Abstract
Grafting is a traditional research and crop production technique used to study the long-distance movement of molecules, reduce disease susceptibility, and improve yield, quality, and nutrient content. Tomato/potato grafts are rare examples of successful interspecies grafting, even resulting in commercially available products. Nevertheless, information on the effect of tomato on the quality parameters of potato tubers is scarce. In this study, the tomato cultivar ‘Mobil’ was grafted with the potato cultivars ‘White Lady’, ‘Hópehely’, and ‘Désirée’, and the phenotype, metabolite composition, and starch and protein contents of the tubers were analysed. Anthocyanins were isolated from the tuber skins, and the expression level of the transcription factor ANTHOCYANIN1 (StAN1) was evaluated. Out of the 112 identified metabolites, the concentrations of twelve compounds were altered in the same direction in all three cultivars. Compared to the self-grafted control, the starch content of tubers was increased in each cultivar, while the protein level remained unaltered in ‘White Lady’ and ‘Hópehely’. The oval tubers became roundish. The tomato scion increased the anthocyanin content of ‘Hópehely’ and ‘Désirée’ tuber skins, which was correlated with the upregulation of StAN1 expression. These results indicate that tomato scion has a significant impact on the quality parameters of potato tubers.
1. Introduction
Grafting is a well-known technique used to improve plant production and reduce disease susceptibility. Grafting is the amalgamation of rootstock and scion so that a vascular continuity is established and the resulting genetically composite organism functions as a single plant [1]. Grafting within a genus is often successful, but the success rate is low within families with the exception of Solanaceae. The best-known example of successful solanaceous interspecies grafting is when tomato scions are grafted to potato rootstocks producing the commercially available TomTato or Ketchup ‘n’ Fries plants, which grow tomato fruits on shoots and potato tubers underground [2].
The first studies on tomato/potato grafts aimed to transfer biotic resistance or study virus transmission [3,4,5]. Then, the interest turned into the spread of mycoplasmas and the distribution of photosynthates and minerals in grafted tomato/potato plants [6,7,8]. Mill studied the distribution of steroidal glycoalkaloids in reciprocal grafts and established that alkaloid transport between roots and shoots does not take place between potato and tomato [9]. Furthermore, grafting experiments demonstrated that the root system plays an important role in defence mechanisms against Verticillium-induced disease in both tomato and potato [10]. Tomato/potato grafts were used to confirm that there is a locus originating from Solanum stoloniferum, called Rysto, which confers broad-spectrum tolerance to potyviruses [11]. Peres et al. used hormone and photomorphogenic tomato mutants to study the process of tuberization through grafting onto potato rootstocks [12]. Tuber number, tuber and shoot dry weight, and sprouting were quantified, and it was concluded that tomato scions are less effective in promoting tuberization than potato scions and that the gibberellin-deficient scion acts as a sprouting inducer. Grafting of tomato on potato, however, in certain variety combinations may have a positive effect on yield, as shown by Negi et al. with rootstock–scion combinations of ‘Kufri Himalini’ potato and ‘GS-600’ tomato [13] and by Arefin et al. with other tomato/potato variety grafts [14]. Zhang and Guo tested the effect of the ‘Zhongyan988’ tomato cultivar on four potato cultivars and found that some characteristics were related to tomato fruit quality traits, i.e., vitamin C and total soluble solids and soluble sugar contents were improved by the potato rootstocks, while the titratable acidity content decreased in fruits of tomato scions [15]. Grafting decreased the tuber number per plant and promoted tuber sprouting. The reducing sugar content of tubers increased, while their starch and vitamin C contents decreased. Transcriptome profiling of ‘Zhongyan988’ tomato and ‘Qingshu9’ potato grafts resulted in the detection of 209 upregulated genes in tomato, some of them related to starch and sucrose biosynthesis, and 1529 up- and 1329 downregulated genes in potato, including genes involved in plant hormone signal transduction [16]. The effect of tomato scions on the potato tuber metabolome, however, has not been investigated thus far.
The primary objective of this study was to assess the influence of the tomato scion on the metabolite composition of tubers grown on potato rootstocks. By testing three different potato cultivars in combination with one tomato cultivar we seek to uncover new insights into the general and cultivar-specific effects of tomato on potato. The results presented below indicate that tomato had a major influence on the metabolite composition of tubers, including the same directional changes in the concentration of twelve metabolites.
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
Three commercial potato (Solanum tuberosum L.) cultivars were used in the experiments. ‘White Lady’ and ‘Hópehely’ originated from the Potato Breeding Centre (Keszthely, Hungary), whereas ‘Désirée’ was obtained from the Fritz Lange KG (Bad Schwartau, Germany). The plants were propagated in vitro from stem segments in MS medium [17] without vitamins (rooting medium, RM) containing 2% (w/v) sucrose and 0.8% agar in 40 mL tubes closed with paper plugs at 24 °C under a photoperiod cycle of 16 h/8 h light/dark at a light intensity of 75 μmol m−2 s−1. Three-week-old plantlets were transplanted into 8 cm diameter pots in Tabaksubstrat sterile soil A200 (Stender GmbH, Schermbeck, Germany) and incubated under a transparent plastic cover in a greenhouse. Seeds from the tomato (Solanum lycopersicum L.) cultivar ‘Mobil’ were sown on the day the in vitro propagation of potato stems began. Tomato seeds were germinated in pots in the same soil as potatoes under greenhouse conditions (18–24 °C, 12 h/12 h light/dark period, ambient light conditions supplemented with artificial lighting by sodium lamps, regular optimal irrigation, and application of pesticides and fungicides for pest and fungal pathogen control). After the potatoes were acclimatized in the greenhouse for one week, splice grafting was carried out. By that time, the tomato shoots were around 10 cm high and 2 mm in diameter with 3–4 compound leaves on them. The tomato shoots were cut just above the ground. Two- to three-centimetre-long potato stems without leaves served as rootstocks and were wrapped in the scions with rubber clips. After grafting, the plants were kept in a plastic box under high humidity in the dark for four days and for an additional ten days under greenhouse conditions before transferring them into larger pots with a 14 cm diameter top and 14 cm depth. The tubers were harvested at the end of the potato vegetation period. After harvesting, tubers were visually evaluated for shape and colour, counted per plant, measured for mass, and sampled for the analysis described below.
2.2. Metabolite Extraction and Profiling
Metabolite extraction and profiling of radial slices cut from the middle of the tubers was carried out as described in [18]. Briefly, after extraction of the nitrogen-frozen tuber powder with methanol followed by the addition of water and chloroform (2:1), derivatization of the resulting two different phases was performed in separate tubes. Ribitol and methyl nonadecanoate were added as internal standards to the metabolites in the water and chloroform phases, respectively. Derivatization of compounds was based on the method of Shepherd et al. [19]. The water soluble compounds were incubated with MEOX (methoxyamine hydrochloride) and MSTFA (N-methyl-N-(trimethylsilyl) trifluoroacetamide), while the nonpolar metabolites were incubated in chloroform, pyridine, and MSTFA. For metabolite analysis, a quadrupole-type GC–MS system (Finnigan Trace GC/DSQ, Thermo Electron Corp., Austin, TX, USA) equipped with a TG-5MS capillary column (30 m × 0.25 mm × 0.25 μm, Thermo Scientific, Waltham, MA, USA), an AI/AS 3000 autosampler, and an electron ionization source (EI) was used. Helium served as the carrier gas. Scans were made in positive ion mode and with total ion chromatogram (TIC) mode set in the mass range between 50 and 650 m/z. During data acquisition, a ramping temperature programme was run.
2.3. Data Transformation and Metabolite Identification
The metabolite data were processed using the Xcalibur Data System Software 1.4.1 SP3 (Thermo Finnigan, San Jose, CA, USA). The relative quantitative analysis was carried out by comparing the peak area of the compound of interest to the peak area of the internal standard. For metabolite identification, C7–C40 saturated alkane standard (Supelco, Bellefonte, PA, USA) measurements were applied, and the retention indices (RIs) of each peak were calculated on the basis of linear interpolation. The NIST MS Search 2.0 software was used for metabolite searches, using mass spectra and RI values for accurate metabolite identification.
2.4. Determination of the Starch Content
The same sets of samples that were used for metabolite extraction were used for starch content measurement. The Starch Assay Kit STA20 (Sigma–Aldrich, St. Louis, MO, USA) was used for tuber starch extraction according to the manufacturer’s instructions, starting from 50 mg of frozen tuber powder. One tenth of the extracted starch was digested with α-amylase and amyloglucosidase reagents. Glucose assays were carried out in microtiter plates. The absorbance was measured at 540 nm using a Clariostar plate reader spectrophotometer (BMG Labtech, Ortenberg, Germany). A calibration curve was prepared from the corn starch available in the Starch Assay Kit, and the starch concentration of potato tubers in mg g−1 fresh weight was predicted based on the calibration curve.
2.5. Determination of the Protein Content
For protein extraction, the method reported in [20] was used with minor modifications. One hundred milligrams of frozen tuber powder from the same sample collection used for metabolite analysis was transferred into a 1.5 mL Eppendorf tube and mixed with 200 µL of extraction buffer containing 50 mM Tris-HCl buffer (pH 8), 0.1 g mL−1 PPVP (polyvinyl polypyrrolidine), 1 mM PMSF (phenylmethylsulfonyl fluoride), and 10 mM 2-mercaptoethanol. The tubes were incubated with continuous shaking at 500 rpm for 25 min at 4 °C, and then, centrifuged for 10 min at 13,000 rpm. Two hundred microlitres of the supernatant was carefully pipetted into a new tube and mixed with 600 µL of ice-cold acetone. Samples were incubated for 20 min at 4 °C with continuous shaking at 350 rpm. Measurements were made using the Bradford assay reagent [21]. Optical density values were measured at 595 nm using a Labsystems Multiskan EX (Thermo Scientific, Waltham, MA, USA) spectrophotometer. Standard measurements for the calibration curve were implemented using different dilutions of 10 mg mL−1 bovine serum albumin (Promega, Wisconsin, MA, USA).
2.6. Determination of the Total Anthocyanin Content
Skins of the same tubers tested for metabolite composition were collected. Only the outermost epidermal layers of the tubers were carefully pulled down without polluting the samples with tuber flesh. A simplified version of the method described in [22] was used for anthocyanin extraction. One gram of skin per sample was incubated in 10 mL of 1% HCl in methanol overnight at 4 °C. Relative concentrations of the chloride forms of the anthocyanin pigments were determined spectrophotometrically by measuring the OD values at 540 nm using a Jenway 6405 UV/VIS spectrophotometer (Jenway Technology Company, Sheung Wan, Hong Kong).
2.7. Sucrose Treatment of In Vitro-Grown Potato Plants
For sucrose treatment of potatoes, the method described in [23] was applied. Internodes with leaves from 1-month-old in vitro plants from each cultivar were propagated in RM medium supplemented with 0 or 120 mM sucrose and grown for 6 days in the culture room under the conditions described in Section 2.1. Two sets of leaf samples each from 5 plants were collected and frozen immediately in liquid nitrogen. The samples were stored at −70 °C until RNA extraction.
2.8. Gene Expression Analysis
The frozen leaf samples of in vitro plantlets were ground in liquid nitrogen. The protocol described in [24] was used for total RNA isolation from 150 mg of leaf sample. In the case of tuber skin, a TRIzol-based protocol was applied for RNA isolation [25]. The purified RNAs from both leaves and tuber skins were dissolved in 30 μL of distilled water, and the concentration was measured at OD260 using an ND-1000 nanodrop spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Complementary DNA (cDNA) was synthesized using the Maxima H minus First Strand cDNA Synthesis Kit with dsDNAse according to the manufacturer’s (Thermo Scientific Molecular Biology, Waltham, MA, USA) instructions.
Expression analysis of the ANTHOCYANIN1 (StAN1) transcription factor involved in the regulation of anthocyanin biosynthesis genes was conducted with quantitative reverse transcription PCR (RT–qPCR) applying ACTIN (Fw 5′ TGGACTCTGGTGATGGTGTG 3′, R 5′ GGTTTCAAGTTCCTGTCTGT 3′) as a reference gene [26]. The same primer pair used in [23] was used in our experiments for the detection of StAN1 expression (Fw 5′ GAGAAGCTGAAAGAACACTACCT 3′, R 5′ CACCATCACCTTGTCCTTGT 3′). The RT–qPCR assays were performed in a Light Cycler-96 thermal cycler (Roche Diagnostics GmbH, Mannheim, Germany) with the Xpert Fast SYBR (uni) 2× Master mix (GRiSP Research Solutions, Porto, Portugal). The transcript level of the StAN1 gene was normalized relative to the transcript level of ACTIN and analysed using Light Cycler-96 software version 1.1 (Roche Diagnostics GmbH, Mannheim, Germany).
2.9. Statistics
Principal component analysis (PCA), dendrogram analysis and volcano plot analysis were carried out using the online MetaboAnalyst 5.0 software (https://www.metaboanalyst.ca/, accessed on 16 June 2023 for dendrogram, on 30 August for volvano plot and on 26 February 2024 for PCA analysis) and 6.0 software accessed on 5 April and 10 June 2024 for heatmaps. Median-normalized and log-transformed data were used for all statistical analyses, implemented by the MetaboAnalyst 5.0 and 6.0 interface. One-way ANOVA with a post hoc Tukey’s HSD test at an adjusted p value of 0.05 was used for analysis of the data shown in heatmaps and the dendrogram. For volcano plot analysis, all the self-grafting and heterografting data from the two groups were used for comparison. Metabolite data that were above the FC 2 and p ≤ 0.01 thresholds in the volcano plot analysis were used for subsequent pathway analysis in KEGG (Kyoto Encyclopedia of Genes and Genomes; https://www.kegg.jp/, accessed on 28 March 2024). Global test and relative betweenness centrality options were set in pathway analysis using the full relative concentration data table of the significant metabolites found in the self-grafted and heterografted treatment comparison. Pathways above the pathway impact threshold of p ≤ 0.01 were considered significant. Statistical significance of the tuber number and mass, starch, protein, anthocyanin, and gene expression measurements was determined by one-way ANOVA with a post hoc Tukey HSD test.
3. Results
3.1. Morphological Characteristics of the Tubers Grown on Tomato/Potato Grafts
To study the metabolite composition of tubers grown on the graft combination with tomato cultivar ‘Mobil’ (TOM), three potato cultivars, ‘White Lady’ (WL), ‘Hópehely’ (HP), and ‘Désirée’ (DES), were used. Selection of the potato cultivars was based on previous studies showing significant cultivar-specific differences in the metabolite composition of tubers [27,28,29], while selection of the tomato cultivar ‘Mobil’ was based on preliminary experiments showing that among the different cultivars tested, ‘Mobil’ was the most compatible with all three selected potato cultivars.
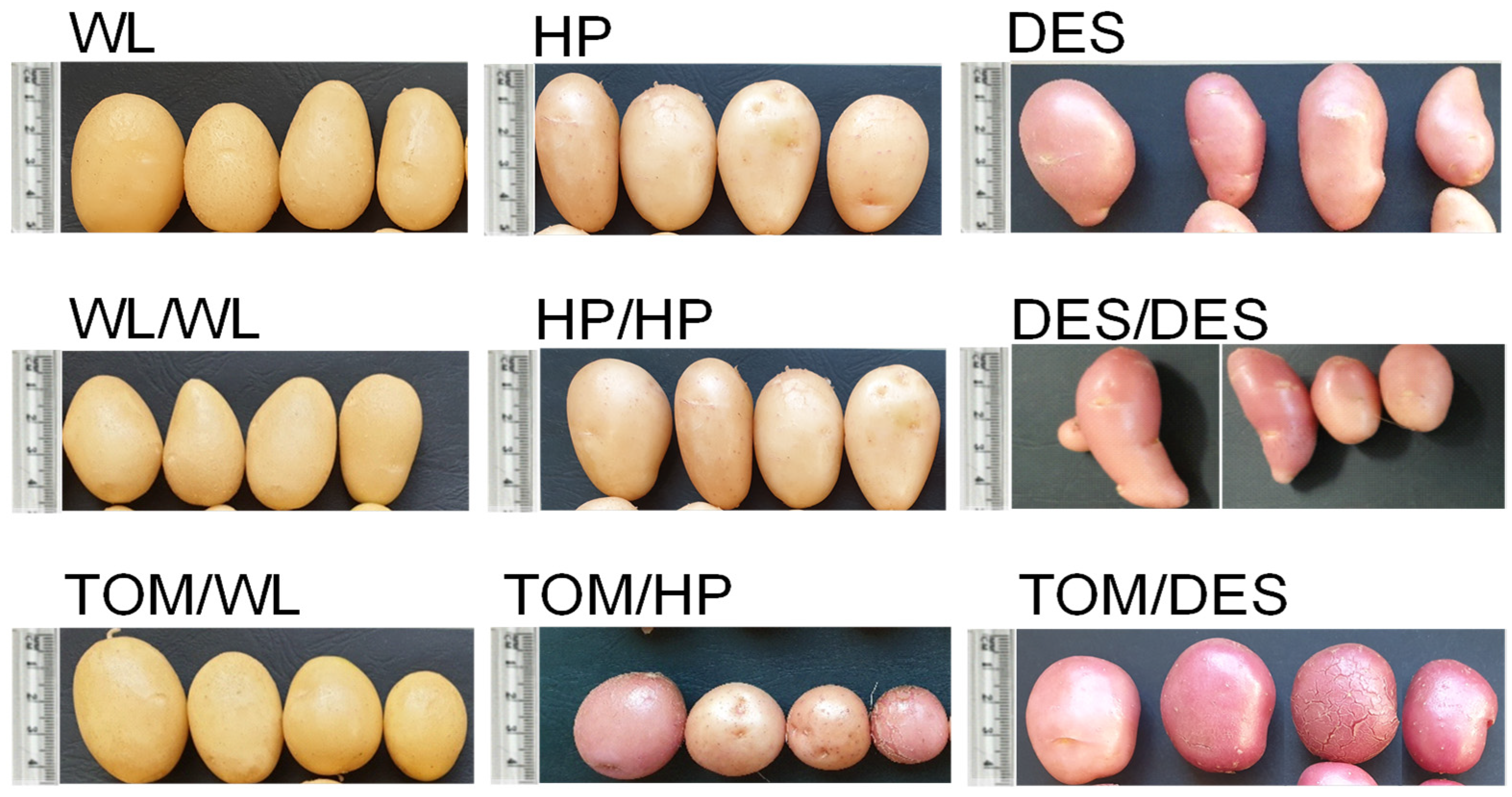
TOM scions grafted with WL (TOM/WL), HP (TOM/HP), and DES (TOM/DES) were grown in a greenhouse in pots simultaneously with self-grafted (WL/WL, HP/HP, DES/DES) and nongrafted (WL, HP, DES) potato controls. Eight potato plants from each cultivar were nongrafted, twelve potato plants were self-grafted as controls, and twenty tomato–potato heterografts were prepared because of the expected lower survivor rate of heterografted than self-grafted and nongrafted plants. Nevertheless, the grafting was so successful that with the exception of TOM/HP and TOM/DES all the grafted plants survived (Table 1). Tubers were harvested at the end of the potato vegetation period, when TOM scions have just started flowering, with ‘Mobil’ being a mid–long season variety. The tuber morphology of the three cultivars was different. WL tubers were oval with light beige skin, HP tubers were short-oval and beige, and DES tubers were long-oval and red-skinned. Self-grafting did not change the shape, colour, or yield of the tubers. In contrast, the TOM scion changed the yield, shape, and, in the case of HP and DES, the colour of the tubers. The tubers became roundish and, with the exception of WL, had intensified skin colour (Table 1 and Figure 1). Compared to the controls, the number of tubers grown on heterografts was approximately half as large, the tuber yield was reduced approximately also to half, while the mean size of the TOM/WL and TOM/DES tubers was slightly increased (Table 1).

Table 1.
Effect of grafting on survival and tuberization of plants compared to the nongrafted controls.
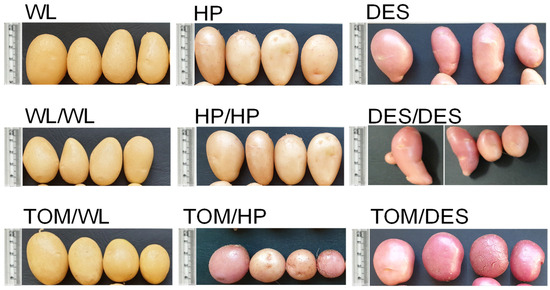
Figure 1.
The four largest tubers harvested from three potato cultivars and their grafts with tomato as a scion. WL, ‘White Lady’; WL/WL, self-grafted WL; TOM/WL, tomato grafted with ‘White Lady’; HP, ‘Hópehely’; HP/HP, self-grafted ‘Hópehely’; TOM/HP, tomato grafted with ‘Hópehely’; DES, ‘Désirée’; DES/DES, self-grafted ‘Désirée’; TOM/DES, tomato grafted with ‘Désirée’.
3.2. Metabolite Composition of Tubers Grown on Tomato/Potato Grafts
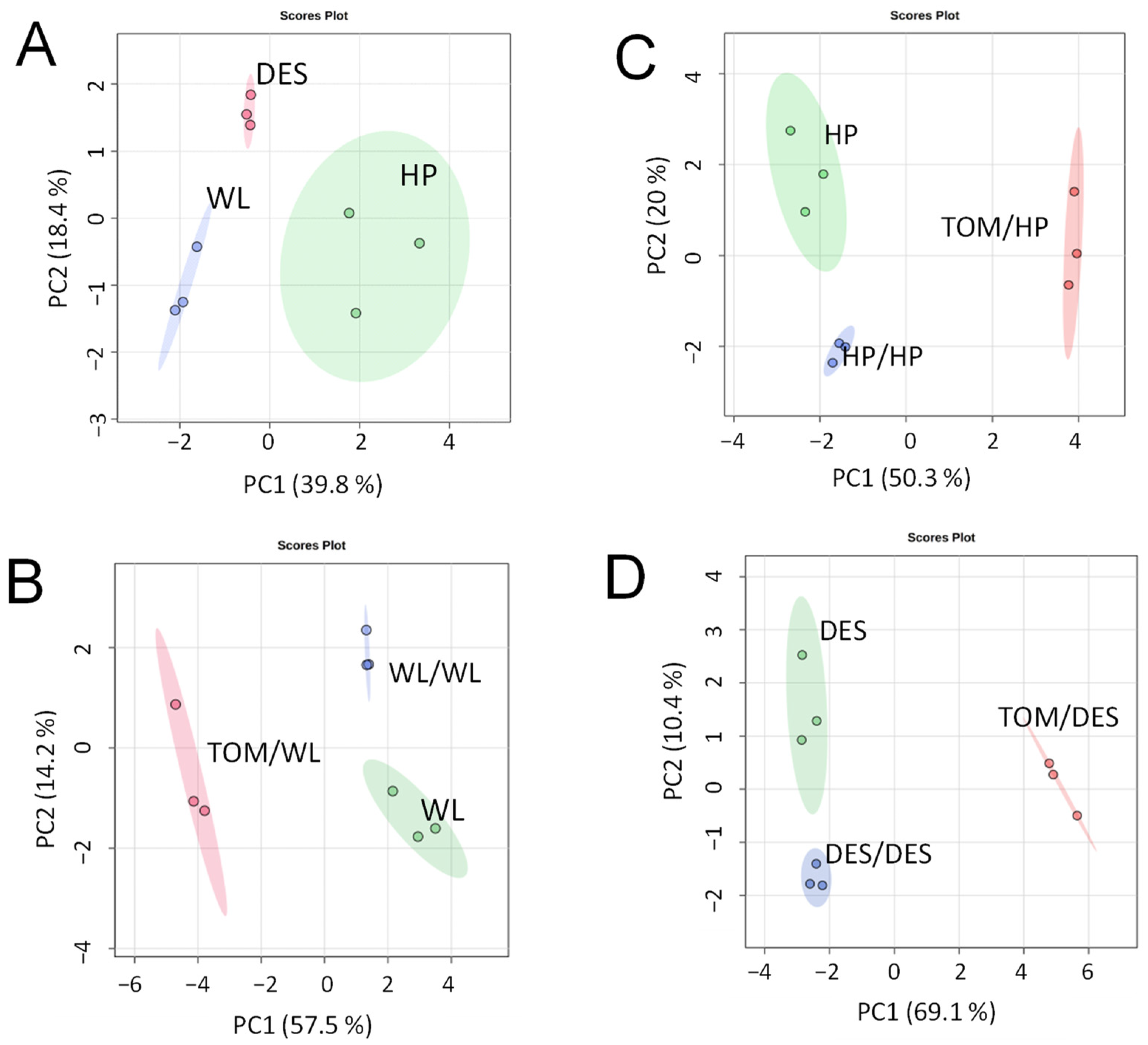
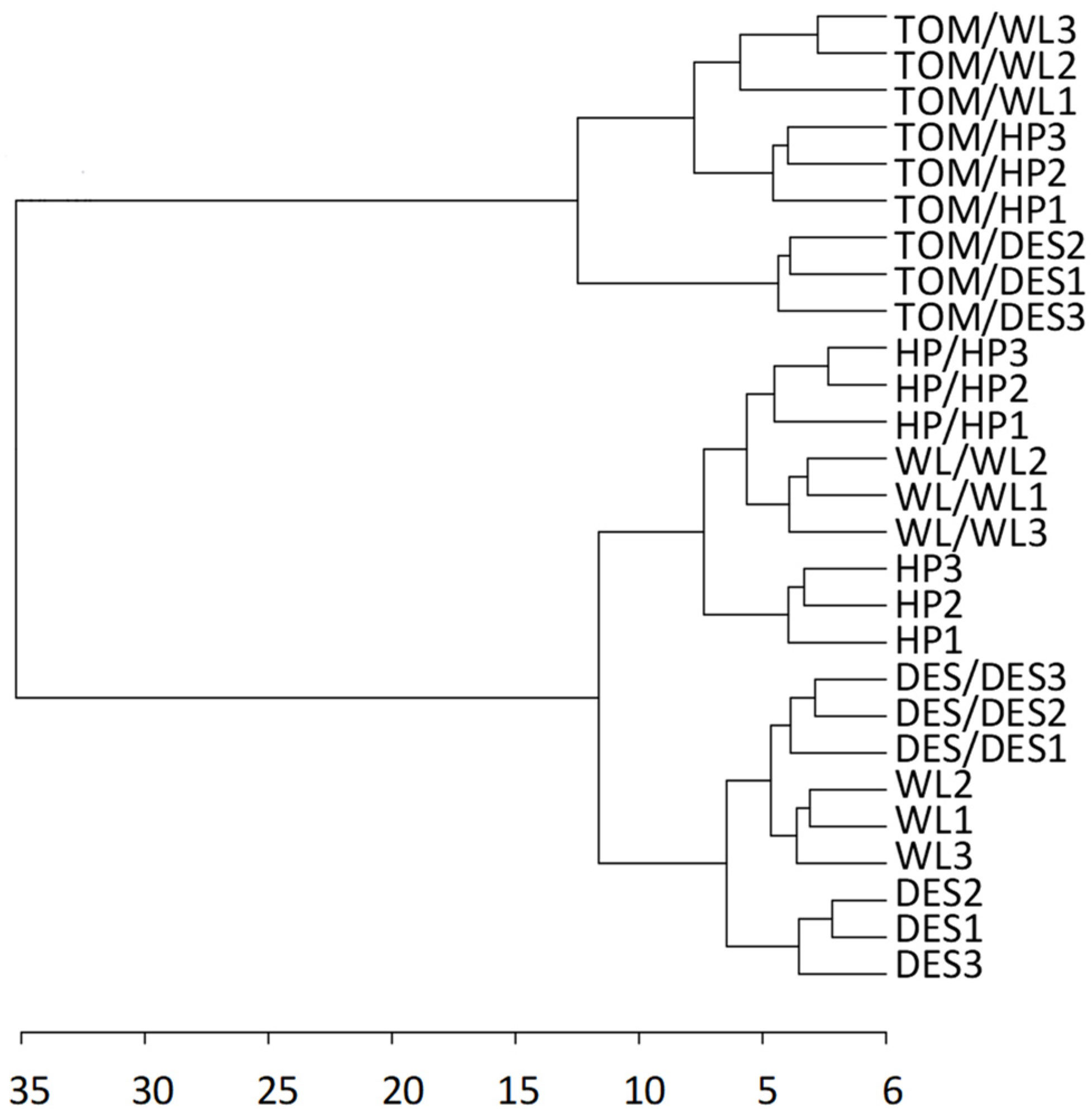
For metabolite analysis, samples were collected from the 12 largest freshly harvested tubers per plant category and divided into three approximately equal groups in terms of tuber size. The metabolite composition of tubers was analysed using GC–MS. A total of 280 metabolite peaks were observed, of which 112 (86 polar and 26 nonpolar compounds) could be identified using the NIST database. Tubers of nongrafted control plants of the three cultivars exhibited distinct groups of metabolite composition in principal component analysis (PCA; Figure 2A). Both self- and heterografting influenced the concentration of the tested compounds, as evidenced by the PCA clustering the tubers of nongrafted, self-grafted, and heterografted plants of each cultivar (Figure 2B–D). However, as shown in Figure 3, hierarchical clustering assigned only the tubers of heterografts into an individual branch in the dendrogram, indicating much less difference between the tubers of nongrafted and self-grafted tubers than between the tubers of self-grafted and heterografted plants. Within the hetergrafted branch, data measured in TOM/DES tubers made up a separate branch from the TOM/WL and TOM/HP tubers.
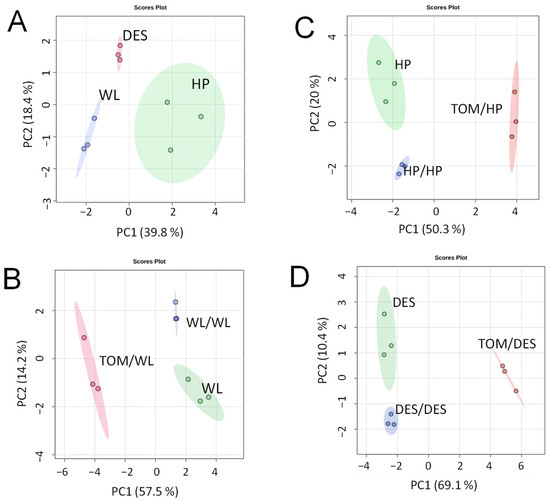
Figure 2.
Differences in metabolite composition of ‘White Lady’ (WL), ‘Hópehely’ (HP), and ‘Désirée (DES) tubers and their self- and tomato (TOM) graft tubers based on PCA scores. (A) Metabolic data of WL, HP, and DES tubers. (B) Metabolic data of WL, self-grafts WL/WL, and heterografts TOM/WL tubers. (C) Metabolic data of HP, self-grafts HP/HP, and heterografts TOM/HP tubers. (D) Metabolic data of DES, self-grafts DES/DES, and heterografts TOM/DES tubers. Dots indicate individual samples each containing metabolites extracted from a group of tubers each consisting of slices of four approximately same-size tubers.
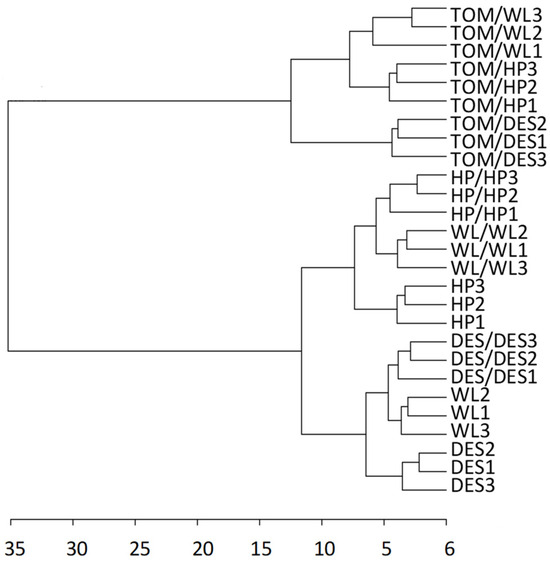
Figure 3.
Dendrogram showing the hierarchical clustering of the three potato cultivars and their self- and heterografts based on the metabolite composition of tubers. The labels are as in Figure 1. The dendrogram was prepared from the same dataset as in Figure 2 with Euclidean distance measure and ward clustering algorithm.
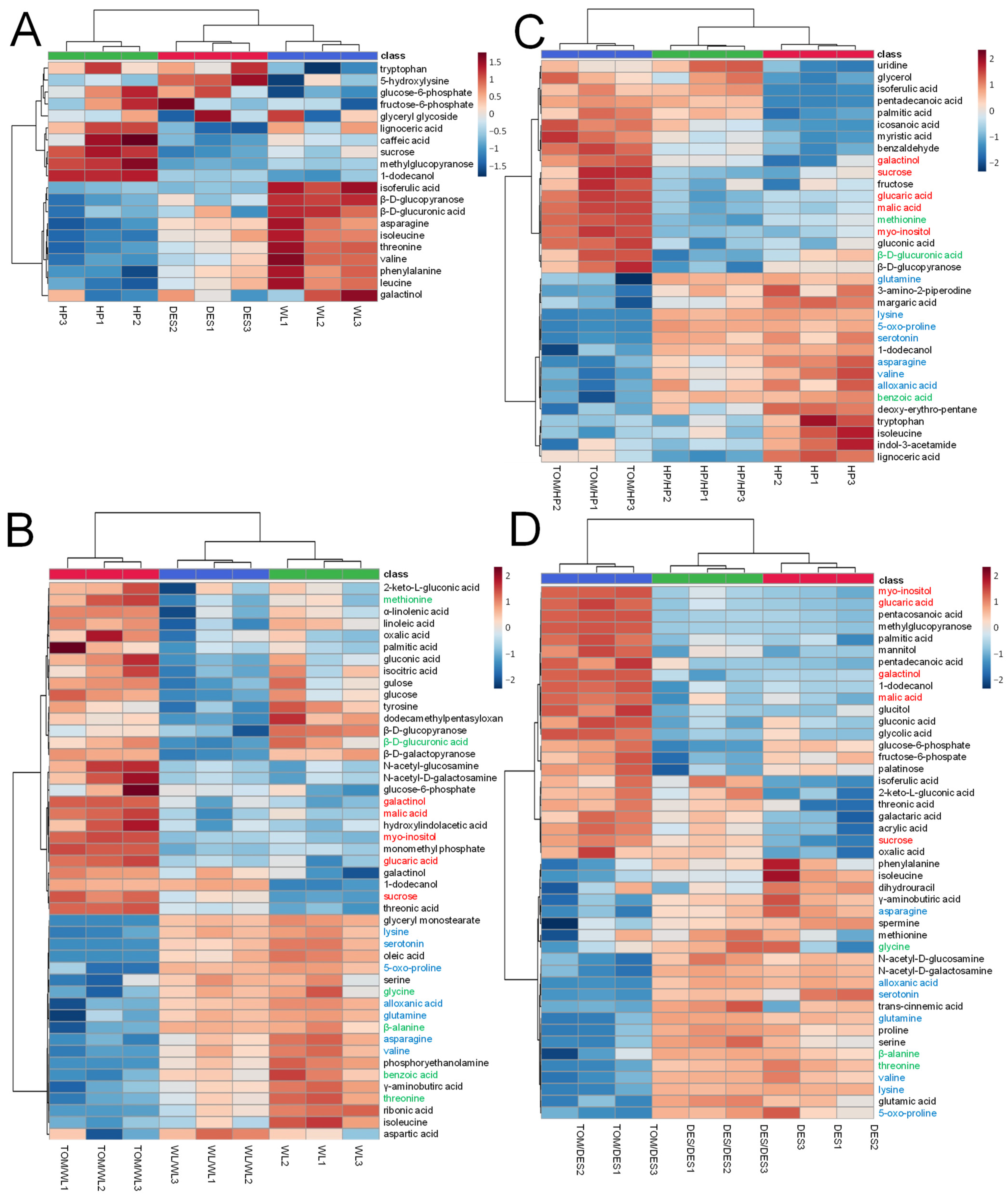
Characteristic differences in the amounts of 20 compounds were found between the cultivars. Tubers of DES and WL were rich in amino acids, tryptophan, asparagine, isoleucine, threonine, valine, phenylalanine, and leucine, while HP was poor in these compounds. In contrast to DES and WL tubers, the level of sucrose, methylglucopyranose 1-dodecanol, and lignoceric acid was high in HP tubers. Glucose-6-phosphate and fructose-6-phosphate concentrations in WL tubers were lower than in HP and DES tubers (Figure 4A).
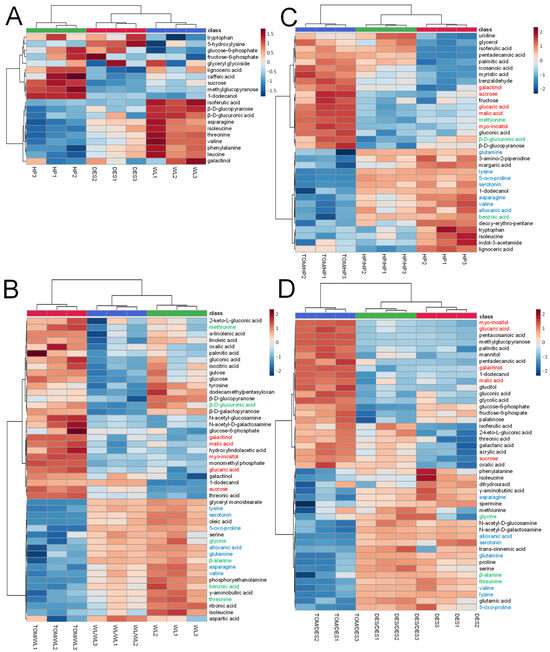
Figure 4.
Heatmap of metabolites significantly differentiating WL, HP, and DES tubers and their self- and TOM graft tubers. The labels are as in Figure 1. (A) Heatmap of WL, HP, and DES. (B) Heatmap of WL, self-grafts WL/WL, and heterografts TOM/WL tubers. (C) Heatmap of HP, self-grafts HP/HP, and heterografts TOM/HP tubers. (D) Heatmap of DES, self-grafts DES/DES, and heterografts TOM/DES tubers. The statistical analysis was carried out with the same dataset as in Figure 2. The statistics was based on one-way ANOVA with a post hoc Tukey’s HSD test at adjusted p value of 0.05. Metabolites increased and decreased in amount in each TOM graft compared to the corresponding self-graft are in red and blue, respectively. Metabolites evenly changed in concentration in heterografts of any two cultivars are in green.
The self-grafting affected the concentration of several compounds in each cultivar. WL/WL tubers were significantly decreased in concentrations of tyrosine, dodecamethylpentasyloxane, β-D-glucopyranose, β-D-glucuronic acid, and β-D-galactopyranose, while the amounts of 1-dodecanol, sucrose, and threonic acid were increased in all three sample groups of WL/WL tubers (Figure 4B). In the case of HP/HP tubers, the levels of uridine, glycerol, isoferulic acid, pentadecanoic acid, palmitic acid, icosanoic acid, myristic acid, and benzaldehyde were increased and β-D-glucopyranose decreased (Figure 4C). In DES/DES tubers, the major changes were detected in glucose-6-phosphate, fructose-6-phospate, and palatinose, which were decreased, and isoferulic acid, 2-keto-L-gluconic acid, threonic acid, galactaric acid, acrylic acid, sucrose, and oxalic acid, which were increased (Figure 4D).
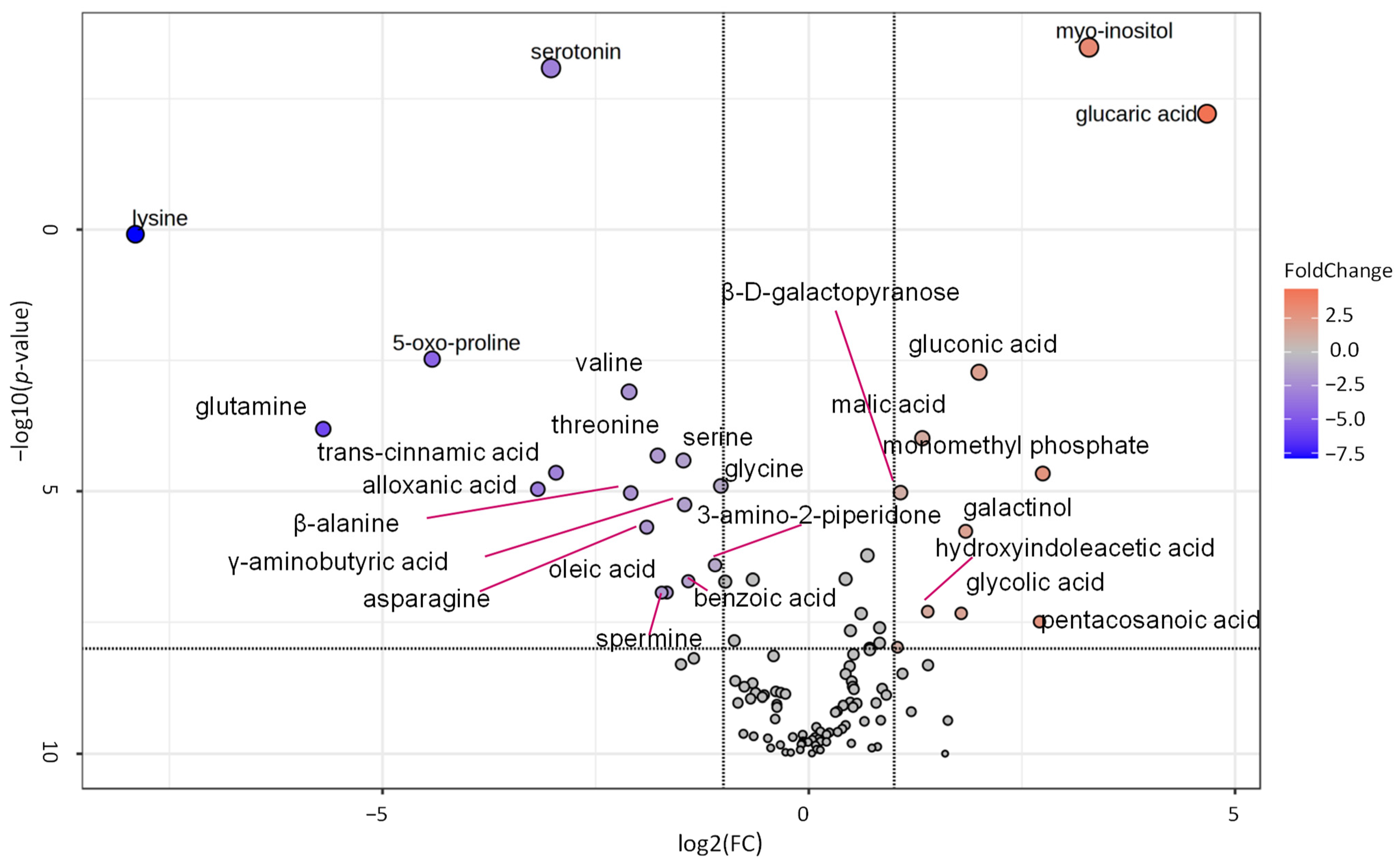
Compared to the tubers of the corresponding self-grafted controls, grafting with TOM significantly altered the concentration of 59 metabolites in the potato tubers. The levels of twelve compounds changed significantly (p ≤ 0.05) in the same direction promoted by TOM in all three cultivars (Figure 4B–D). Metabolites of carbon metabolism, glucaric acid, myo-inositol, galactinol, malic acid, and sucrose, were present in higher concentrations in tubers of TOM grafts than in tubers of self-grafts. Compounds with reduced levels were mainly amino acids, namely, asparagine, valine, 5-oxo-proline, glutamine, and lysine. In addition to amino acids, the levels of serotonin and alloxanic acid were reduced in the tubers of the heterografts of all three cultivars. Threonine, β-alanine, and glycine evenly decreased in WL and DES, whereas methionine, β-D-glucuronic acid, and benzoic acid were the components that evenly changed in WL and HP but remained unchanged in concentration in DES. Only 3 out of the 59 metabolites with significantly altered levels showed opposite alterations between any two cultivars.
3.3. Pathways Altered in Tubers Grown on Tomato/Potato Grafts
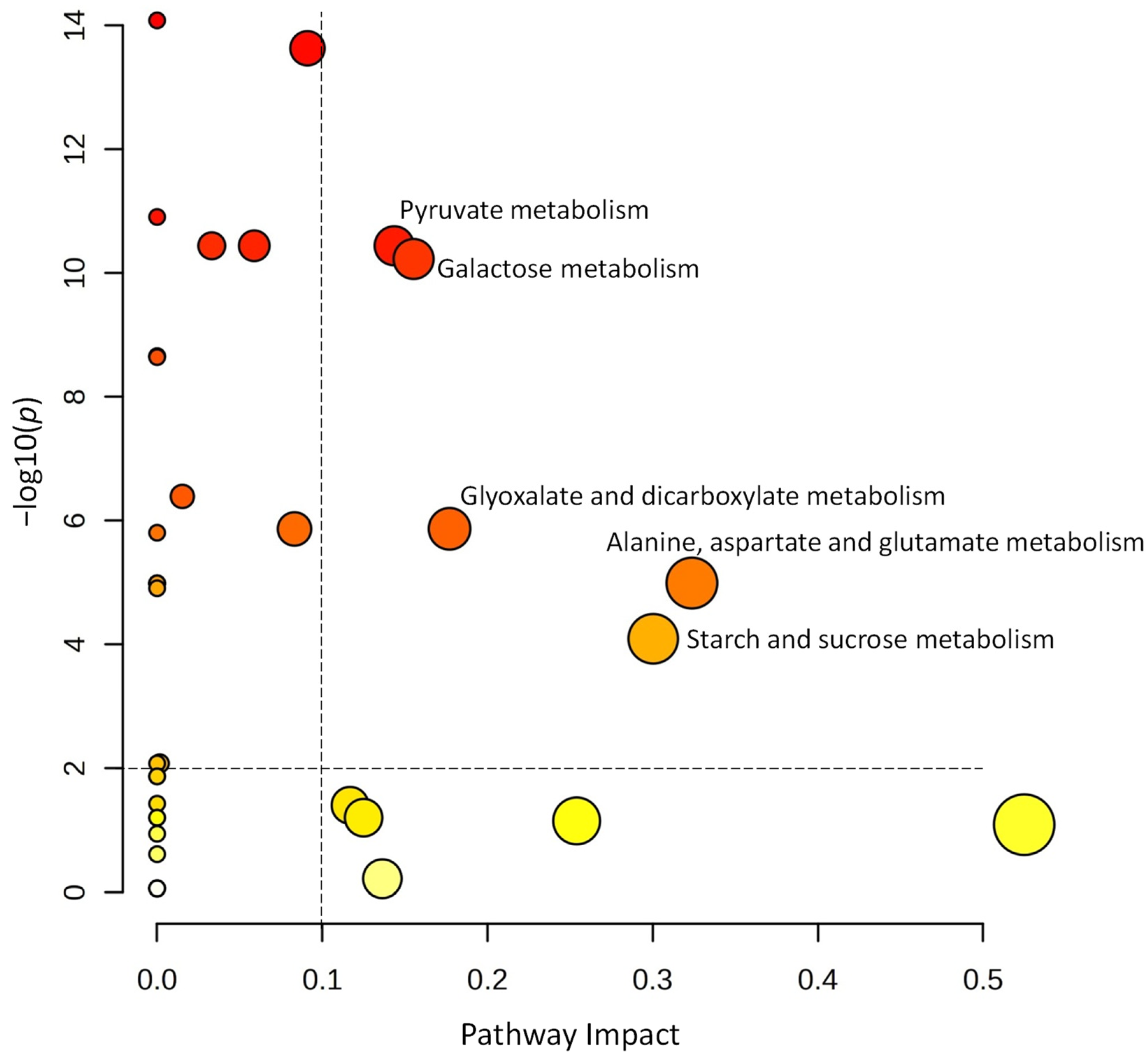
A volcano plot analysis was used to narrow the list of metabolites used for the pathway analysis (Figure 5). Comparing the metabolite data of tubers harvested from the self-grafted and TOM-grafted plants of all three potato cultivars, 27 compounds were identified with FC thresholds of 2 and p ≤ 0.01. The data of these compounds were uploaded to Metaboanalyst’s pathway analysis tool as a data table containing the relative concentration data of the two treatments, i.e., TOM-grafted compared to self-grafted plants. Data were analysed using the global test and relative betweenness centrality options and the pathway library of Arabidopsis thaliana available in KEGG. Pathways were considered significantly altered when the pathway impact was above 0.1 and −log(10)p was above 2. Five pathways corresponded to these criteria: ‘pyruvate metabolism’, ‘galactose metabolism’, ‘glyoxalate and dicarboxylate metabolism’, ‘alanine, aspartate, and glutamate metabolism’, and ‘starch and sucrose metabolism’ had the highest impact (Figure 6).
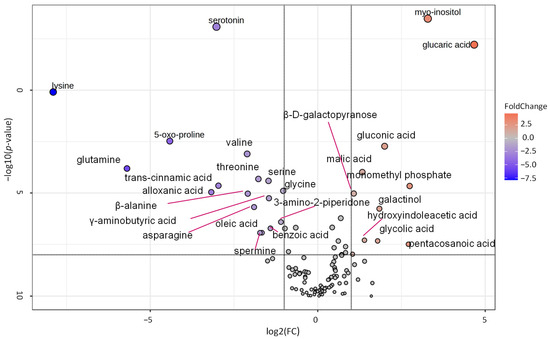
Figure 5.
Volcano plot showing fold change of the different metabolites found in tubers of tomato grafts relative to the self-grafts of the cultivars ‘White Lady’, ‘Hópehely’, and ‘Désirée’. Fold change (FC) threshold log2 = 1 and p ≤ 0.01. The statistical analysis was carried out with the same dataset as in Figure 2.
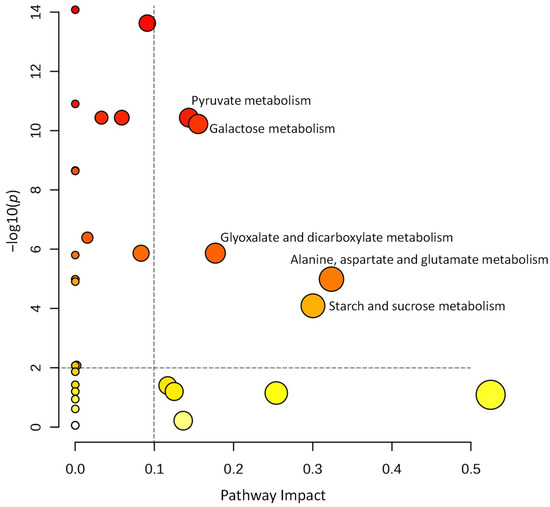
Figure 6.
Pathway analysis using the metabolites significantly altered in amount from the volcano plot shown in Figure 5. Pathway impact of significant metabolites is above 0.01 and −log10(p) ≥ 2. The size of the circles serves to signify the magnitude of pathway impact, while the significance is in connection with the colour, the darker ones having lower p values.
3.4. Starch and Protein Contents of Tubers Grown on Tomato/Potato Grafts
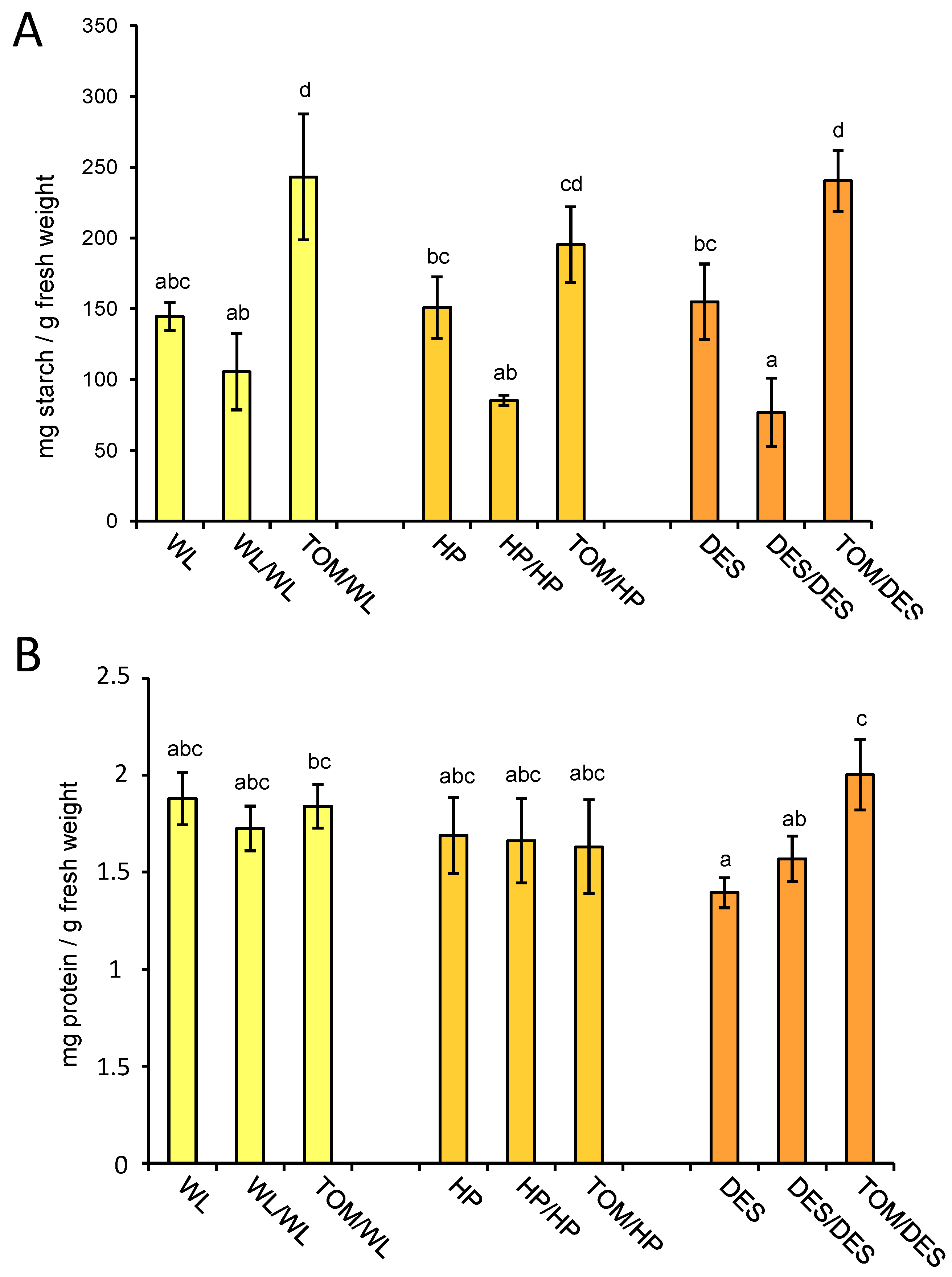
The major storage compound in tubers is starch, accounting for approximately 10–25% of fresh tuber weight. To test the influence of the TOM scion on the starch content of tubers, the same set of samples tested for metabolite composition was analysed for starch concentration. The starch amounts in tubers of the three potato cultivars were similar, approximately 15% of the fresh weight. The self-grafting decreased the starch content of tubers in each cultivar; however, to a significant manner only in DES. In contrast, the heterografting increased the starch content of tubers in each cultivar, which was significant compared to the nongrafted control in WL and DES, while compared to the self-grafted control it was significant in each cultivar (Figure 7A).
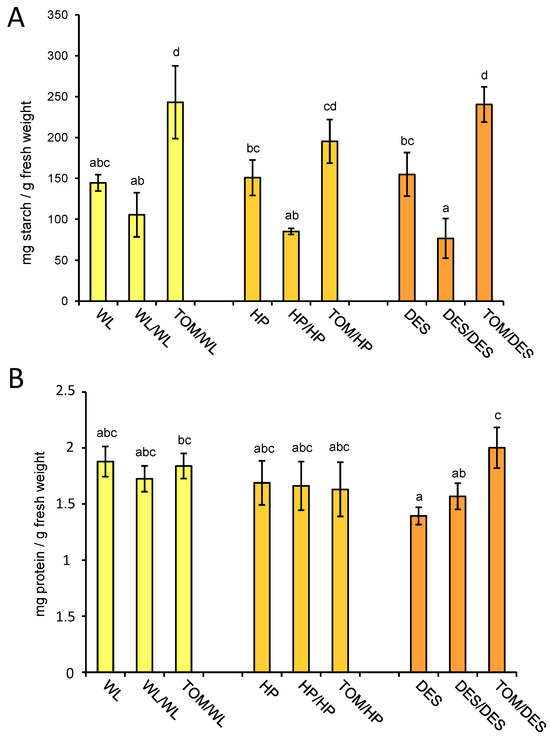
Figure 7.
Starch and protein contents of tubers. The same tuber samples, i.e., three groups of tubers each consisting of slices of four approximately same-size tubers, were analysed for starch (A) and protein contents (B) as for metabolites. Means presented with the same letter are not significantly different at p ≤ 0.05. The standard deviations are indicated by the error bars. The labels are as in Figure 1.
Figure 7B shows the protein content of tuber tissues of the different grafting combinations. DES is the only cultivar that shows significant alteration in protein concentration as an effect of heterografting. Compared to the nongrafted and self-grafted control, TOM increased the protein content of DES tubers by 44% and 28%, respectively.
3.5. Anthocyanin Content and Expression of StAN1 in the Tuber Skin of Tomato/Potato Grafts
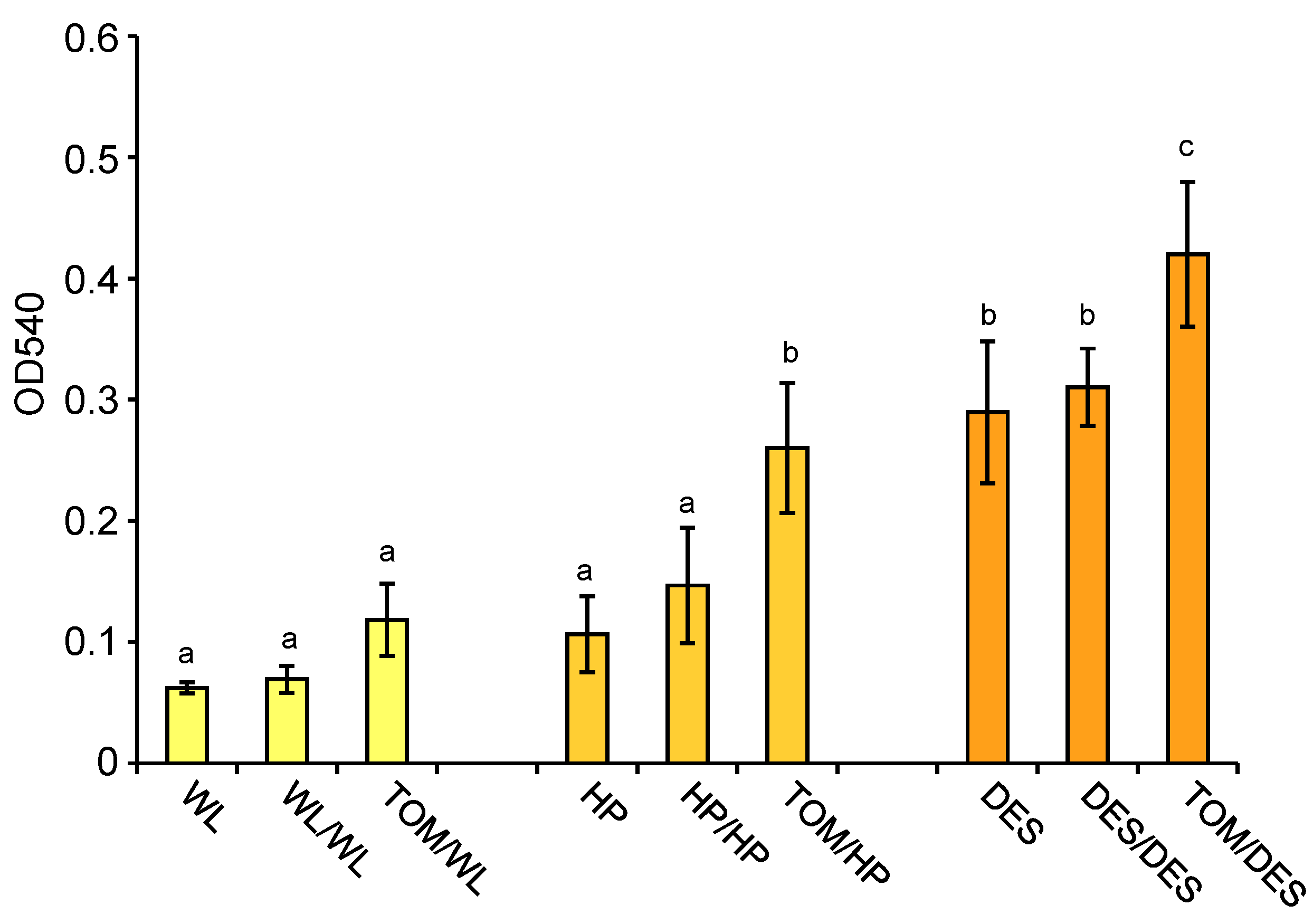
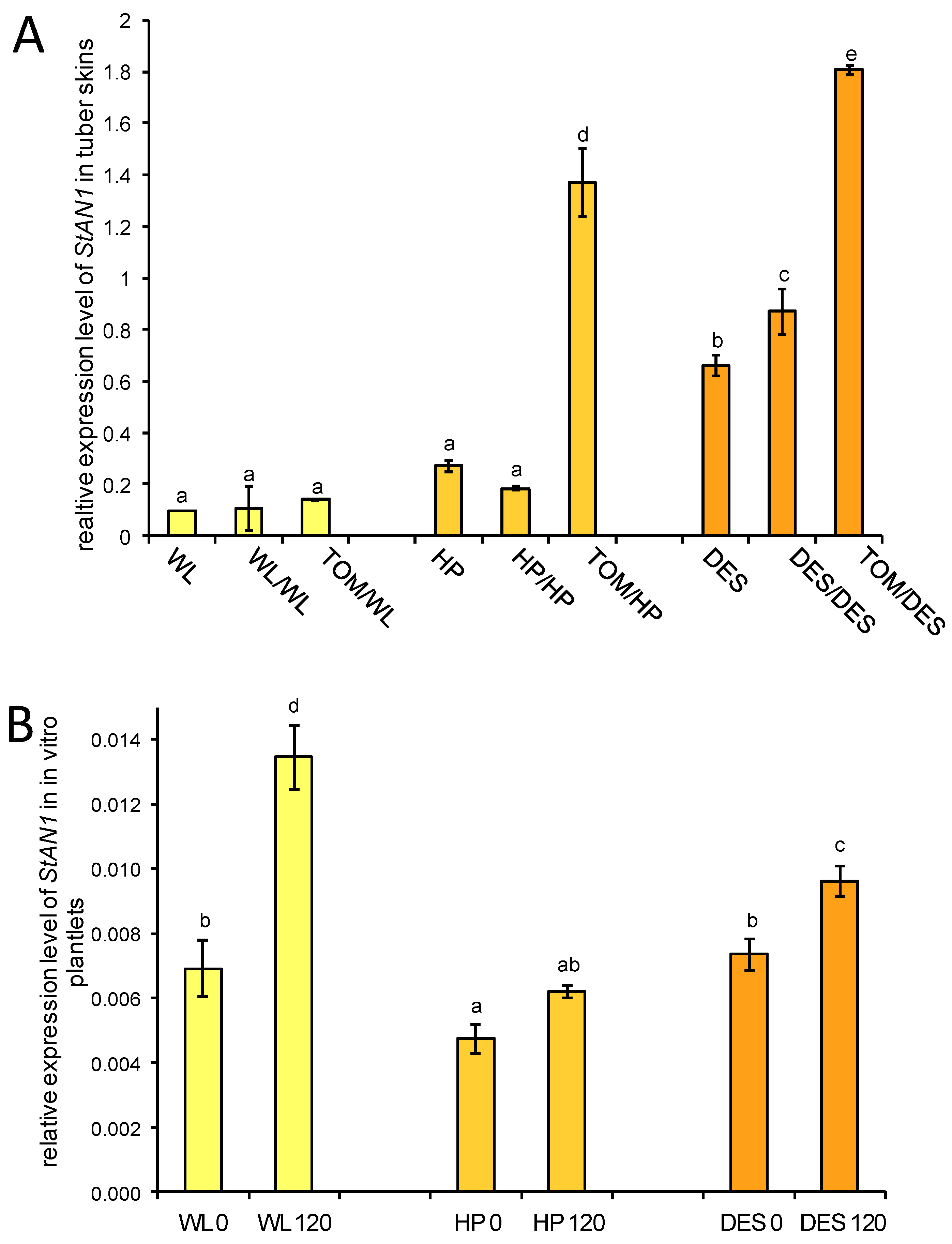
The skin colours of tubers grown on HP and DES heterografts were more intense than those of the tubers grown on control plants (Figure 1). Skin colour is determined by anthocyanins. These compounds were extracted from tuber skins, and their relative quantity was measured spectrophotometrically. Figure 8 shows that the levels of anthocyanins were significantly increased in the tuber skins of both HP and DES heterografts.
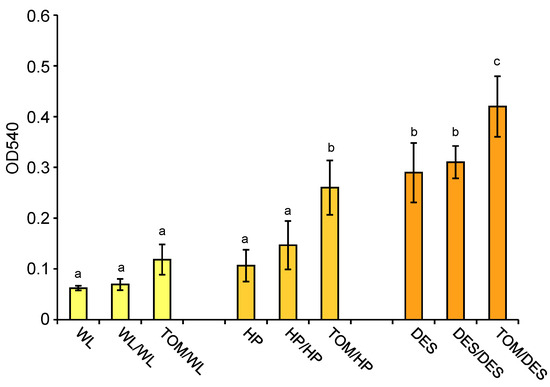
Figure 8.
Anthocyanin contents of tuber skins shown as optical density (OD) measured at the wavelength of 540 nm. Skins of the same tubers, i.e., three groups of tubers each consisting of slices of four approximately same-size tubers, analysed at metabolite level, were analysed for anthocyanin content. The standard deviations are indicated by the error bars. Means presented with the same letter are not significantly different at p ≤ 0.05. The labels are as in Figure 1.
ATHOCYANIN1 (StAN1) is a major transcriptional regulator of genes involved in anthocyanin biosynthesis [23]. The expression of StAN1 was tested in tuber skins with RT–qPCR. Approximately 7-fold and 2-fold increases in StAN1 mRNA levels were observed in the tuber skins of HP and DES heterografts, respectively, whereas no difference was found in StAN1 expression in the tuber skin of WL heterografts compared to the corresponding self-grafted controls (Figure 9A).
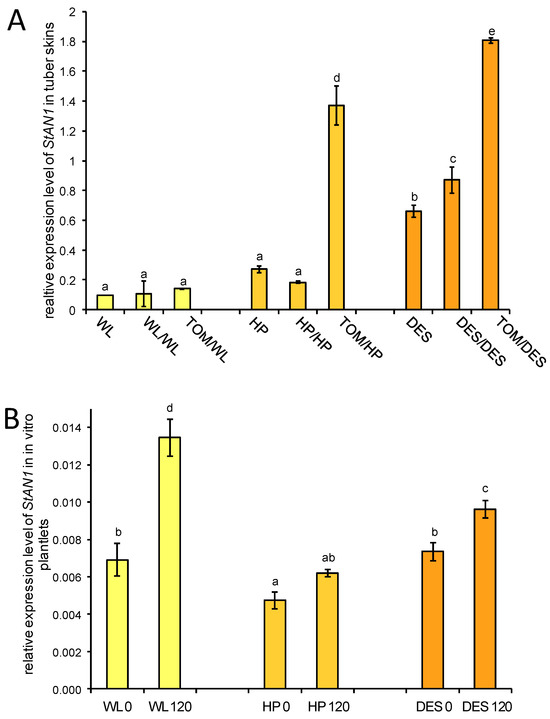
Figure 9.
Expression of StAN1 in three potato cultivars and their grafts. (A) Expression of StAN1 in tuber skins. RNA was isolated from the same group of samples used for anthocyanin measurement (Figure 8). The labels are as in Figure 1. (B) Expression of StAN1 in the leaves of sucrose-fed in vitro plantlets. WL 0, ‘White Lady’ grown with 0 mM sucrose; WL 120, ‘White Lady’ grown with 120 mM sucrose; HP 0, ‘Hópehely’ grown with 0 mM sucrose; HP 120, ‘Hópehely’ grown with 120 mM sucrose; DES 0, ‘Désirée’ grown with 0 mM sucrose; DES 120, ‘Désirée’ grown with 120 mM sucrose. Expression of StAN1 is related to that of ACTIN. Two sets of leaf samples each from five plants were tested in three technical replicates. The standard deviations are indicated by the error bars. Means presented with the same letter are not significantly different at p ≤ 0.05.
Payyavula et al. demonstrated that the expression of StAN1 is sucrose inducible in the potato cultivar ‘Purple Majesty’ [23]. To test the sucrose inducibility of StAN1 in the WL, HP, and DES cultivars, a sucrose-feeding experiment was performed with plantlets grown in vitro. RNA was isolated from leaves of non-sucrose-fed and sucrose-fed plantlets, and the relative amount of StAN1 mRNA compared to the expression level of ACTIN as a reference gene was analysed with RT–qPCR. Elevation of StAN1 expression was observed in all three cultivars: 2.2-fold in WL, 1.3-fold in HP, and 1.2-fold in DES leaves (Figure 9B).
4. Discussion
To study the effects of the tomato cultivar ‘Mobil’ (TOM) on the morphology and metabolite composition of tubers, three potato cultivars were used as rootstocks in grafting experiments. The three potato cultivars, ‘White Lady’ (WL), ‘Hópehely’ (HP), and ‘Désirée’ (DES), differed in tuber shape and skin colour as well as in tuber metabolite composition. WL and HP are genetically similar Hungarian cultivars, having one parent in common (Potato Pedigré Database; https://www.plantbreeding.wur.nl/PotatoPedigree/index.html, accessed on 25 October 2023), while DES is a Dutch cultivar, having ascendants genetically distant from the other two varieties. Despite the genetic similarity of the two Hungarian cultivars, their tubers showed contrasting concentrations of several amino acids and carbohydrates, whereas both WL and DES had higher amino acid contents than HP.
The three potato cultivars were different not only in the metabolite composition of tubers but also in their reactions evoked by the TOM scion. The highest level of influence was detected in tubers of DES heterografts, including the number of metabolites that changed in concentration and the significant increase in starch and protein contents. Previous studies also demonstrated cultivar-dependent effects of tomato scions, including even opposite directions of yield changes, as demonstrated in [12,15] versus [13,14], in which decreased yield versus increased yield is reported, respectively. Our experiment demonstrated a negative effect on the yield of all three cultivars tested.
Not only nongrafted but also self-grafted potato plants were applied in our study as controls. Changes in the concentrations of several metabolites, including sucrose, were detected in tubers of the self-grafted plants compared to the nongrafted controls. Nevertheless, none of the compounds had an elevated concentration in all three potato cultivars. The observed differences might be explained by the biological variation in the tubers and/or the lower analytical reproducibility of certain metabolites. Similar variation in the metabolite composition of tubers was found in one of our earlier grafting experiments [18] and in [30] studying Arabidopsis thaliana. The starch content of tubers of self-grafted plants was lower in all three potato cultivars than in the tubers of nongrafted plants. Although the difference was significant only in the case of DES, we think that this has a physiological background because the tendency was the same in all three cultivars. Plant development might be slowed down for a while by grafting, which could lead to delayed tuber development and lower starch content of tubers of self-grafted plants compared to the tubers of nongrafted plants.
Although to a different extent, TOM grafting increased the concentration of five compounds compared to the corresponding self-grafted controls in each potato cultivar. The highest increase (21–41-fold) was found in the glucaric acid concentration, followed by myo-inositol (10–11-fold) and galactinol (3–10-fold). The synthesis of these compounds is interconnected, as both glucaric acid and myo-inositol are produced in the ascorbate and aldarate pathways, and myo-inositol can be derived directly from galactinol, it being 1-α-D-galactosyl-myo-inositol. Grafting with TOM also increased the concentrations of malic acid and sucrose in tubers by 2.4–4.1-fold and 1.5–2.5-fold, respectively. Malic acid is present in substantial amounts in potato tubers, whereas sucrose is the dominant metabolite [27]. Thus, increases in the concentrations of these two compounds have a significant impact on the metabolite composition of tubers. In addition to the above mentioned five compounds, the starch content was significantly increased in tubers of heterografted cultivars compared to the corresponding self-grafted control. The pathway for the conversion of sucrose to starch in growing tubers is under flux control [31]. Since the tomato variety ‘Mobil’ that we used for the experiments grows more vigorously in greenhouse conditions than potatoes, it is conceivable that it delivered more assimilates from the leaves to the developing tubers than potatoes, and this is the explanation for the higher starch content of the tubers harvested from the heterografts than from controls.
The levels of five amino acids, including asparagine, were significantly reduced in the tubers of the TOM grafts compared to the control tubers in all three potato cultivars. It is known that acrylamide, with acute toxicity, can form from free asparagine and reducing sugars during potato frying or chips production [32]. Thus, the low asparagine concentration of TOM/DES tubers in which the concentration of reducing sugars is not changed significantly compared to the controls might be advantageous in terms of human health.
Grafting with TOM increased the anthocyanin content of the skins of the HP and WL tubers. Anthocyanins are produced, starting from phenylalanine, by the phenylpropanoid biosynthesis pathway. Numerous factors mediate the expression of phenylpropanoid genes, including transcription factors and sugars. MYB, bHLH, and WD40 are the three transcription factor families known to play major roles in the regulation of anthocyanin synthesis [33]. StAN1 (previously named StAN2 in some studies) is an R2R3 MYB transcription factor that can independently regulate anthocyanin synthesis in potato through the formation of the MYB-bHLH or the MYB-bHLH-WD40 complex [34]. Testing the expression of StAN1, we found a 7-fold and a 2-fold upregulation of StAN1 in the skins of tubers grown on TOM-grafted HP and DES plants, respectively, indicating that StAN1 is involved in the elevation of the tuber skin anthocyanin content in these cultivars. In line with the lack of colour change, the expression of StAN1 was not upregulated in TOM-grafted WL tuber skins in spite of the fact that the StAN1 gene turned out to be sucrose-inducible not only in HP and DES but also in WL. Regulation of StAN1 in different potato varieties might be different. Strygina et al. found high variability in the promoter region of StAN1 genes and identified 19 different cis-acting regulatory elements [35]. Mutation of any of these elements might cause the lack of transcriptional activation by the TOM scion in tubers of WL rootstock. In Petunia hybrida, the repressor PHMYB27 prevents the activation of PhAN2 [36]. Thus, an alternative explanation might be that a repressor keeps the expression of StAN1 low in WL.
An interesting finding was the rounding of rootstock tubers as an effect of TOM scions. Cultivated potato is diverse in shape, from elongated to round. This phenotypic variability allowed the genetic analysis of this important trait and identification of the Ro locus on chromosome 10 conferring round tuber shape [37]. This locus carries the StOFP20 marker, which identifies a gene similar to OVATE FAMILY PROTEIN 20 (SlOFP20), underlying fruit shape in tomato [38]. In potato, loss of function of StOFP20 alters tuber shape [39]. Nevertheless, how it is regulated by a TOM scion signal needs further investigation.
5. Conclusions
Potato tubers are sink organs whose metabolite compositions are influenced by the source organ, the vegetative part of the plant. We were interested in how tomato, as a scion, can influence the quality parameters of tubers. Testing three different potato cultivars as rootstocks, we found 59 metabolites, mainly carbohydrates and amino acids, altered in amounts in the tubers of the heterografts. Twelve compounds had the same strand of alteration in each cultivar. Tomato scions significantly increased the starch content of tubers compared to the tubers of self-grafted controls. Since tomato grows more vigorously in greenhouse conditions than potato, it is conceivable that it delivers more assimilates from the leaves to the developing tubers than potato, leading to increased starch content. The anthocyanin content of tuber skins in two cultivars was increased in correlation with the transcriptional activation of the transcription factor StAN1, which has a central role in the regulation of anthocyanin synthesis. As a further experiment, analysis of phloem sap is required to identify the inducing compound. Finally, we can conclude that consumption of fries and chips prepared from tomato grafts’ tubers has lower health risk than from nongrafted tubers due to the reduced asparagine content.
Author Contributions
Data curation, Z.B.; funding acquisition, Z.B.; investigation, V.V., K.O. and C.O.O.; methodology, V.V.; supervision, Z.B.; visualization, V.V.; writing—original draft, V.V.; writing—review and editing, Z.B. All authors have read and agreed to the published version of the manuscript.
Funding
The research was financed by the National Research, Development and Innovation Office (Grant No. NN_124441 and K_146328).
Data Availability Statement
The datasets generated and analysed during the current study are available in the Metabolights database under the study identifier MTBLS7601.
Acknowledgments
We thank András Kis for advising in isolation of RNA with TRIZOL reagent and Monika Kiss for the excellent technical assistance and plant propagation.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nie, W.; Wen, D. Study on the applications and regulatory mechanisms of grafting on vegetables. Plants 2023, 12, 2822. [Google Scholar] [CrossRef] [PubMed]
- Melnyk, C.W. Plant grafting: Insights into tissue regeneration. Regeneration 2017, 4, 3–14. [Google Scholar] [CrossRef]
- Bond, T.E.T. Phytophthora infestans (mont.) debary and Cladosporium fulvum cooke on varieties of tomato and potato and on grafted solanaceous plants. Ann. Appl. Biol. 1936, 23, 11–29. [Google Scholar] [CrossRef]
- Mai, W.F. Virus X in the newer potato varieties and the transmission of this virus by the cutting knife. Am. Potato J. 1947, 24, 341–351. [Google Scholar] [CrossRef]
- Raymer, W.B.; O’Brien, M.J. Transmission of potato spindle tuber virus to tomato. Am. Potato J. 1962, 39, 401–408. [Google Scholar] [CrossRef]
- Ulrychová, M.; Limberk, J. Some factors affecting the spread of mycoplasma in plants. Biol. Plant. 1972, 14, 238–240. [Google Scholar] [CrossRef]
- Khan, A.; Sagar, G.R. Alteration of the pattern of distribution of photosynthetic products in the tomato by manipulation of the plant. Ann. Bot. 1969, 33, 753–762. [Google Scholar] [CrossRef]
- Bünemann, G.; Grassia, A. Growth and mineral distribution in grafted tomato/potato plants according to sink number. Sci. Hortic. 1973, 1, 13–24. [Google Scholar] [CrossRef]
- Mill, J.G. Distribution of steroidal glycoalkaloids in reciprocal grafts of Solanum tuberosum L. and Lycopersion esculentum Mill. Experientia 1982, 38, 460–462. [Google Scholar] [CrossRef]
- Tsror, L.; Nachmias, A. Significance of the root system in verticillium wilt tolerance in potato and resistance in tomato. Israel J. Plant Sci. 1995, 43, 315–323. [Google Scholar]
- Barker, H. Inheritance of resistance to potato viruses Y and A in progeny obtained from potato cultivars containing gene Ry: Evidence for a new gene for extreme resistance to PVA. Theor. Appl. Genet. 1997, 95, 1258–1262. [Google Scholar] [CrossRef]
- Peres, L.E.P.; Carvalho, R.F.; Zsögön, A.; Bermúdez-Zambrano, O.D.; Robles, W.G.R.; Tavares, S. Grafting of tomato mutants onto potato rootstocks: An approach to study leaf-derived signaling on tuberization. Plant Sci. 2005, 169, 680–688. [Google Scholar] [CrossRef]
- Negi, V.; Sharma, P.; Raj, D.; Singh, A.; Vats, B. Horticultural and yield related traits as influenced by grafting tomato cultivars on potato rootstocks for higher returns. Indian J. Ecol. 2017, 44, 364368. [Google Scholar]
- Arefin, S.M.A.; Zeba, N.; Solaiman, A.H.; Naznin, M.T.; Azad, M.O.K.; Tabassum, M.; Park, C.H. Evaluation of compatibility, growth characteristics, and yield of tomato grafted on potato (‘pomato’). Horticulturae 2019, 5, 37. [Google Scholar] [CrossRef]
- Zhang, G.; Guo, H. Effects of tomato and potato heterografting on photosynthesis, quality and yield of grafted parents. Hortic. Environ. Biotechnol. 2019, 60, 9–18. [Google Scholar] [CrossRef]
- Zhang, G.; Mao, Z.; Wang, Q.; Song, J.; Nie, X.; Wang, T.; Zhang, H.; Guo, H. Comprehensive transcriptome profiling and phenotyping of rootstock and scion in a tomato/potato heterografting system. Physiol. Plant. 2019, 166, 833–847. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Plant Physiol. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Villányi, V.; Gondor, O.K.; Bánfalvi, Z. Metabolite profiling of tubers of an early- and a late-maturing potato line and their grafts. Metabolomics 2022, 18, 88. [Google Scholar] [CrossRef]
- Shepherd, T.; Dobson, G.; Verral, S.R.; Conner, S.; Griffiths, D.W.; McNicol, J.W.; Davies, H.V.; Stewart, D. Potato metabolomics by GC–MS: What are the limiting factors? Metabolomics 2007, 3, 475–488. [Google Scholar] [CrossRef]
- Lewis, C.E. Anthocyanins and Related Compounds in Potatoes (Solanum tuberosum L.). Ph.D. Dissertation, University of Canterbury, Christchurch, New Zealand, 1996; pp. 205–206. [Google Scholar]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Toguri, T.; Umemoto, N.; Kobayashi, O.; Ohtani, T. Activation of anthocyanin synthesis genes by white light in eggplant hypocotyl tissues, and identification of an inducible P-450 cDNA. Plant Mol. Biol. 1993, 23, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Payyavula, R.S.; Singh, R.K.; Navarre, D.A. Transcription factors, sucrose, and sucrose metabolic genes interact to regulate potato phenylpropanoid metabolism. J. Exp. Bot. 2013, 64, 5115–5131. [Google Scholar] [CrossRef]
- Stiekema, W.J.; Heidekamp, F.; Dirkse, W.G.; van Beckum, J.; de Haan, P.; Bosch, C.T.; Louwerse, J.D. Molecular cloning and analysis of four potato tuber mRNAs. Plant Mol. Biol. 1988, 11, 255–269. [Google Scholar] [CrossRef]
- Rio, D.C.; Ares, M.; Hannon, G.J.; Nilsen, T.W. Purification of RNA using TRIzol (TRI Reagent). Cold Spring Harb. Protoc. 2010, 2010, 5439. [Google Scholar] [CrossRef]
- Nicot, N.; Hausman, J.F.; Hoffmann, L.; Evers, D. Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress. J. Exp. Bot. 2005, 56, 2907–2914. [Google Scholar] [CrossRef]
- Roessner, U.; Wagner, C.; Kopka, J.; Trethewey, R.N.; Willmitzer, L. Simultaneous analysis of metabolites in potato tuber by gas chromatography-mass spectrometry. Plant J. 2000, 23, 131–142. [Google Scholar] [CrossRef]
- Uri, C.; Juhász, Z.; Polgár, Z.; Bánfalvi, Z. A GC-MS-based metabolomics study on the tubers of commercial potato cultivars upon storage. Food Chem. 2014, 159, 287–292. [Google Scholar] [CrossRef]
- Odgerel, K.; Bánfalvi, Z. Metabolite analysis of tubers and leaves of two potato cultivars and their grafts. PLoS ONE 2021, 16, e0250858. [Google Scholar] [CrossRef]
- Fiehn, O.; Kopka, J.; Dörmann, P.; Altmann, T.; Trethewey, R.N.; Willmitzer, L. Metabolite profiling for plant functional genomics. Nat. Biotechnol. 2000, 18, 1157–1161. [Google Scholar] [CrossRef]
- Geigenberger, P.; Stitt, M.; Fernie, A.R. Metabolic control analysis and regulation of the conversion of sucrose to starch in growing potato tubers. Plant Cell Environ. 2004, 27, 655–673. [Google Scholar] [CrossRef]
- Halford, N.G.; Raffan, S.; Oddy, J. Progress towards the production of potatoes and cereals with low acrylamide-forming potential. Curr. Opin. Food Sci. 2022, 47, 100887. [Google Scholar] [CrossRef]
- Wu, Y.; Han, T.; Lyu, L.; Li, W.; Wu, W. Research progress in understanding the biosynthesis and regulation of plant anthocyanins. Sci. Hortic. 2023, 321, 112374. [Google Scholar] [CrossRef]
- Liu, Y.; Tikunov, Y.; Schouten, R.E.; Marcelis, L.F.M.; Visser, R.G.F.; Bovy, A. Anthocyanin biosynthesis and degradation mechanisms in solanaceous vegetables: A review. Front. Chem. 2018, 6, 52. [Google Scholar] [CrossRef]
- Strygina, K.V.; Alex, V.; Kochetov, A.V.; Khlestkina, E.K. Genetic control of anthocyanin pigmentation of potato tissues. BMC Genet. 2019, 20, 27. [Google Scholar] [CrossRef]
- Albert, N.W.; Lewis, D.H.; Zhang, H.; Schwinn, K.E.; Jameson, P.E.; Davies, K.M. Members of an R2R3-MYB transcription factor family in Petunia are developmentally and environmentally regulated to control complex floral and vegetative pigmentation patterning. Plant J. 2011, 65, 771–784. [Google Scholar] [CrossRef]
- van Eck, H.J.; Jacobs, J.M.; Stam, P.; Ton, J.; Stiekema, W.J.; Jacobsen, E. Multiple alleles for tuber shape in diploid potato detected by qualitative and quantitative genetic analysis using RFLPs. Genetics 1994, 137, 303–309. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, B.; Keyhaninejad, N.; Rodríguez, G.R.; Kim, H.J.; Chakrabarti, M.; Illa-Berenguer, E.; Taitano, N.K.; Gonzalo, M.J.; Díaz, A.; et al. A common genetic mechanism underlies morphological diversity in fruits and other plant organs. Nat. Commun. 2018, 9, 4734. [Google Scholar] [CrossRef]
- Ai, J.; Wang, Y.; Yan, Y.; Li, C.; Luo, W.; Ma, L.; Shang, Y.; Gao, D. StOFP20 regulates tuber shape and interacts with TONNEAU1 recruiting motif proteins in potato. J. Integr. Agric. 2023, 22, 752–776. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).