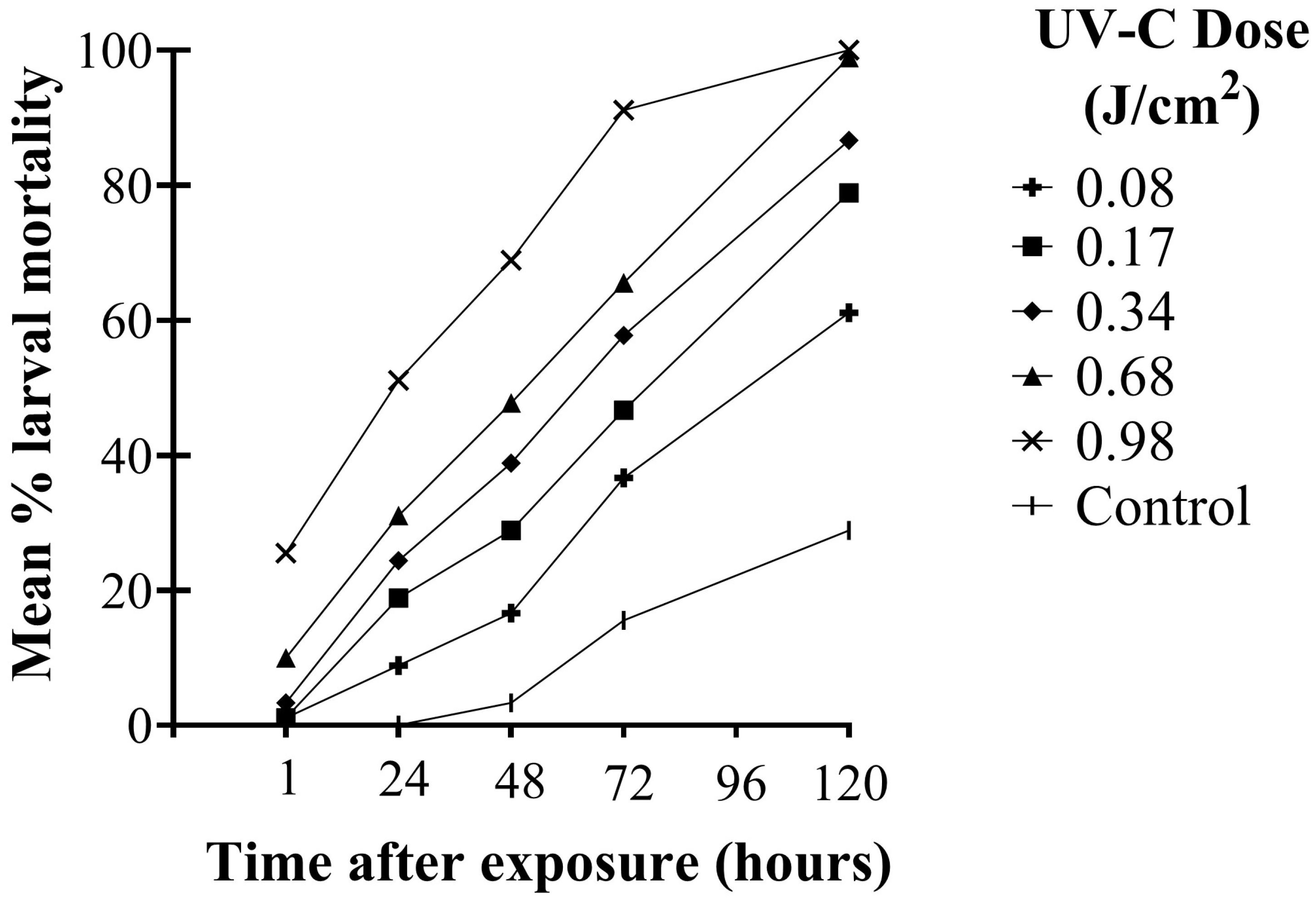
There were significant differences in the mortality of second instar WFT larvae among UV-C doses over time after exposure (F20,144 = 14.95; p < 0.001). Larval mortality increased significantly over time (F4,144 = 765.82; p < 0.001). Significant differences in larval mortality among UV-C doses were observed as early as 1 h after exposure and remained significant at each observation time up to 120 h (F5,36 = 38.91; p < 0.001). One hour after UV-C exposure, the two highest doses (0.68 and 0.98 J/cm2) caused significantly greater mortality than the control and two lowest doses (0.08 and 0.17 J/cm2) (p ≤ 0.004). There were no significant differences in mortality among the three lowest rates and the control (p ≥ 0.776) and no significant difference between 0.34 and 0.68 J/cm2 (p = 0.116). Forty-eight hours after exposure, all treatment doses were significantly different from the control (p ≤ 0.028) and remained significant thereafter up to 120 h post-exposure (p < 0.001). Peak larval mortality was observed in all treatments at 120 h after exposure. There were no significant differences between doses 0.17 and 0.34 J/cm2 or 0.68 and 0.98 J/cm2 after 120 h, though significantly greater mortality was observed for those doses than the lowest dose (0.08 J/cm2) (p < 0.001) (Figure 3).
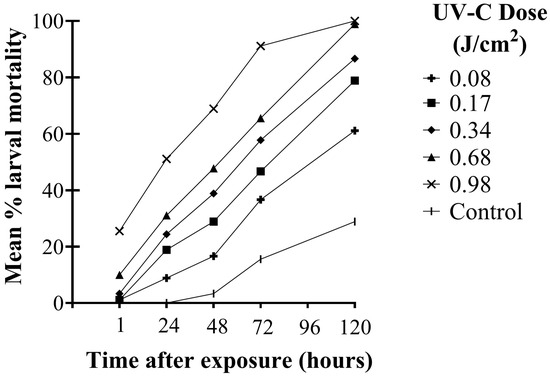
Figure 3.
Mean % mortality of WFT larvae at different time points up to 120 h after exposure to UV-C doses of 0.08, 0.17, 0.34, 0.68, and 0.98 J/cm2 (30 s, 60 s, 2 min, 4 min, and 5 min exposure, respectively) and an untreated control.
3.1.2. Adults
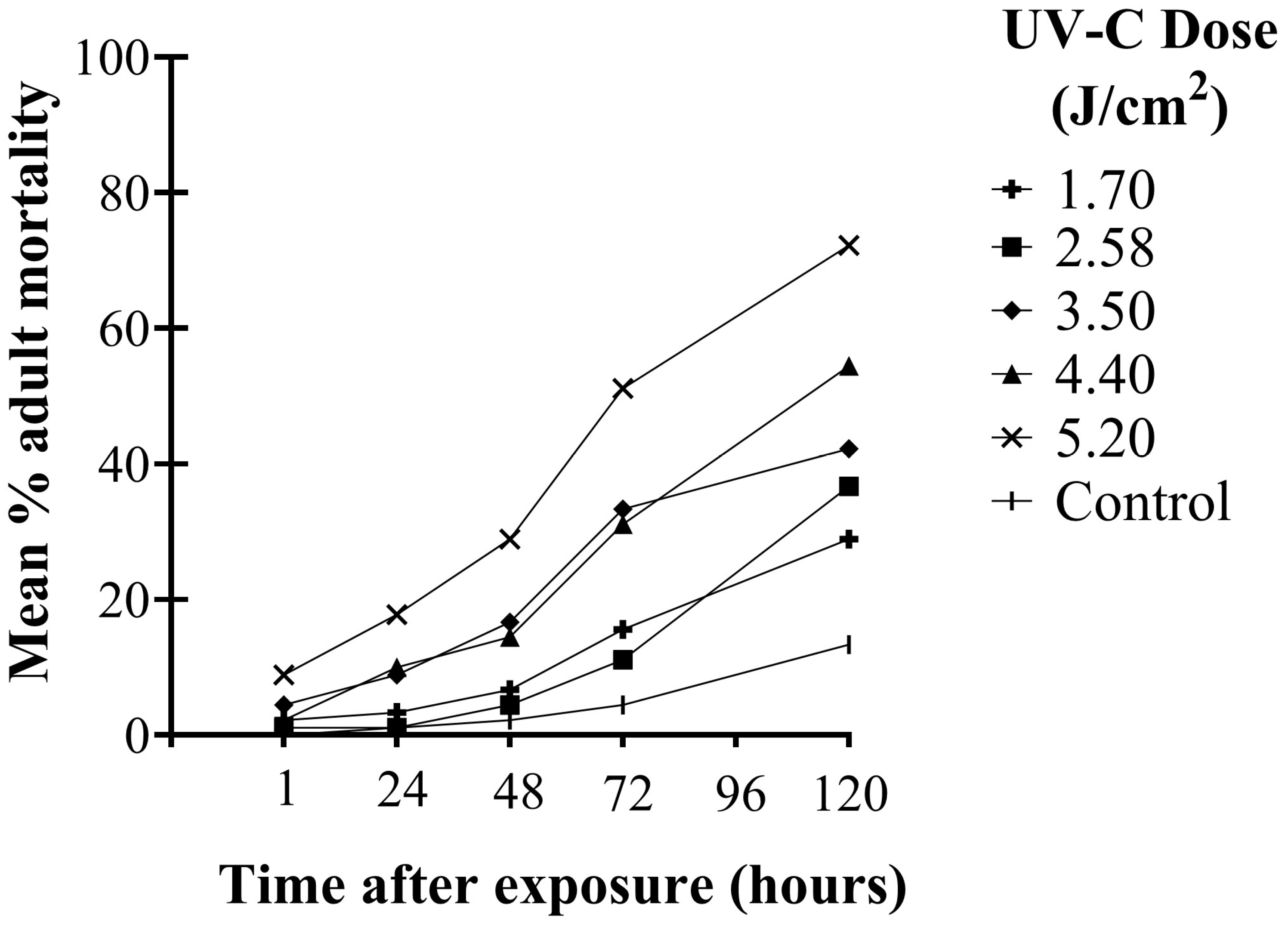
There were significant differences in the mortality of WFT adults among UV-C doses over time after exposure (F20,144 = 11.63; p < 0.001). Higher UV-C doses were required to kill WFT adults than second instars. Adult mortality increased significantly over time (F4,144 = 281.52; p < 0.001). Significant differences in adult mortality among UV-C doses were observed as early as 1 h after exposure and remained significant at each observation time up to 120 h (F5,36 = 3.27; p ≤ 0.016). One hour after UV-C exposure, the highest dose (5.2 J/cm2) caused significantly greater mortality than the control (p = 0.012). After 72 h, the three highest doses (3.5, 4.4, and 5.2 J/cm2) had significantly greater adult mortality compared to the control (p < 0.001). It was not until 120 h after exposure that all UV-C doses showed significantly greater adult mortality compared to the control (p ≤ 0.025). At the two highest doses, 5.2 and 4.4 J/cm2, 50% of the adults died within 72 and 120 h, respectively, whereas no other doses achieved 50% mortality over the experimental period (Figure 4).
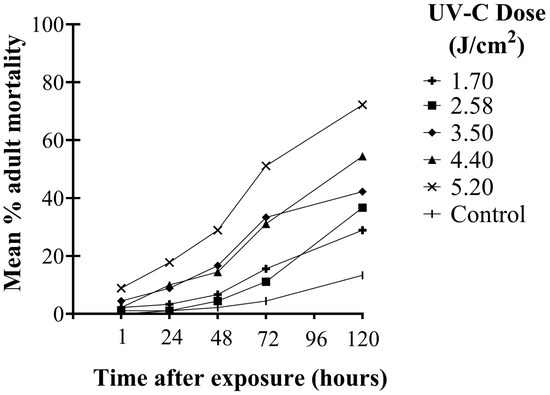
Figure 4.
Mean percent (%) mortality of WFT adults at different times up to 120 h after exposure to UV-C doses of 1.70, 2.58, 3.50, 4.40, and 5.20 J/cm2 (10, 15, 20, 25, and 30 min exposure, respectively).
3.2. Effect of UV-C Exposure on WFT Fecundity and Egg Survival
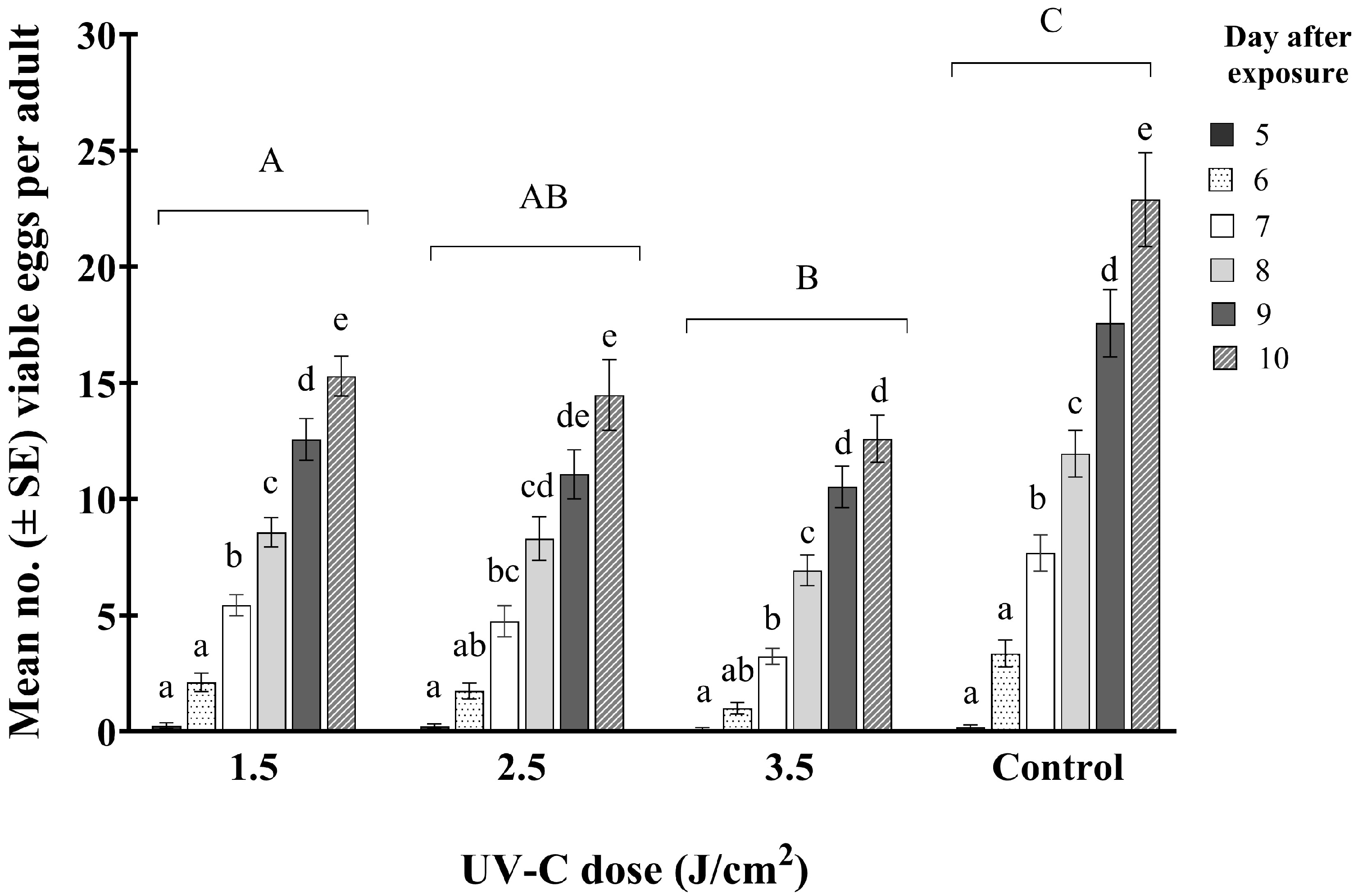
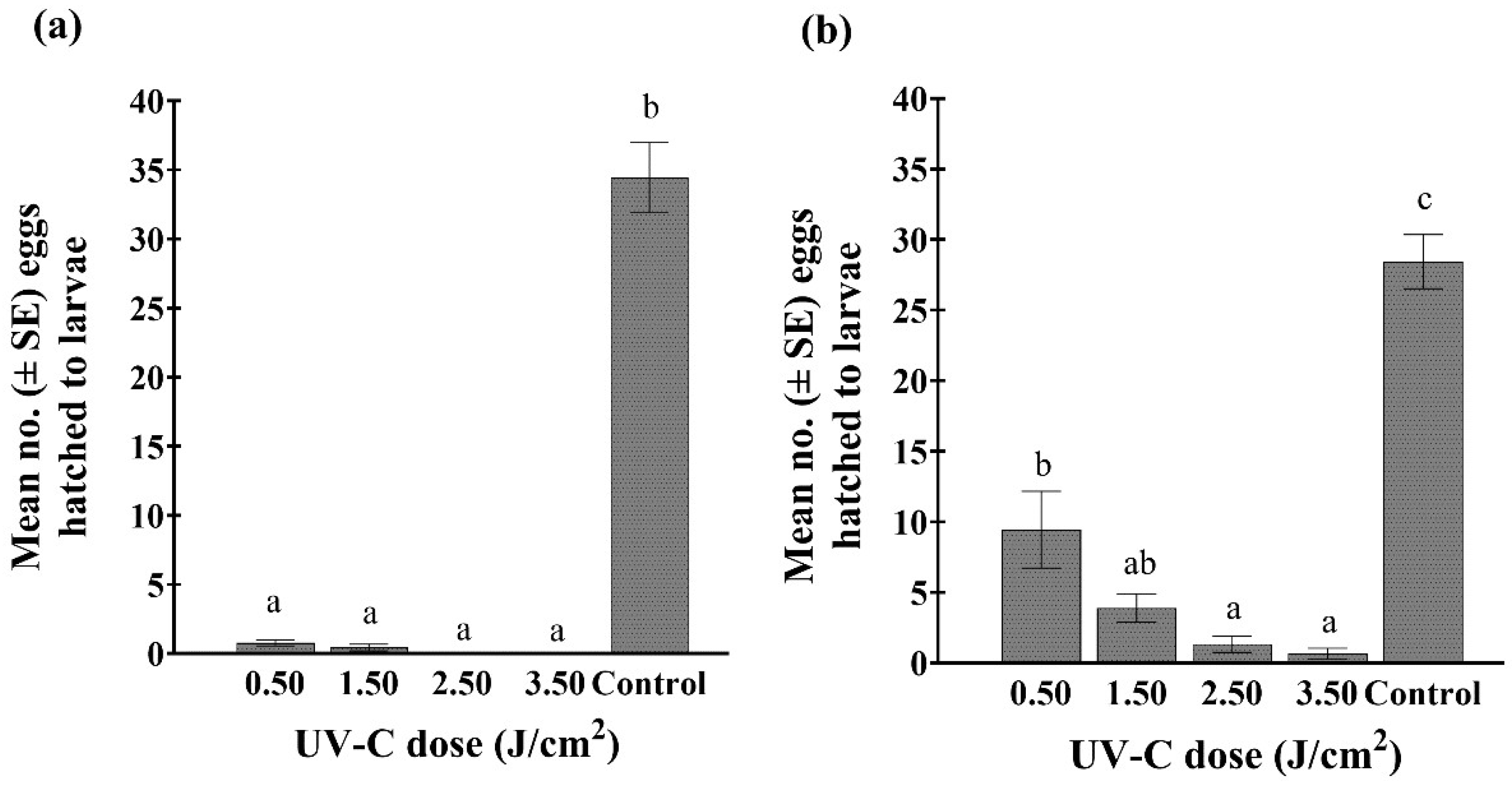
Results from our efficacy trials indicated that even at the higher UV-C doses, a small percentage of WFT survived. However, it was found that these surviving WFT laid significantly fewer viable eggs than the controls and the total egg laying period was significantly shorter than that for the controls over time (F15,505 = 7.60; p < 0.001) (Figure 5).
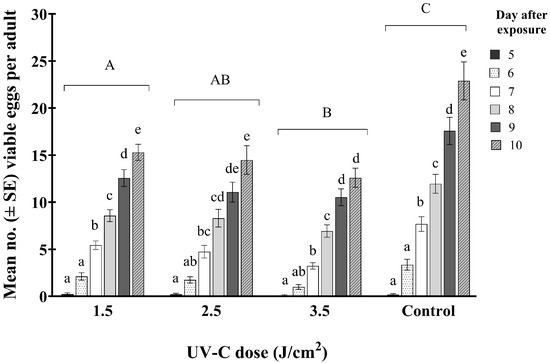
Figure 5.
Mean number of viable WFT eggs (±SE) (based on larval hatch) per adult at 5–10 days after exposure to one of three doses of UV-C (1.5, 2.5, and 3.5 J/cm2 (8, 15, and 22 min exposure, respectively)) and an untreated control. Bars with the same lowercase letters within a dose indicate no significant differences among treatments. The same uppercase letters indicate no significant differences among UV-C treatments (Tukey’s HSD; p < 0.05).
The number of viable eggs, based on successful larval hatch, laid by adult females after exposure to UV-C radiation was significantly less than from untreated ones. The average number of eggs that hatched 10 days post-exposure was 13, 14, and 15 per adult for females treated with 3.5, 2.5, and 1.5 J/cm2, respectively, compared to a mean of 23 hatched eggs per adult in the controls. Results of these fecundity trials demonstrated that though around 60% of adults treated with a UV-C dose of 3.5 J/cm2 survived (as shown with the efficacy trial), they would be expected to have a significantly lower reproductive rate than untreated ones. Therefore, exposure of females to sublethal doses of UV-C resulted in fewer larvae, either because of reduced egg laying or laying of non-viable eggs.
Significantly fewer eggs hatched from bean leaf tissue exposed to various UV-C doses compared to the untreated controls, whether treatments were applied to the upper or lower leaf surface (treatment location: leaf upper side (F4,45 = 52.89; p < 0.001); underside (F4,45 = 165.41; p < 0.001)) (Figure 6). Based on the relative number of eggs that hatched from untreated green bean leaves, a reduction of 67, 86, 95, and 98% in hatch was observed after the upper side of leaves were exposed to 0.5, 1.5, 2.5, and 3.5 J/cm2, respectively. The hatch rate was considerably lower when the undersides of leaves were exposed to the UV-C treatments (98, 99, 100, and 100% less, respectively). For the trials with marigolds, no WFT larvae were observed in Petri dishes that contained marigold leaves treated with UV-C doses of 1.5, 2.5, and 3.5 J/cm2, whether exposure was directed at the upper or undersides of the leaves. However, in the controls, an average of 25 and 30 eggs hatched from the upper and undersides of the leaves, respectively.
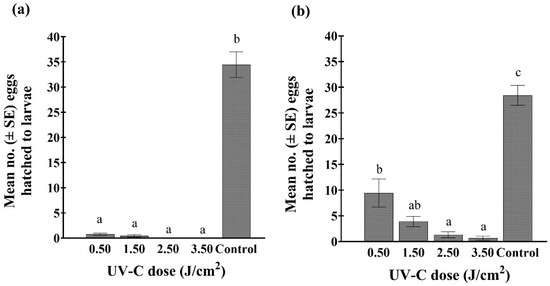
Figure 6.
Mean number (±SE) of viable WFT eggs, based on larval hatch: (a) 7 days after the underside of the bean leaf was exposed to one of four doses of UV-C (0.5, 1.5, 2.5, and 3.5 J/cm2 (2.9, 8, 15, and 22 min exposure, respectively)) and an untreated control, (b) 7 days after the upper side of the bean leaf was exposed to one of four doses of UV-C (0.5, 1.5, 2.5, and 3.5 J/cm2 (2.9, 8, 15, and 22 min exposure, respectively)) and an untreated control. Bars with differing lowercase letters indicate significant differences among treatments within a graph (Tukey’s HSD; p < 0.05).
3.3. Effect of UV-C Exposure on Plants
The non-flowering and flowering stages of the three ornamental plants tested, Portulaca, African marigold, and Calibrachoa, exhibited a variety of signs and levels of damage that differed significantly among plant types and dose over time (non-flowering: F72,810 = 6.68, p < 0.001; flowering: F32,360 = 20.98, p < 0.001). In non-flowering Calibrachoa and African marigold, leaves curled and yellowed and appeared abnormally small 3 days after exposure. Portulaca was considerably more susceptible to UV-C exposure, with many leaves curling and dropping off within 24 h of treatment at 2.5 and 3.5 J/cm2. After 10 days, the Calibrachoa and African marigold plants outgrew much of the damage in all but the highest dose, 3.5 J/cm2. However, damage remained visible on Portulaca even after 10 days (Table 1). In the flowering stage, African marigold was the most resistant, exhibiting no visible damage after exposure to all UV-C doses. Flowers of Calibrachoa and Portulaca were susceptible to UV-C doses of 2.5 and 3.5 J/cm2. A few petals on the flowers of these plants changed color within 3 days after treatment, but most recovered after 30 days (Table 2). Unlike Portulaca, the flowering Calibrachoa only showed minor damage from UV-C exposure, and within 14 days of treatment they had refoliated, appeared normal, and were of saleable quality.

Table 1.
Mean damage rating 1 on African marigold, Calibrachoa, and Portulaca plants in the non-flowering stage for up to 10 days after exposure to one of four doses of UV-C (0.5, 1.5, 2.5, and 3.5 J/cm2 (2.9, 8, 15, and 22 min exposure, respectively)) and an untreated control.

Table 2.
Mean damage rating 1 on African marigold, Calibrachoa, and Portulaca plants in the flowering stage for up to 30 days after exposure to one of four doses of UV-C (0.5, 1.5, 2.5, and 3.5 J/cm2 (2.9, 8, 15, and 22 min exposure, respectively)) and untreated controls.
4. Discussion
The efficacy trial results showed WFT mortality was dependent on UV-C dose and life stage with higher doses causing greater WFT mortality in a shorter time period for both larvae and adults. Higher doses were required to kill WFT adults than larvae, and larvae died more rapidly than adults after exposure. Rapid knockdown of WFT is advantageous for growers because it prevents further feeding damage and reduces virus transmission. Several aspects of WFT biology may influence susceptibility of WFT to UV-C radiation. Adults have a thicker exoskeleton than the more soft-bodied immatures. This exoskeleton layer may protect them from damage and confer resistance. The molecular mechanisms of the insect exoskeleton in adaptation to environmental factors such as UV radiation stress has been investigated in several insect groups, but not thrips [32,33,34]. In the aphid Macrosiphum euphorbiae, some exoskeletal proteins were induced in response to a combination of high temperatures and UV-B exposure [33].
Other researchers have tested the effect of UV-C exposure on thrips and other pests. Montemayor et al. [18] reported that low doses of UV-C (200 and 350 J/m2) applied to field-grown strawberries had little to no effect on WFT or Scirtothrips dorsalis compared with untreated controls. However, the UV-C dose used in that study was considerably less than those we tested. Results from our efficacy trials indicated that UV-C exposure killed over 50% of adults and larvae at the higher doses tested, though a percentage of them survived. These results demonstrate the importance of establishing the effective UV-C dose required to cause mortality before testing under field conditions. Leskey et al. [22] tested UV-C exposure against all life stages of the greenhouse whitefly, Trialeurodes vaporariorum, and observed significantly lower pest numbers on tomatoes treated nightly with UV-C compared to unexposed plants. The UV-C dose used in that study was considerably lower than what we used. The efficacy might have been explained by the repeated treatments over the 6 week trial period. In a similar study, the exposure of T. vaporariorum adults to UV-C for 12 min resulted in >90% mortality after 48 h (irradiation intensity not reported) [35]. However, in our study, the highest mortality rate for adults reached 78% within 120 h when exposed to UV-C for 30 min (dose: 5.20 J/cm2). Additionally, for WFT larvae, the mortality rate was 100% within 120 h when exposed to UV-C for 6 min (dose: 0.98 J/cm2). The conflicting results between studies show that the effects of UV-C exposure vary among arthropod species, doses applied, and frequency of exposures. These variations must be factored in when considering the full potential of UV-C as part of an IPM program that targets multiple pests and diseases.
Our results indicate that UV-C radiation had a significant impact on laying of viable eggs and hatch of larval WFT in vitro and has the potential to reduce population density. The number of larvae from eggs that hatched from females exposed to UV-C was 40% lower in those subjected to the highest dose (3.5 J/cm2) compared to untreated ones. This secondary effect from UV-C exposure offers additional management benefits by reducing the reproductive potential of adults that survive or escape the full impact of exposure. Our findings aligned with a study by Tuncbilek et al. [36] in which exposure to UV-C caused a gradual decrease in the number of hatched eggs laid by Trichogramma euproctidis, a parasitoid of several lepidopteran pests. Adult emergence also decreased as radiation exposure increased. In a similar study, exposure to UV-B and UV-C radiation decreased oriental fruit fly, Bactrocera dorsalis, egg laying by 36% and 55%, respectively [37]. These findings suggest that UV-C exposure could be a suitable treatment for leaf miners and other pests that hide within the leaf tissue. The hatchability of eggs exposed to UV radiation was previously reported in the greenhouse whitefly, Trialeurodes vaporariorum; two-spotted spider mite, Tetranychus urticae; and Neoseiulus californicus [12,18,22]. These studies suggest that insect eggs suffer high mortality rates from exposure to UV-C radiation and thus they support the value of further investigation of UV-C as a potential tool to manage whitefly, mites, and WFT during their vulnerable life stages.
The detrimental effects of UV radiation to plant tissue are well known and must be factored in when developing UV-C to manage WFT. Typically, the impact of UV-C radiation on plants varies, including stress-associated damage, secondary metabolite production, as well as growth and flowering responses. UV-C irradiation has the potential to damage or kill plants, with the critical factors being the duration and frequency of exposure. In one study, African marigolds and pansies exposed to UV-C for 60 min weekly (irradiation intensity and UV-C dose not reported) for up to 8 weeks suffered complete burning [38]. However, a suitable weekly dose, as brief as 15 min, enhanced branching and increased the number of flowers, although the timing of flowering varied based on the plant species [38]. UV radiation can also influence the quality of plant tissue for herbivores through factors such as changes in leaf chemistry or reinforcement of the plant cell wall [23,39]. Ultraviolet radiation can induce the production of phenolic compounds in several plant species, which negatively affect herbivorous arthropods. Recent evidence has demonstrated that UV light can augment key and inducible defense-related hormones in plants that also impact arthropod pests. These responses are highly plant genotype and UV light dose dependent [26,40,41,42].
Western flower thrips is a major pest of greenhouse ornamentals, and crop plants in this industry are sold for their beauty and thus should be without blemishes, either from pests or treatments against pests. For this reason, trials were conducted on potential phytotoxic effects of UV-C on three popular ornamental plant species (Portulaca, African marigold, and Calibrachoa). Both plants in the non-flowering and flowering stage were tested as these are two different floriculture production phases and plant tissues may be differentially sensitive depending on their maturity. In addition, the plants also may have different capacity for recovery depending on their age. As expected, the effect of UV-C exposure varied among plant types and age. Though some of the plants tested exhibited damage following exposure to the selected UV-C doses, in most cases, damage was minimal, and most recovered fully. In general, UV-C doses that killed >80% of WFT immatures caused little or no damage to the plants, or the plants recovered within 14 days. Most plants exposed to doses that killed WFT adults recovered from damage within 30 days. Studies are needed to assess the effect of UV-C on whole plants to better understand the practical application of UV-C in commercial settings.
5. Conclusions
The results reported herein show UV-C offers promise as a strategy that could be tested as part of a comprehensive WFT IPM program in the future. At some doses, UV-C exposure showed increased mortality of WFT adults or larvae, with minimal phytotoxic effects. A relationship between UV-C dose and WFT mortality was observed, with lethal impacts on adults, larvae, and eggs increasing as UV-C dose increased. Adults exhibited less susceptibility to UV-C irradiation than larvae and eggs, and it took them longer to die after exposure than larvae. However, sublethal doses of UV-C effectively reduced females’ fecundity, leading to a reduction in WFT populations over time.
Introducing UV-C as a treatment against WFT on living plants and exploring its potential to induce plant defense mechanisms introduces a new element for IPM. Further research is needed to assess potential impacts of UV-C on WFT physiology and other components of WFT IPM. Additionally, investigations into plant responses to UV-C, including induced defense mechanisms and accumulation of UV-C-induced phenolic compounds, are crucial for a comprehensive understanding of the interactions between UV-C and WFT. Based on the promising results of using UV-C against WFT reported herein, further research is needed to refine its use under controlled greenhouse conditions to elucidate the effectiveness of UV irradiation against WFT over time and its compatibility with biological control (beneficial insects, biopesticides, etc.) and other IPM practices.
Author Contributions
Conceptualization, B.L.P., M.S., A.D. and M.S.R.; investigation, A.D.; formal analysis, A.D. and C.F.S.; visualization, C.F.S.; writing—original draft, A.D. and C.F.S.; writing—review and editing, B.L.P., M.S. and M.S.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by grants from the American Floral Endowment and the USDA HATCH program, multistate project #NCERA222.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Available upon a reasonable request.
Acknowledgments
The authors are grateful to the American Floral Endowment, and in particular Terril Nell, research director, for the interest in and financial backing of this innovative initiative. They thank the Ball Horticultural Company for providing the plant material and the ongoing support of the University of Vermont, College of Agriculture and Life Sciences. They also appreciate the assistance of Andrew Bierman, Lighting Research Center, Rensselaer Polytechnic Institute, for the construction of the test chamber used for the research reported herein and for the assistance with interpreting the results.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- Reitz, S.R.; Gao, Y.; Kirk, W.D.J.; Hoddle, M.S.; Leiss, K.A.; Funderburk, J.E. Invasion biology, ecology, and management of western flower thrips. Annu. Rev. Entomol. 2020, 65, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Pappu, H.R.; Jones, R.A.C.; Jain, R.K. Global status of tospovirus epidemics in diverse cropping systems: Successes achieved and challenges ahead. Virus Res. 2009, 141, 219–236. [Google Scholar] [CrossRef] [PubMed]
- Webster, C.G.; Reitz, S.R.; Perry, K.L.; Adkins, S. A Natural M RNA Reassortant arising from two species of plant- and insect-infecting bunyaviruses and comparison of its sequence and biological properties to parental species. Virology 2011, 413, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Prins, M.; Goldbach, R. The emerging problem of tospovirus infection and nonconventional methods for control. Trends Microbiol. 1998, 6, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Riley, D.G.; Joseph, S.V.; Srinivasa, R.; Diffie, S. Thrips vectors of tospoviruses. J. Integr. Pest Manag. 2011, 1, I1–I10. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Meng, J.Y.; Wang, X.P.; Zhu, F.; Lei, C.L. Effects of UV-A exposures on longevity and reproduction in Helicoverpa armigera, and on the development of its F1 generation. Insect Sci. 2011, 18, 697–702. [Google Scholar] [CrossRef]
- Meng, J.Y.; Zhang, C.Y.; Lei, C.L. A Proteomic Analysis of Helicoverpa armigera Adults after exposure to UV light irradiation. J. Insect Physiol. 2010, 56, 405–411. [Google Scholar] [CrossRef]
- Llabrés, M.; Agustí, S.; Alonso-Laita, P.; Herndl, G.J. Synechococcus and Prochlorococcus cell death induced by UV radiation and the penetration of lethal UVR in the Mediterranean Sea. Mar. Ecol. Prog. Ser. 2010, 399, 27–37. [Google Scholar] [CrossRef]
- Zeeshan, M.; Prasad, S.M. Differential response of growth, photosynthesis, antioxidant enzymes and lipid peroxidation to UV-B radiation in three cyanobacteria. S. Afr. J. Bot. 2009, 75, 466–474. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Kumar, A.; Tyagi, M.B.; Sinha, R.P. Molecular mechanisms of ultraviolet radiation-induced DNA damage and repair. J. Nucleic Acids 2010, 2010, 592980. [Google Scholar] [CrossRef]
- Sanha, R.P.; Hader, D.P. UV-induced DNA damage and repair: A review. Photochem. Photobiol. Sci. 2002, 1, 225–236. [Google Scholar] [CrossRef]
- Tachi, F.; Osakabe, M. Spectrum-specific UV egg damage and dispersal responses in the phytoseiid predatory mite Neoseiulus californicus (Acari: Phytoseiidae). Environ. Entomol. 2014, 43, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Suthaparan, A.; Stensvand, A.; Torre, S.; Herrero, M.L.; Pettersen, R.I.; Gadoury, D.M.; Gislerod, H.R. Continuous lighting reduces conidial production and germinability in the rose powdery mildew Pathosystem. Plant Dis. 2010, 94, 339–344. [Google Scholar] [CrossRef] [PubMed]
- de Mello, P.P.; Onofre, R.B.; Rea, M.; Bierman, A.; Gadoury, D.M.; Ivors, K. Design, construction, and evaluation of equipment for nighttime applications of UV-C for management of strawberry powdery mildew in Florida and California. Plant Health Prog. 2022, 1, 321–327. [Google Scholar] [CrossRef]
- Needham, G.; Begg, C.; Buchanan, S. Ultraviolet C exposure is fatal to American house dust mite eggs. J. Allergy Clin. Immunol. 2006, 117, 28. [Google Scholar] [CrossRef]
- Collins, D.A.; Kitchingman, L. The effect of ultraviolet C radiation on stored-product pests. In Proceedings of the 10th International Working Conference on Stored Product Protection, Estoril, Portugal, 27 June–2 July 2010; Julius-Kuhn-Archiv. Volume 425, pp. 632–636. [Google Scholar]
- Faruki, S.; Dass, D.; Khan, A.R.; Khatun, M. Effects of ultraviolet (254 nm) irradiation on egg hatching and adult emergence of the flour beetles, Tribolium castaneum, T. confusum and the almond moth, Cadra cautella. J. Insect Sci. 2007, 7, 36. [Google Scholar] [CrossRef]
- Montemayor, J.D.; Smith, H.A.; Peres, N.A.; Lahiri, S. Potential of UV-C for management of two-spotted spider mites and thrips in Florida strawberry. Pest Manag. Sci. 2023, 79, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Osakabe, M. Biological impact of ultraviolet-B radiation on spider mites and its application in integrated pest management. Appl. Entomol. Zool. 2021, 56, 139–155. [Google Scholar] [CrossRef]
- Murata, Y.; Osakabe, M. Photo-enzymatic repair of UVB-induced DNA damage in the two-spotted spider mite Tetranychus urticae. Exp. Appl. Acarol. 2017, 71, 15–34. [Google Scholar] [CrossRef]
- Suzuki, T.; Watanabe, M.; Takeda, M. UV tolerance in the two-spotted spider mite, Tetranychus urticae. J. Insect Physiol. 2009, 55, 649–654. [Google Scholar] [CrossRef]
- Leskey, T.C.; Short, B.D.; Emery, M.; Evans, B.; Janisiewicz, W.; Takeda, F. Effect of UV-C irradiation on greenhouse whitefly, Trialeurodes vaporariorum (Hemiptera: Aleyrodidae). Fla. Entomol. 2021, 104, 148–150. [Google Scholar] [CrossRef]
- Robson, T.; Klem, K.; Urban, O.; Jansen, M.A. Re-interpreting plant morphological responses to UV-B radiation. Plant Cell Environ. 2015, 38, 856–866. [Google Scholar] [CrossRef] [PubMed]
- Lindroth, R.L.; Hofman, R.W.; Campbell, B.D.; McNabb, W.C.; Hunt, D.Y. Population differences in Trifolium repens L. response to ultraviolet-B radiation: Foliar chemistry and consequences for two lepidopteran herbivores. Oecologia 2000, 122, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Zhang, M.; Lu, C.; Hettenhausen, C.; Tan, Q.; Cao, G.; Zhu, X.; Wu, G.; Wu, J. Ultraviolet-B enhances the resistance of multiple plant species to lepidopteran insect herbivory through the jasmonic acid pathway. Sci. Rep. 2018, 8, 277. [Google Scholar] [CrossRef] [PubMed]
- Demkura, P.V.; Abdala, G.; Baldwin, I.T.; Ballare, C.L. Jasmonate Dependent and independent pathways mediate specific effects of solar ultraviolet B radiation on leaf phenolics and antiherbivore defense. Plant Physiol. 2010, 152, 1084–1095. [Google Scholar] [CrossRef] [PubMed]
- Mazza, C.A.; Zavala, J.; Scopel, A.L.; Ballare, C.L. Perception of solar UVB radiation by phytophagous insects: Behavioral responses and ecosystem implications. Proc. Natl. Acad. Sci. USA 1999, 96, 980–985. [Google Scholar] [CrossRef] [PubMed]
- Izaguirre, M.M.; Mazza, C.A.; Svatos, A.; Baldwin, I.T.; Ballaré, C.L. Solar ultraviolet-B radiation and insect herbivory trigger partially overlapping phenolic responses in Nicotiana attenuata and Nicotiana longiflora. Ann. Bot. 2007, 99, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Williams, M.M.; Loh, Y.T.; Lee, G.I.; Howe, G.A. Resistance of cultivated tomato to cell content-feeding herbivores is regulated by the octadecanoid-signaling pathway. Plant Physiol. 2002, 130, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Ament, K.; Kant, M.R.; Sabelis, M.W.; Haring, M.A.; Schuurink, R.C. Jasmonic acid is a key regulator of spider mite-induced volatile terpenoid and methyl salicylate emission in tomatoes. Plant Physiol. 2004, 135, 2025–2037. [Google Scholar] [CrossRef]
- Sullivan, C.F.; Davari, A.; Kim, J.S.; Parker, B.L.; Skinner, M. Evaluation of a guardian plant system to suppress Frankliniella occidentalis (Thysanoptera: Thripidae) in greenhouse ornamentals. Pest Manag. Sci. 2023, 79, 3559–3569. [Google Scholar] [CrossRef]
- Yang, C.; Meng, J.; Yao, M.S.; Zhang, C.Y. Transcriptome analysis of Myzus persicae to UV-B stress. J. Insect Sci. 2021, 21, 7. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Michaud, D.; Cloutier, C. A Proteomic analysis of the aphid Macrosiphum euphorbiae under heat and radiation stress. Insect Biochem. Mol. Biol. 2009, 39, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.J.; Zhu, Z.H.; Liu, Z.X.; Ma, W.H.; Desneux, N.; Lei, C.L. Identification and transcriptional profiling of differentially expressed genes associated with response to UVA radiation in Drosophila melanogaster (Diptera: Drosophilidae). Environ. Entomol. 2013, 42, 1110–1117. [Google Scholar] [CrossRef] [PubMed]
- Poushand, F.; Aramideh, S.; Forouzan, M. Effect of ultraviolet (UV-C) in different times and heights on adult stage of whitefly (Trialeurodes vaporariorum). J. Entomol. Zool. Stud. 2017, 5, 864–868. [Google Scholar]
- Tuncbilek, A.S.; Ercan, S.F.; Canpolat, U. Effect of Ionizing (gamma) and non-ionizing (UV) radiation on the development of Trichogramma euproctidis (Hymenoptera: Trichogrammatidae). Arch. Biol. Sci. 2012, 64, 287–295. [Google Scholar] [CrossRef]
- Cui, H.; Zeng, Y.; Reddy, G.V.; Gao, F.; Li, Z.; Zhao, Z. UV Radiation increases mortality and decreases the antioxidant activity in a Tephritid Fly. Food Energy Secur. 2021, 10, e297. [Google Scholar] [CrossRef]
- Bridgen, M.P. Utilization of Ultraviolet-C (UV-C) Irradiation on Ornamental Plants for Growth Regulation. American Floral Endowment Special Research Report 2016, #534. Available online: https://endowment.org/wp-content/uploads/2013/03/534_BridgenReport-copy.pdf (accessed on 13 January 2024).
- Rousseaux, M.C.; Julkunen-Tiitto, R.; Searles, P.S.; Scopel, A.L.; Aphalo, P.J.; Ballare, C.L. Solar UV-B radiation affects leaf quality and insect herbivory in the southern beech tree Nothofagus antarctica. Oecologia 2004, 138, 505–512. [Google Scholar]
- Dinh, S.T.; Galis, I.; Baldwin, I.T. UVB Radiation and 17-hydroxygeranyllinalool diterpene glycosides provide durable resistance against mirid (Tupiocoris notatus) attack in field-grown Nicotiana attenuata plants. Plant Cell Environ. 2013, 36, 590–606. [Google Scholar] [CrossRef]
- Dillon, F.M.; Chludil, H.D.; Reichelt, M.; Mithöfer, A.; Zavala, J.A. Field-grown soybean induces jasmonates and defensive compounds in response to thrips feeding and solar UV-B radiation. Environ. Exp. Bot. 2018, 156, 1–7. [Google Scholar] [CrossRef]
- Escobar-Bravo, R.; Nederpel, C.; Naranjo, S.; Kim, H.K.; Rodriguez-Lopez, M.J.; Chen, G.; Glauser, G.; Leiss, K.A.; Klinkhamer, P.G.L. Ultraviolet radiation modulates both constitutive and inducible plant defenses against thrips but is dose and plant genotype dependent. J. Pest Sci. 2019, 94, 69–81. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).