The Induction of Salt Stress Tolerance by Gibberellic Acid Treatment in Stevia rebaudiana Bertoni Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Growth Conditions and Treatments
- −
- Control (C): 0 mM NaCl
- −
- Salt stress (S): 80 mM NaCl
- −
- 50 ppm GA3 (G1)
- −
- 100 ppm GA3 (G2)
- −
- Combination of GA3 with salt stress (G1+S and G2+S)
2.2. Parameters Measured
2.3. Mineral Ions Estimation (K, Ca, and Na)
2.4. Chlorophyll Fluorescence and Stomatal Conductance Measurements
2.5. Chlorophyll Content Determination
2.6. Leaf Relative Water Content
2.7. Evaluation of Proline, Glycine Betaine, and Total Soluble Sugar Contents
2.8. Lipid Peroxidation, Electrolyte Leakage, and Hydrogen Peroxide
2.9. Measurement of Antioxidant Enzyme Activities
2.10. Statistical Analysis
3. Results
3.1. Effect of Gibberellic Acid on Growth Traits
3.2. Effect of Gibberellic Acid on Nutrients
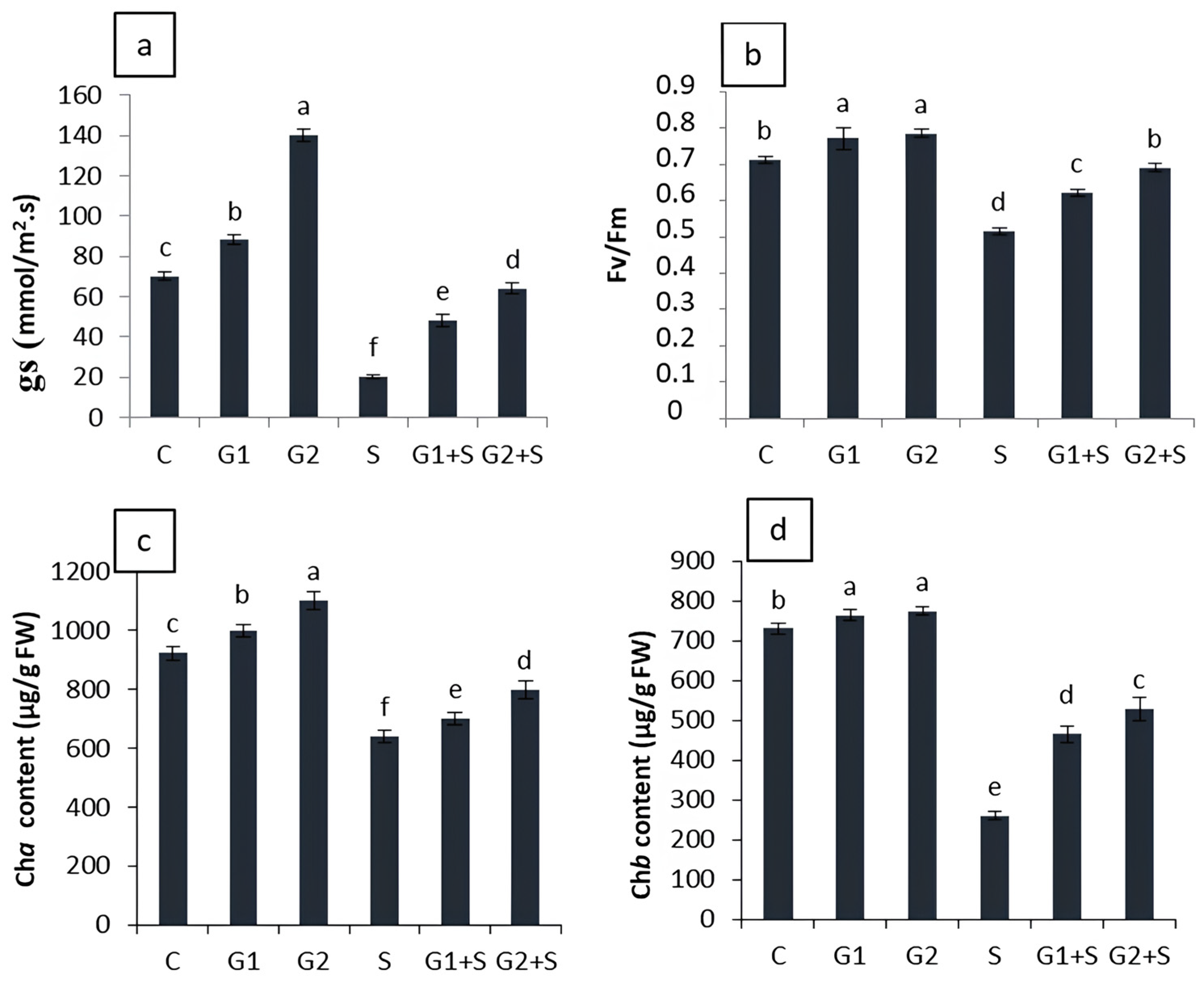
3.3. Effect of Gibberellic Acid on Photosynthesis-Related Performance
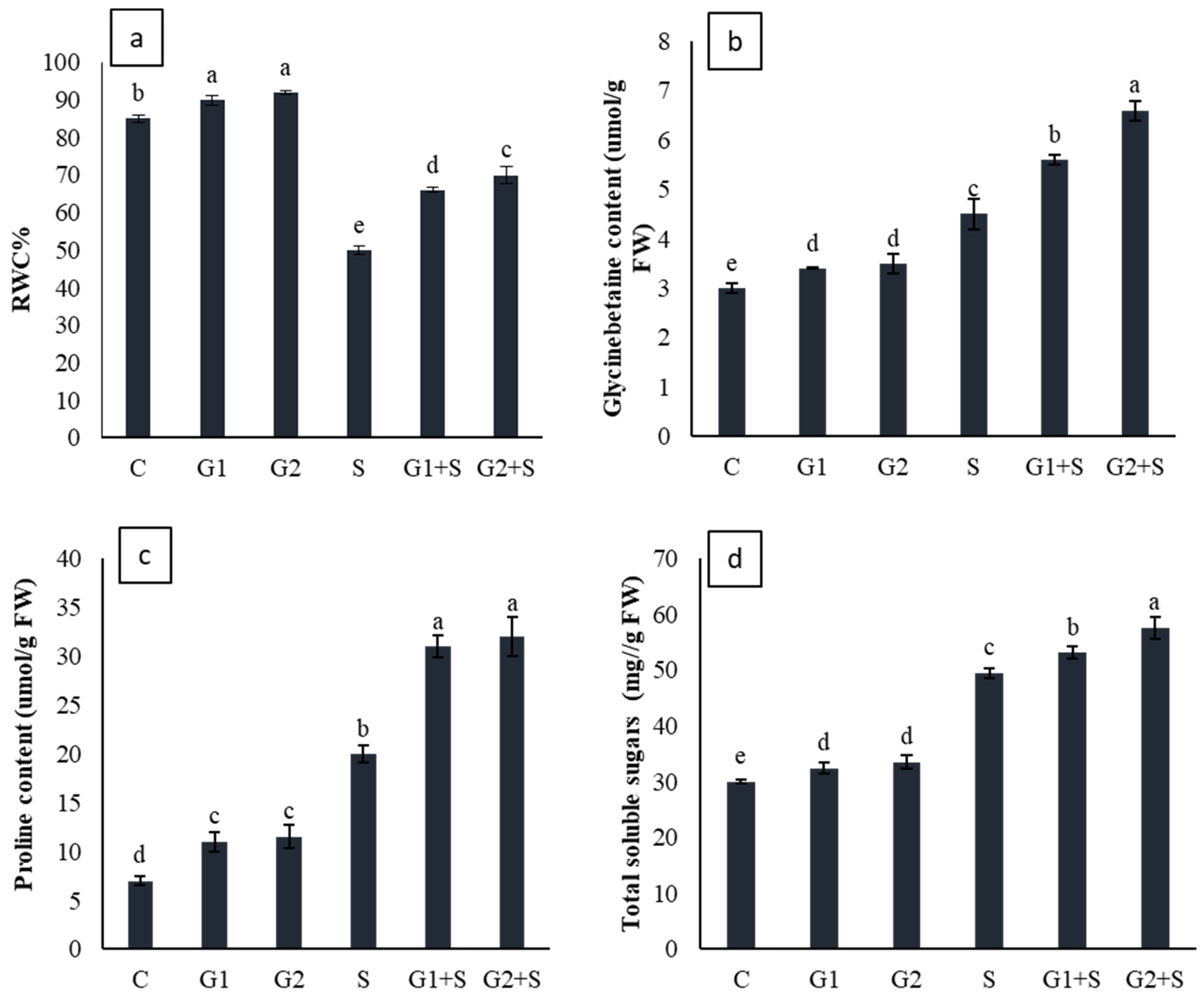
3.4. Effect of Gibberellic Acid on Osmotic Molecules and Leaf Relative Water Content
3.5. Effect of Gibberellic Acid on Oxidative Damage
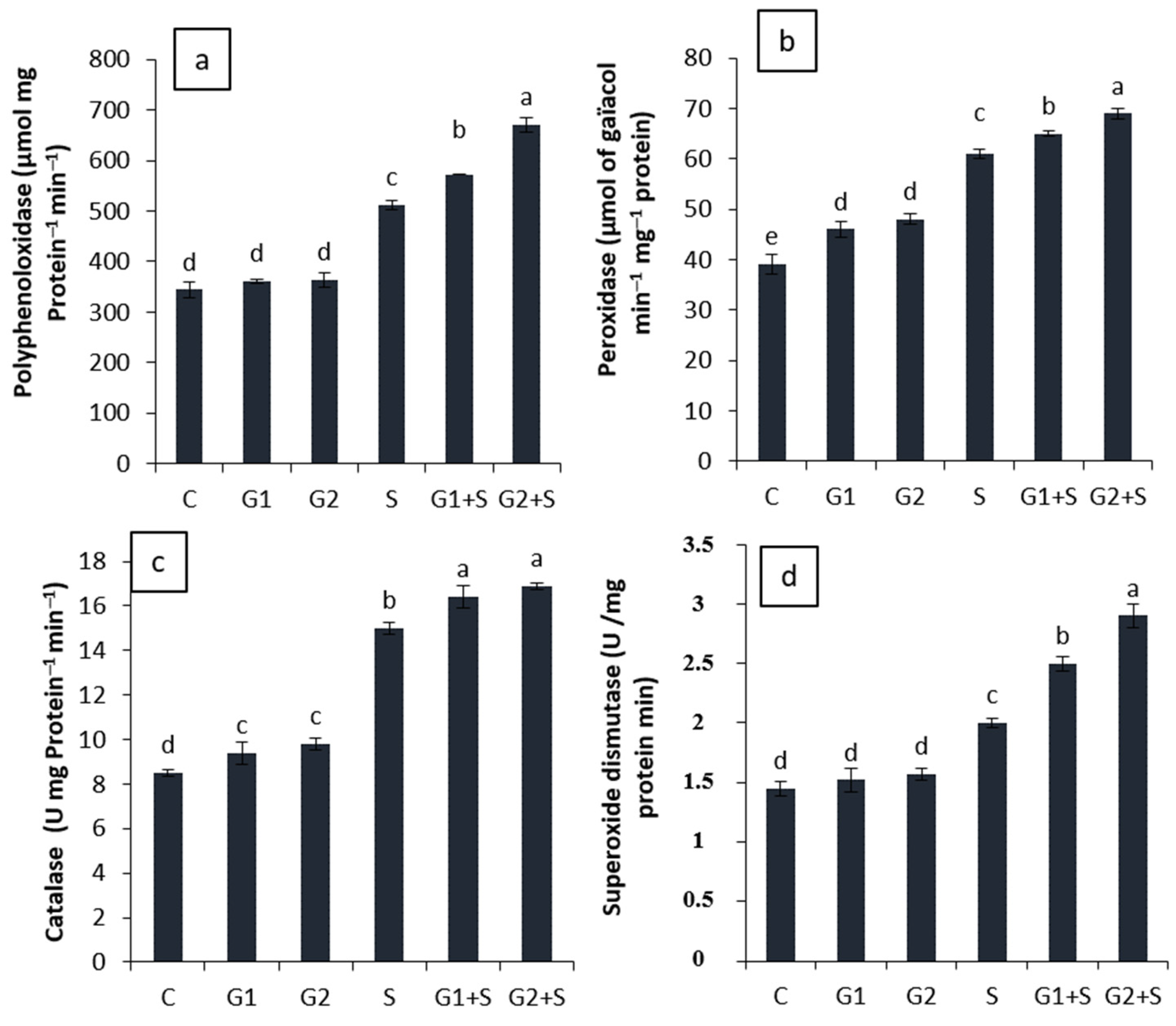
3.6. Effect of Gibberellic Acid on Antioxidant Enzyme Activities
4. Discussion
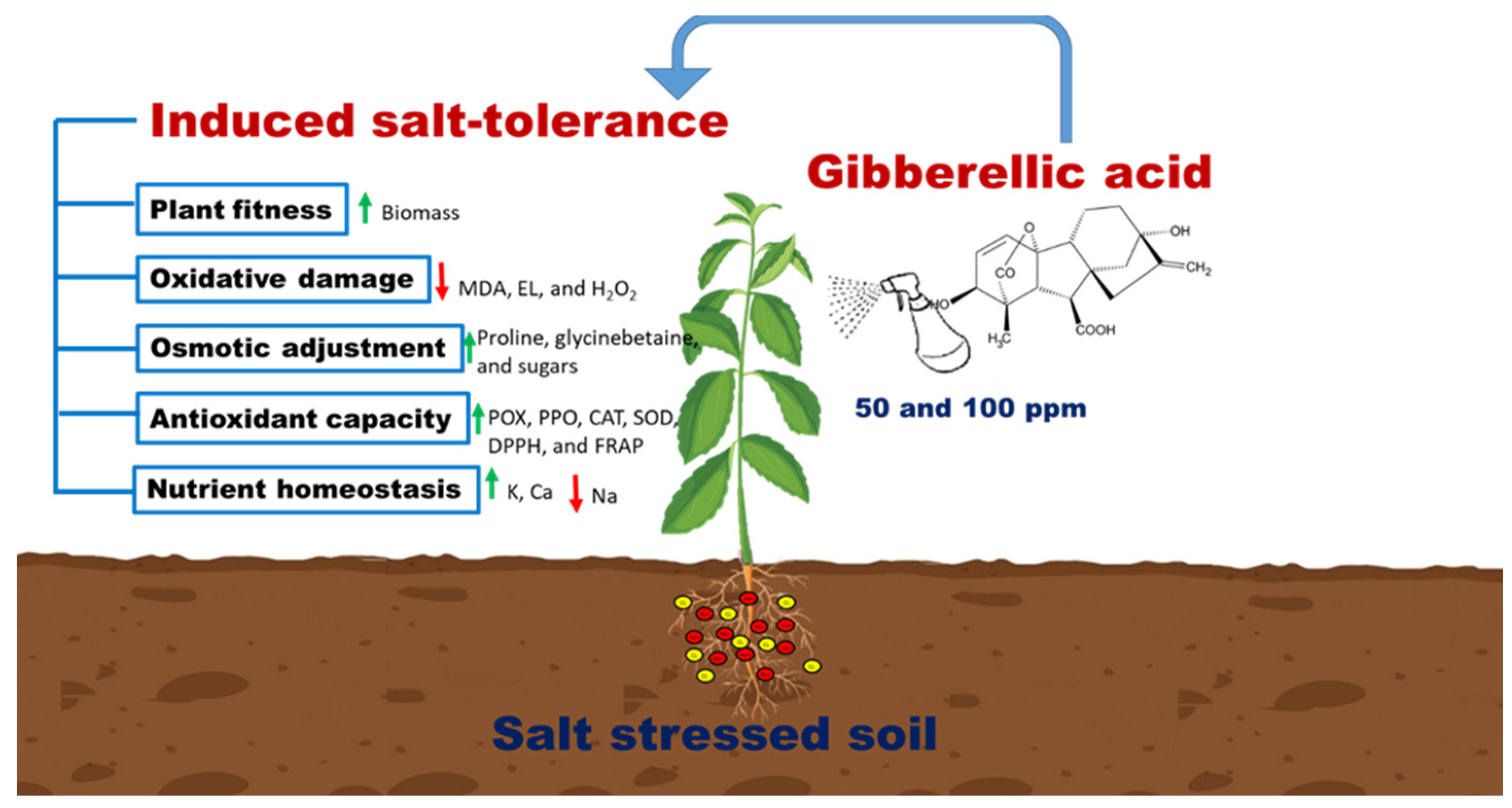
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef]
- Ouhaddou, R.; Ben-Laouane, R.; Lahlali, R.; Anli, M.; Ikan, C.; Boutasknit, A.; Meddich, A. Application of indigenous rhizospheric microorganisms and local compost as enhancers of lettuce growth, development, and salt stress tolerance. Microorganisms 2022, 10, 1625. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Akbar, A.; Parveen, A.; Rasheed, R.; Hussain, I.; Iqbal, M. Phenological application of selenium differentially improves growth, oxidative defense and ion homeostasis in maize under salinity stress. Plant Physiol. Biochem. 2018, 123, 268–280. [Google Scholar] [CrossRef]
- Liu, M.; Lv, Y.; Cao, B.; Chen, Z.; Xu, K. Physiological and molecular mechanism of ginger (Zingiber officinale Roscoe) seedling response to salt stress. Front. Plant Sci. 2023, 14, 1073434. [Google Scholar] [CrossRef]
- Vishal, B.; Kumar, P.P. Regulation of seed germination and abiotic stresses by gibberellins and abscisic acid. Front. Plant Sci. 2018, 9, 838. [Google Scholar] [CrossRef]
- Ritonga, F.N.; Zhou, D.; Zhang, Y.; Song, R.; Li, C.; Li, J.; Gao, J. The roles of gibberellins in regulating leaf development. Plants 2023, 12, 1243. [Google Scholar] [CrossRef]
- Saleem, M.H.; Fahad, S.; Adnan, M.; Ali, M.; Rana, M.S.; Kamran, M.; Hussain, R.M. Foliar application of gibberellic acid endorsed phytoextraction of copper and alleviates oxidative stress in jute (Corchorus capsularis L.) plant grown in highly copper-contaminated soil of China. Environ. Sci. Pollut. Res. 2020, 27, 37121–37133. [Google Scholar] [CrossRef]
- Chandel, A.; Thakur, M.; Rakwal, A.; Chauhan, S.; Bhargava, B. Exogenous applications of gibberellic acid modulate the growth, flowering and longevity of calla lily. Heliyon 2023, 9, 5. [Google Scholar] [CrossRef]
- Han, Z.; Jin, Y.; Wang, B.; Guo, Y. Multi-omics revealed the molecular mechanism of Maize (Zea mays L.) seed germination regulated by GA3. Agronomy 2023, 13, 1929. [Google Scholar] [CrossRef]
- Fu, J.; Li, L. Effect of gibberellic acid on photosynthesis and oxidative stress response in maize under weak light conditions. Front. Plant Sci. 2023, 14, 1128780. [Google Scholar] [CrossRef]
- Shahzad, K.; Hussain, S.; Arfan, M.; Hussain, S.; Waraich, E.A.; Zamir, S.; El-Esawi, M.A. Exogenously applied gibberellic acid enhances growth and salinity stress tolerance of maize through modulating the morpho-physiological, biochemical and molecular attributes. Biomolecules 2021, 11, 1005. [Google Scholar] [CrossRef]
- Rodríguez, A.A.; Stella, A.M.; Storni, M.M.; Zulpa, G.; Zaccaro, M.C. Effects of cyanobacterial extracellular products and gibberellic acid on salinity tolerance in Oryza sativa L. Saline Syst. 2006, 2, 7. [Google Scholar] [CrossRef]
- Milani, P.G.; Formigoni, M.; Lima, Y.C.; Piovan, S.; Peixoto, G.M.L.; Camparsi, D.M.; da Costa, S.C. Fortification of the whey protein isolate antioxidant and antidiabetic activity with fraction rich in phenolic compounds obtained from Stevia rebaudiana (Bert.). Bertoni leaves. J. Food Sci. Technol. 2017, 54, 2020–2029. [Google Scholar] [CrossRef]
- Janah, I.; Meddich, A.; Elhasnaoui, A.; Khayat, S.; Anli, M.; Boutasknit, A.; Loutfi, K. Arbuscular mycorrhizal fungi mitigates salt stress toxicity in Stevia rebaudiana Bertoni through the modulation of physiological and biochemical responses. J. Soil Sci. Plant Nut. 2021, 23, 152–162. [Google Scholar] [CrossRef]
- Ghorbani, A.; Ghasemi-Omran, V.O.; Chen, M. The effect of glycine betaine on nitrogen and polyamine metabolisms, expression of glycoside-related biosynthetic enzymes, and K/Na balance of stevia under salt stress. Plants 2023, 12, 1628. [Google Scholar] [CrossRef]
- Yin, L.; Wang, S.; Tanaka, K.; Fujihara, S.; Itai, A.; Den, X.; Zhang, S. Silicon-mediated changes in polyamines participate in silicon-induced salt tolerance in Sorghum bicolor L. Plant Cell Environ. 2016, 39, 245–258. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1. [Google Scholar] [CrossRef]
- Turner, N.C.; Begg, J.E. Plant-water relations and adaptation to stress. Plant Soil. 1981, 58, 97–131. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.A.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil. 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Rao, K.M.; Sresty, T.V.S. Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci. 2000, 157, 113–128. [Google Scholar]
- Lutts, S.; Kinet, J.M.; Bouharmont, J. NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann. Bot. 1996, 78, 389–398. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A.J.P.S. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Hori, K.; Wada, A.; Shibuta, T. Changes in phenoloxidase activities of the galls on leaves of Ulmus davidana formed by Tetraneura fuslformis (Homoptera: Eriosomatidae). Appl. Entomol. Zool. 1997, 32, 365. [Google Scholar] [CrossRef]
- Oktay, M.; Küfreviolu, I.; Kocaçalişkan, I.; Şaklrolu, H. Polyphenoloxidase from Amasya apple. J. Food Sci. 1995, 60, 494–496. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Montavon, P.; Kukic, K.R.; Bortlik, K. A simple method to measure effective catalase activities: Optimization, validation, and application in green coffee. Anal. Biochem. 2007, 360, 207–215. [Google Scholar] [CrossRef]
- Farsaraei, S.; Moghaddam, M.; Pirbalouti, A.G. Changes in growth and essential oil composition of sweet basil in response of salinity stress and superabsorbents application. Sci. Hortic. 2020, 271, 109465. [Google Scholar] [CrossRef]
- Al-Taisan, W.A.A.; Alabdallah, N.M.; Almuqadam, L. Moringa leaf extract and green algae improve the growth and physiological attributes of Mentha species under salt stress. Sci. Rep. 2022, 12, 14205. [Google Scholar] [CrossRef]
- Janah, I.; Elhasnaoui, A.; Issa Ali, O.; Lamnai, K.; Aissam, S.; Loutfi, K. Physiochemical responses of Stevia rebaudiana Bertoni subjected to sodium chloride (NaCl) salinity and exogenous salicylic acid application. Gesunde Pflanz. 2021, 73, 509–520. [Google Scholar] [CrossRef]
- Tuna, A.L.; Kaya, C.; Dikilitas, M.; Higgs, D. The combined effects of gibberellic acid and salinity on some antioxidant enzyme activities, plant growth parameters and nutritional status in maize plants. Environ. Exp. Bot. 2008, 62, 1–9. [Google Scholar] [CrossRef]
- Sharan, A.; Dkhar, J.; Singla-Pareek, S.L.; Pareek, A. Crosstalk between gibberellins and abiotic stress tolerance machinery in plants. Mech. Plant Horm. Signal. Under Stress 2017, 1, 101–126. [Google Scholar]
- Llanes, A.; Biava, S.; Travaglia, C.; Masciarelli, O.; Luna, V. Do Gibberellins Mediate Growth Responses of the Halophytic Woody Prosopis Strombulifera (Lam.) Benth Plants Exposed to Different Sodium Salts? J. Plant Growth Regul. 2023, 42, 2545–2557. [Google Scholar] [CrossRef]
- Shah, S.H.; Islam, S.; Mohammad, F.; Siddiqui, M.H. Gibberellic acid: A versatile regulator of plant growth, development and stress responses. J. Plant Growth Regul. 2023, 42, 7352–7373. [Google Scholar] [CrossRef]
- Abdel-Hamid, A.M.; Mohamed, H.I. The effect of the exogenous gibberellic acid on two salt stressed barley cultivars. Eur. Sci. J. 2014, 10, 6. [Google Scholar]
- Singh, N.B.; Singh, A.; Khare, S.; Yadav, V.; Bano, C.; Yadav, R.K. Mitigating strategies of gibberellins in various environmental cues and their crosstalk with other hormonal pathways in plants: A review. Plant Mol. Biol. Report. 2021, 39, 34–49. [Google Scholar]
- Gao, X.H.; Xiao, S.L.; Yao, Q.F.; Wang, Y.J.; Fu, X.D. An updated GA signaling ‘relief of repression’ regulatory model. Mol. Plant 2011, 4, 601–606. [Google Scholar] [CrossRef]
- Banerjee, A.; Roychoudhury, A. The regulatory signaling of gibberellin metabolism and its crosstalk with phytohormones in response to plant abiotic stresses. In Plant Signaling Molecules; Woodhead Publishing: Sawston, UK, 2019; pp. 333–339. [Google Scholar]
- Colebrook, E.H.; Thomas, S.G.; Phillips, A.L.; Hedden, P. The role of gibberellin signalling in plant responses to abiotic stress. J. Exp. Boil. 2014, 217, 67–75. [Google Scholar] [CrossRef]
- Hamayun, M.; Khan, S.A.; Khan, A.L.; Shin, J.H.; Ahmad, B.; Shin, D.H.; Lee, I.J. Exogenous gibberellic acid reprograms soybean to higher growth and salt stress tolerance. J. Agric. Food Chem. 2010, 58, 7226–7232. [Google Scholar] [CrossRef]
- Javid, M.G.; Sorooshzadeh, A.; Moradi, F.; Modarres Sanavy, S.A.M.; Allahdadi, I. The role of phytohormones in alleviating salt stress in crop plants. Aust. J. Crop Sci. 2011, 5, 726. [Google Scholar]
- Rafique, M.; Naveed, M.; Mustafa, A.; Akhtar, S.; Munawar, M.; Kaukab, S.; Salem, M.Z. The combined effects of gibberellic acid and rhizobium on growth, yield and nutritional status in chickpea (Cicer arietinum L.). Agronomy 2021, 11, 105. [Google Scholar] [CrossRef]
- Wang, Y.H.; Zhang, G.; Chen, Y.; Gao, J.; Sun, Y.R.; Sun, M.F.; Chen, J.P. Exogenous application of gibberellic acid and ascorbic acid improved tolerance of okra seedlings to NaCl stress. Acta Physiol. Plant 2019, 41, 93. [Google Scholar] [CrossRef]
- Arruda, T.F.D.L.; Lima, G.S.D.; Silva, A.A.R.D.; Azevedo, C.A.V.D.; Souza, A.R.D.; Soares, L.A.D.A.; Saboya, L.M.F. Salicylic acid as a salt stress mitigator on chlorophyll fluorescence, photosynthetic pigments, and growth of precocious-dwarf cashew in the post-grafting phase. Plants 2023, 12, 2783. [Google Scholar] [CrossRef]
- Ali, A.Y.A.; Ibrahim, M.E.H.; Zhou, G.; Nimir, N.E.A.; Elsiddig, A.M.I.; Jiao, X.; Elradi, S.B.M. Gibberellic acid and nitrogen efficiently protect early seedlings growth stage from salt stress damage in Sorghum. Sci. Rep. 2021, 11, 6672. [Google Scholar] [CrossRef]
- Keawmanee, N.; Ma, G.; Zhang, L.; Yahata, M.; Murakami, K.; Yamamoto, M.; Kato, M. Exogenous gibberellin induced regreening through the regulation of chlorophyll and carotenoid metabolism in Valencia oranges. Plant Physiol. Biochem. 2022, 173, 14–24. [Google Scholar] [CrossRef]
- Youssef, M.H.; Raafat, A.; El-Yazied, A.A.; Selim, S.; Azab, E.; Khojah, E.; El Nahhas, N.; Ibrahim, M.F. Exogenous Application of Alpha-Lipoic Acid Mitigates Salt-Induced Oxidative Damage in Sorghum Plants through Regulation Growth, Leaf Pigments, Ionic Homeostasis, Antioxidant Enzymes, and Expression of Salt Stress Responsive Genes. Plants 2021, 10, 2519. [Google Scholar] [CrossRef]
- Fregonezi, B.F.; Pereira, A.E.; Ferreira, J.M.; Fraceto, L.F.; Gomes, D.G.; Oliveira, H.C. Seed priming with nanoencapsulated gibberellic acid triggers beneficial morphophysiological and biochemical responses of tomato plants under different water conditions. Agronomy 2024, 14, 588. [Google Scholar] [CrossRef]
- Dinler, B.S.; Cetinkaya, H.; Akgun, M.; Korkmaz, K. Simultaneous treatment of different gibberellic acid doses induces ion accumulation and response mechanisms to salt damage in maize roots. J. Plant Biochem. Physiol. 2021, 9, 258. [Google Scholar]


| Shoot Height (cm) | Shoot Dry Weight (g) | Root Dry Weight (g) | Leaf Area (cm2/Plant) | ||
|---|---|---|---|---|---|
| 0 mM NaCl | G0 | 65.66 ± 2.31 b | 4.17 ± 0.10 c | 4.80 ± 0.07 b | 629.11 ± 12.8 c |
| G1 | 81.65 ± 3.28 a | 5.01 ± 0.09 b | 5.66 ± 0.04 a | 671.03 ± 16.20 b | |
| G2 | 82.50 ± 2.16 a | 5.48 ± 0.11 a | 5.68 ± 0.10 a | 680.54 ± 12.16 a | |
| 80 mM NaCl | G0 | 38.82 ± 1.78 e | 1.51 ± 0.08 e | 2.30 ± 0.05 d | 316.16 ± 14.11 f |
| G1 | 42.00 ± 1.12 d | 2.73 ± 0.07 d | 3.31 ± 0.06 c | 498.23 ± 10.43 e | |
| G2 | 60.43 ± 1.47 c | 3.28 ± 0.11 d | 3.34 ± 0.02 c | 520.07 ± 11.52 d |
| Sodium Content (mg/g DW) | Potassium Content (mg/g DW) | Calcium Content (mg/g DW) | K/Na Ratio | ||
|---|---|---|---|---|---|
| 0 mM NaCl | G0 | 9.99 ± 0.18 d | 26.99 ± 0.32 b | 5.97 ± 0.09 c | 2.71 ± 0.02 b |
| G1 | 10.04 ± 0.12 d | 28.30 ± 0.12 a | 7.44 ± 0.03 b | 2.81 ± 0.03 a | |
| G2 | 10.07 ± 0.15 d | 28.46 ± 0.53 a | 7.91 ± 0.13 a | 2.79 ± 0.01 a | |
| 80 mM NaCl | G0 | 22.17 ± 1.09 a | 14.16 ± 0.11 e | 3.01 ± 0.11 f | 0.63 ± 0.04 e |
| G1 | 17.54 ± 0.22 b | 18.15 ± 0.82 d | 4.76 ± 0.10 e | 1.03 ± 0.02 d | |
| G2 | 16.15 ± 0.51 c | 20.47 ± 0.10 c | 5.10 ± 0.07 d | 1.26 ± 0.03 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janah, I.; Ben-Laouane, R.; Elhasnaoui, A.; Anli, M.; Meddich, A. The Induction of Salt Stress Tolerance by Gibberellic Acid Treatment in Stevia rebaudiana Bertoni Plants. Int. J. Plant Biol. 2024, 15, 505-516. https://doi.org/10.3390/ijpb15020038
Janah I, Ben-Laouane R, Elhasnaoui A, Anli M, Meddich A. The Induction of Salt Stress Tolerance by Gibberellic Acid Treatment in Stevia rebaudiana Bertoni Plants. International Journal of Plant Biology. 2024; 15(2):505-516. https://doi.org/10.3390/ijpb15020038
Chicago/Turabian StyleJanah, Iman, Raja Ben-Laouane, Abdelhadi Elhasnaoui, Mohamed Anli, and Abdelilah Meddich. 2024. "The Induction of Salt Stress Tolerance by Gibberellic Acid Treatment in Stevia rebaudiana Bertoni Plants" International Journal of Plant Biology 15, no. 2: 505-516. https://doi.org/10.3390/ijpb15020038






